Abstract
Context: Daucus carota Linn (Apiaceae), a useful vegetable, is traditionally used in treating kidney and hepatic dysfunctions.
Objective: To evaluate the protective and curative potential of D. carota root extract on renal ischemia reperfusion injury in rats.
Materials and methods: Wistar rats were selected with 8 + 8 groups (n = 6). Renal pedicles of rats were occluded for 45 min and allowed for reperfusion period. In protective and curative studies, 14 days prior and 14 days after the induction of ischemia/reperfusion (I/R), rats received petroleum ether extract (PEE 250 and 500 mg/kg), fractional methanol extract (FME 250 and 500 mg/kg) and direct methanol extract (DME 250 and 500 mg/kg) of Daucus carota root, orally, once daily.
Results: PEE at a dose of 500 mg/kg significantly (p < 0.001) reduced the levels of serum creatinine (0.853–3.090 mg/dl), uric acid (1.300–3.500 mg/dl) and urea (58.26–132.00 mg/dl) compared to disease control. FME at a dose of 500 mg/kg body weight significantly (p < 0.001) reduced the levels of serum creatinine (0.960–3.090 mg/dl), uric acid (1.700–3.500 mg/dl) and urea (77.17–132.00 mg/dl) compared to disease control. DME at a dose of 500 mg/kg body weight significantly (p < 0.001) reduced the levels of serum creatinine (1.173–3.090 mg/dl), uric acid (2.267–3.500 mg/dl) and urea (84.75–132.00 mg/dl) compared to disease control.
Discussion and conclusion: Findings demonstrate that postconditioning with the D. carota root extract significantly improves kidney function in I/R rats.
Introduction
Ischemic injury to vital organs such as heart, brain and kidneys is a major cause of morbidity and mortality rate in the twenty-first century. Acute kidney injury is a devastating disease with clinical, economical and ethical dimensions that are emerging as a major public health problem worldwide (Esson et al., Citation2002; Thadhani et al., Citation1996). Recently, prospective studies on overall incidence of acute renal failure is almost 500 million per year (Hegarty et al., Citation2005; Stevens et al., Citation2001) and an incidence of acute renal failure needing dialysis of more than 200 million per year (Metcalfe et al., Citation2002).
Renal ischemia, a consequence of arterial occlusion shock and organ transplantation, is a common cause of renal cell death and renal failure. After an acute renal ischemic event, early reperfusion remains the most effective strategy to limit organ damage. However, reperfusion of the kidney has the potential to cause lethal cell death similar to that in heart. Novel protective strategies are applied at the time of reperfusion to prevent this injury (Serviddio et al., Citation2008).
The mechanisms of renal ischemia/reperfusion (I/R) injury involve both vascular and tubular factors, but despite advances in preventive strategies, this disease continues to be associated with significant morbidity and mortality (Kelly et al., Citation2006) and there is no successful specific therapy except for supportive care (Friedewald et al., Citation2004).
Daucus carota Linn. (Apiaceae), commonly known as carrot, has undergone extensive phytochemical studies and a large number of active ingredients have been isolated indicating its pharmacological activities (Vasudevan et al., Citation2006). Reported phytoconstituents present in carrot are phenolic compounds, terpenes and carotenoids, which act as free radical quenchers and scavengers (Desobry et al., Citation1998; Heinonen, Citation1990; Johnson, Citation2003). Daucus carota is used as an antiasthmatic, anthelmintic, anticancer, anti-inflammatory agent that gives protection against kidney dysfunctions or hepatic injury (Bishayee et al., Citation1995; Lokar & Poldini, Citation1988; Zaini et al., Citation2011). Recent studies also proved the efficacy of D. carota for hypoglycemic, antifertility and aphrodisiac activities (Singh et al., Citation2012). Two new sesquiterpenoids containing an interesting epoxy unit, daucuside and daucusol, isolated from the fruits and fresh juice of D. carota, have been used for the treatment of leukemia (Fu et al., Citation2010).
Consequently, the authors evaluated the capacity of D. carota in ischemic preconditioning and ischemic postconditioning to minimize renal tissue injury in the procedure of renal ischemia and reperfusion.
Materials and methods
Collection of plant material and preparation of extract
Fresh roots of orange colored D. carota were procured in the month of January 2011, from the local merchants outside Dehradun. The collected D. carota roots were identified by Dr. Imran Kazmi, Assistant Professor, Siddhartha Institute of Pharmacy, Dehradun (SIP/DPP/consult/-21-01-11/375/98). Roots were washed with fresh water, crushed, dried under shade, finely powdered and stored at room temperature. The powdered material was extracted successively with petroleum ether, chloroform and methanol by continuous hot extraction method. Direct methanol extraction of fresh powder was also carried and all the extracts were concentrated under reduced pressure.
Experimental animals
Healthy male/female Wistar rats weighing 200–250 g were procured from the central animal house facility of Siddhartha Institute of Pharmacy. All animals were housed at an ambient temperature (22 ± 1 °C) and relative humidity (55 ± 5%) with standard diet and water ad libitium. The protocol was approved by the Institutional Animal Ethics Committee (IAEC) as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests (Animal Welfare Division), Government of India. The work was divided into two protocols: protective (48 rats) and curative (48 rats). The rats were divided into eight groups (n = 6): sham control (SC), disease control (DC) and treatment group of petroleum ether extract (PEE 250 and 500 mg/kg), fractional methanol extract (FME 250 and 500 mg/kg) and direct methanol extract (DME 250 and 500 mg/kg) of D. carota root for both of the studies.
Induction of renal ischemia/reperfusion injury in rat
Animals were anesthetized with ketamine (60 mg/kg i.p.) and diazepam (5 mg/kg i.p). Body temperature was maintained throughout the surgery at 37 ± 0.5 °C with a lamp. The skin on the back of the animals was shaved and disinfected with the povidone iodine solution. All rats underwent surgical exposure of the left and right renal pedicles via midline incision. To induce renal ischemia, both renal pedicles were occluded for 45 min with vascular clamps. After 45 min of occlusion, the clamps were removed and kidneys were reperfused for 24 h. The abdominal muscle layer was closed with an interrupted suture, and the skin layer was closed with a continuous subcutaneous suture. Topically 2% of lidocaine jelly was applied for analgesia in experimental rats. The wound for 24 h and one dose of acetaminophen (6.8 mg/kg per rectum) as deemed by the Animal Care Staff was also administered. After 24 h of reperfusion for protective study (preconditioning of the kidney) and 14 days of reperfusion in the curative study (postconditioning of kidney), the rats were sacrificed and the kidney was rapidly removed for further analysis.
Estimation of biochemical parameters
Blood samples were collected on the termination day of the experiment from retro-orbital plexus under light ether anesthesia without any anticoagulant and allowed to stand for 30 min at room temperature, centrifuged at 2500 rpm for 10 min to separate serum. Serum obtained was kept at 2–4 °C for further use. Serum creatinine, urea and uric acid levels were estimated using Standard kits (Nicholas India Pvt. Ltd., Hyderabad, India) with semi-auto analyzer (photometer 5010, Nicholas India Pvt. Ltd).
Antioxidant enzyme estimation
After sacrificing the animals, kidneys were quickly removed, perfused immediately with ice cold hypertonic saline solution and homogenized in chilled potassium chloride (1.17%) using a Potter Elvehjem Homogenizer (Remi, Mumbai, India). The homogenate was centrifuged at 10,500g for 20 min at 4 °C to get the postmitochondrial supernatant, which was used to assay superoxide dismutase (SOD) (Misra et al., Citation1972), catalase (Aebi et al., Citation1974), reduced glutathione (GSH) (Moran et al., Citation1979) and lipid peroxidation activities (Ohkawa et al., Citation1979).
Histopathological examination
The kidneys were preserved in phosphate-buffered 10% formalin, embedded in paraffin and were used for histopathological examination. Sections were cut (5 µm thick), deparaffinized, hydrated and stained with hematoxylin and eosin. The renal sections were examined blindly for tubular cell swelling, interstitial edema, tubular dilatation and to determine moderate to severe necrosis in all treatments.
Statistical analysis
Results were expressed as mean ± SEM. Statistical significance between more than two groups was tested using one-way ANOVA followed by the Bonferroni multiple comparison test or an unpaired two-tailed Student’s t-test as appropriate using computer-based fitting program (GraphPad Software, Inc., La Jolla, CA). Differences were considered to be statistically significant when p < 0.05.
Results
Protective study
Serum creatinine, uric acid, urea and blood urea nitrogen (BUN) were evaluated as indices of renal function and their profiles are reported in . Renal I/R produces significant increase (p < 0.001) in the levels of serum creatinine, uric acid, urea and BUN when compared to sham control, which indicates that renal I/R leads to the generation of oxidative stress, leading to kidney damage. Pretreatment with PEE, FME and DME of D. carota root at 250 and 500 mg/kg, for 14 days, significantly decreased (p < 0.001) serum creatinine, urea, uric acid and BUN levels found in renal I/R rats, compared to renal I/R control rats, which indicates the protective activity of D. carota root extract against renal I/R-induced oxidative stress in the kidney.
Table 1. Data showing comparison of serum creatinine, uric acid, urea and blood urea nitrogen (BUN) in sham control (SC), disease control (DC) and treated group of petroleum ether extract (PEE 250 and 500 mg/kg), fractional methanol extract (FME 250 and 500 mg/kg) and direct methanol extract (DME 250 and 500 mg/kg) of D. carota root.
depicts the ischemia and reperfusion on renal SOD and catalase activities as well as renal GSH and malondialdehyde (MDA) levels. In preconditioning of the kidney, there was a significant increase (p < 0.001 or 0.01) in SOD, catalase and GSH, while significant decrease (p < 0.001) in MDA was found in renal I/R rats when pretreated with PEE, FME and DME of D. carota root at 250 and 500 mg/kg, compared to renal I/R control rats. It is speculated that pretreatment with the D. carota root extract prevented renal I/R-induced lipid peroxidation and protected the kidneys from severe increasing of ROS products.
Table 2. Data showing comparison of SOD, catalase, MDA and glutathione in sham control (SC), disease control (DC) and treated group of petroleum ether extract (PEE 250 and 500 mg/kg), fractional methanol extract (FME 250 and 500 mg/kg) and direct methanol extract (DME 250 and 500 mg/kg) of D. carota root.
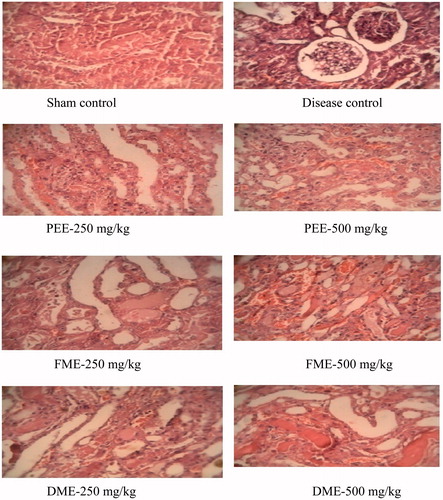
Sections of the rat kidney (hematoxylin-eosin, ×100) are shown in . A kidney section of a rat in the sham control group shows normal glomeruli and tubuli. A kidney section of a rat exposed to renal I/R shows interstitial hemorrhage surrounding the glomeruli, loss of tubular epithelial cells and lumen of tubule shows cell debris. Kidney section of the rats with I/R injury treated with 500 mg/kg PEE, DME and FME, respectively, showed moderate congestion of glomeruli and moderate damage to the tubules. Kidney section of the rats with I/R injury treated with 250 mg/kg PEE, DME and FME, respectively, showed mild congestion of glomeruli and damaged tubules.
Curative study
Data depicted in show that in the curative (postconditioning of kidney) study, a significant increase (p < 0.001) in the levels of serum creatinine, uric acid, urea and BUN was observed in renal I/R rats when compared to sham control, which indicates that renal I/R leads to the generation of oxidative stress, causing kidney damage. Postconditioning of kidney resulted in a significant decrease (p < 0.001) in serum creatinine, urea, uric acid and BUN levels found in renal I/R rats post-treated with PEE, FME and DME of D. carota root at 500 mg/kg, compared to renal I/R control rats, which indicates the curative potential of D. carota root extract against renal I/R-induced oxidative stress in the kidney. Post-treatment of PEE, FME and DME of D. carota root at 250 mg/kg also showed a significant decrease (p < 0.05 or 0.01 or 0.001) in creatinine, urea, uric acid and BUN levels compared to renal I/R control rats, but the levels were still high compared to 500 mg/kg post-treated animals. The above results show that at 500 mg/kg dose D. carota root extract demonstrated the best curative activity against renal I/R in rats.
Table 3. Data showing comparison of serum creatinine, uric acid, urea and BUN in sham control (SC), disease control (DC) and treated group of petroleum ether extract (PEE 250 and 500 mg/kg), fractional methanol extract (FME 250 and 500 mg/kg) and direct methanol extract (DME 250 and 500 mg/kg) of D. carota root in curative study.
Data in show that in a curative study (postconditioning of the kidney), there was a significant increase (p < 0.001 or 0.01 or 0.05) of SOD, catalase and GSH. However, significant decreases (p < 0.001 or 0.01) in MDA were observed in renal I/R rats pretreated with PEE, FME and DME of D. carota root at 500 mg/kg, compared to renal I/R control rats. It can be speculated that post-treatment with D. carota root extract prevented renal I/R-induced lipid peroxidation and protected the kidneys from a severe increase in ROS products. Post-treatment of D. carota root extract at 250 mg/kg also showed antioxidant activity but it is not as significant as at a dose of 500 mg/kg.
Table 4. Data showing comparison of SOD, catalase, MDA and glutathione in sham control (SC), disease control (DC), and treated group of petroleum ether extract (PEE 250 and 500 mg/kg), fractional methanol extract (FME 250 and 500 mg/kg), direct methanol extract (DME 250 and 500 mg/kg) of D. carota root in curative study.
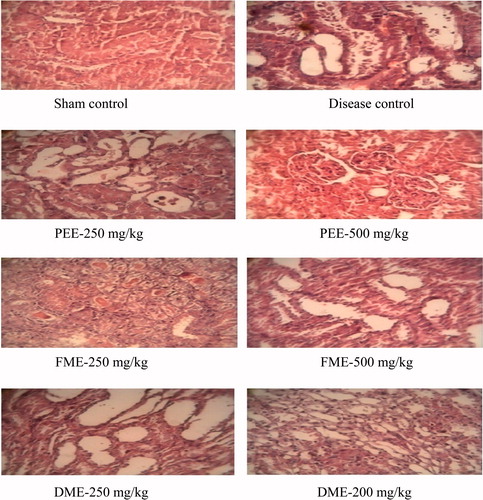
Sections of the rat kidney (hematoxylin-eosin, ×100) show the normal tubular structure of the kidney, showing normal tubular epithelium attached with baseline in sham control (). Kidney sections of a rat exposed to renal I/R show loss of tubular epithelial cells and lumen of tubule shows cell debris. Treatment with PEE 500 mg/kg depicts good recovery and regenerative tubules after ischemic injury. It was observed that recovered tubules along with ongoing recovery after induced ischemia when treated with FME and DME 500 mg/kg. Treatment with FME and DME 250 mg/kg shows damaged tubules along with ongoing recovery after ischemic injury.
Discussion
Some of the earlier methods of intervention have outlined free radical scavengers, enzyme inhibitors, receptor antagonists, cell adhesion molecule blockade, molecular manipulation of cells, controlled reperfusion, preconditioning: ischemic, pharmacological, postconditioning, to name a few. Preconditioning is a promising avenue to limit I/R injury-induced damage and can be classified as ischemic and pharmacologic preconditioning. Further, recent trials with the novel mechanism of postconditioning have shown some encouraging results. Clinically, postconditioning is well suited for vasculo-occlusive emergencies and elective surgical settings involving clamping of arteries and subsequent release (Singh et al., Citation2007). Postconditioning involves a series of brief mechanical interruptions of reperfusion that follow a specific prescribed algorithm applied at the very onset of reperfusion (Zhen-Xiao et al., Citation2007).
The mechanical procedure of postconditioning seems to induce a multiplicity of events that together attenuate reperfusion injury at many cellular and intracellular sites, as opposed to inhibiting an individual biochemical or molecular targets (Zhao et al., Citation2006).
However, despite the various phytochemical constituents and diverse medicinal activities attributed to D. carota plant, no comparative studies have been carried out to shed light on the role of D. carota in renal I/R. In the light of the above, the present study was undertaken for comparative evaluation of both pre- and postconditioning of kidney with D. carota root extract and their effects on serum creatinine, urea, uric acid and BUN levels and antioxidant enzymes in I/R rats.
Plasma concentrations of urea and uric acid were measured as an indicator of impaired renal function and/or increased catabolism (Baum et al., Citation1975; Ejaz et al., Citation2007). BUN and serum creatinine are very common parameters for the evaluation of renal function. However, increases in BUN or creatinine levels are considered to be indirect findings of renal dysfunction, because BUN and creatinine are waste products which are cleared from the blood into the urine, and therefore, a time lag is observed between the onset of renal impairment and the elevation of BUN or serum creatinine (El daly, Citation1996).
The present study suggests the involvement of antioxidant in the protection exerted by both pre- and postconditioning. There is a wide consensus that the protective mechanism of postconditioning involves a prevention of the burst or prolonged elevation of free radical generation. Data from the present study are consistent with reduction in oxygen radical species, and further suggest that those oxidants derived from D. carota root extracts reduced biochemical and antioxidant enzyme levels more by postconditioning as compared to preconditioning. This was the first report demonstrating that postconditioning is associated with protection of kidneys more as compared to preconditioning. In the postconditioning group, an almost complete recovery of the kidney is observed. In addition to potentially attenuating oxidant injury, postconditioning may have favorably altered mechanical events during reperfusion independent of altering oxidant-mediated injury.
Although experimental studies demonstrate that ischemic and pharmacological preconditioning attenuate ischemia/reperfusion injury, these interventions must be applied before the prolonged “index” ischemia. In the clinical setting, however, pretreatment is rarely an option. The results of the present study show that the salvage of the oxidative stress and renal damage can be achieved when the kidney is postconditioned with D. carota root extracts, briefly interrupted perfusion during the early moments of reflow. The degree of renal damage salvages with postconditioning was more comparable to that observed with preconditioning.
Conclusion
The present study shows, for the first time, that pre- and postconditioning with D. carota root extract improves in vivo renal function after prolonged ischemia, which is associated with attenuation of renal bioenergetics dysfunction, ROS synthesis and protein damage. The postconditioning procedure is very simple, and therefore, may be clinically applicable. Postconditioning, in contrast to preconditioning, which requires a foreknowledge of the ischemic event, can be clinically applicable at the onset of reperfusion at the point of clinical service, i.e., renal artery and kidney surgery or transplantation, where reperfusion injury is expressed. Future studies are needed to define the “gold algorithm” of postconditioning sequences, as well as other mechanisms involved in the protective effect.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Acknowledgements
The authors are greatly thankful to Mr. Durga Verma (Chairman) and Mr. R.R. Aggarwal (Director) of Siddhartha Institute of Pharmacy, Dehradun, India, for providing laboratory and library facilities for research work.
References
- Aebi H. (1974). Catalase estimation. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. Weinheim, New York: Verlag Chemic, 673–84
- Baum N, Dichoso CC, Carlton CE. (1975). Blood urea nitrogen and serum creatinine, physiology and interpretations. Urology 5:583–8
- Bishayee A, Sarkar A, Chatterjee M. (1995). Hepatoprotective activity of carrot (Daucus carota L.) against carbon tetrachloride intoxication in mouse liver. J Ethnopharmacol 47:69–74
- Desobry SA, Netto FM, Labuza TP. (1998). Preservation of β-carotene from carrots. Crit Rev Food Sci Nutr 38:381–96
- Ejaz AA, Mu W, Kang DH, et al. (2007). Could uric acid have a role in acute renal failure? Clin J Am Soc Nephrol 2:16–21
- El daly ES. (1996). Effect of methimazole and fish oil treatment on gentamicin nephrotoxicity in rats. J Islamic Acad Sci 9:37–48
- Esson ML, Schrier RW. (2002). Diagnosis and treatment of acute tubular necrosis. Ann Intern Med 137:744–52
- Friedewald JJ, Rabb H. (2004). Inflammatory cells in ischemic acute renal failure. Kidney Int 66:486–91
- Fu HW, Zhang L, Yi T, et al. (2010). Two new guaiane-type sesquiterpenoids from the fruits of Daucus carota L. Fitoterapia 81:443–6
- Hegarty J, Middleton RJ, Krebs M, et al. (2005). Severe acute renal failure in adults: Place of care, incidence and outcomes. Q J Med 98:661–6
- Heinonen MI. (1990). Carotenoids and provitamin A activity of carrot (Daucus carota L.) cultivars. J Agric Food Chem 38:609–12
- Johnson J. (2003). Phytochemical Functional Foods. New York: CRC Press
- Kelly KJ. (2006). Acute renal failure: Much more than a kidney disease. Semin Nephrol 26:105–13
- Lokar LC, Poldini L. (1988). Herbal remedies in the traditional medicine of the Venezia Giulia region (North East Italy). J Ethnopharmacol 22:231–78
- Metcalfe W, Simpson M, Khan IH, et al. (2002). Acute renal failure requiring renal replacement therapy: Incidence and outcome. Q J Med 95:579–83
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the antioxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:31–70
- Moran MS. (1979). Assay of reduced glutathione in animal tissue by dithio nitrobenzoic reaction. Biochem Biophys Res Commun 212:367–71
- Ohkawa I, Ohisi N, Yagi K. (1979). Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Serviddio G, Romano AD, Gesualdo L, et al. (2008). Postconditioning is an effective strategy to reduce renal ischaemia/reperfusion injury. Nephrol Dial Transplant 23:1504–12
- Singh HR, Ahilathirunayagam S, Andrew ID. (2007). Reperfusion syndrome: Cellular mechanisms of microvascular dysfunction and potential therapeutic strategies. Vasc Endovascular Surg 41:277–93
- Singh K, Singh N, Chandy A, Manigauha A. (2012). In vivo antioxidant and hepatoprotective activity of methanolic extracts of Daucus carota seeds in experimental animals. Asian Pac J Trop Biomed 2:385–8
- Stevens PE, Tamimi NA, Al-Hasani MK, et al. (2001). Non-specialist management of acute renal failure. Q J Med 94:533–40
- Thadhani R, Pascual M, Bonventre JV. (1996). Acute renal failure. N Engl J Med 334:1448–60
- Vasudevan M, Kumar KG, Parle M. (2006). Antinociceptive and anti-inflammatory properties of Daucus carota seed extract. J Health Sci 52:598–606
- Zaini R, Clench MR, Le Maitre CL. (2011). Bioactive chemicals from carrot (Daucus carota) juice extracts for the treatment of leukemia. J Med Food 14:1303–12
- Zhao ZQ, Vinten-Johansen J. (2006). Postconditioning: Reduction of reperfusion-induced injury. Cardiovasc Res 70:200–11
- Zhen-Xiao J, Jing-Jun Z, Mei X, et al. (2007). Postconditioning the human heart with adenosine in heart valve replacement surgery. Ann Thorac Surg 83:2066–73