Abstract
Context: Artichoke, Cynara scolymus L. (Asteraceae), has many natural antioxidants and multiple pharmacological actions. Recent studies have shown that it has antitoxic activity.
Objective: Lead (Pb) is a dangerous environmental toxicant that induces a broad range of dysfunctions in human. This study evaluated the protective effect of the hydroethanolic extract of artichoke against altered biochemical parameters in rats fed with lead-containing diet.
Materials and methods: Thirty-two rats were randomly divided into four groups. The first (control) group received standard diet. The second, third and fourth groups received 500 mg lead/kg diet, 500 mg lead/kg diet plus 300 mg/kg b.w. artichoke extract daily, and 500 mg lead/kg diet plus 1 mg vitamin C/100 g b.w. daily for 6 weeks, respectively. Serum lead, lipoprotein profile, ALT (alanine transaminase), AST (aspartate transaminase), ALP (alkaline phosphatase), malondialdehyde (MDA) and liver histopathology assessments were conducted.
Results: Serum lead, triglyceride (TG), VLDL, ALT, AST, ALP and MDA levels decreased significantly (p < 0.05) in the artichoke-treated group (35.85, 38.26, 38.38, 21.90, 12.81, 26.86 and 46.91%, respectively) compared to lead-intoxicated rats without treatment. No significant change was observed in serum lead, ALP and ALT between artichoke and vitamin C-treated groups (p > 0.05). Furthermore, the liver histopathology in rats treated with artichoke showed a mild degree of lymphocyte infiltration that was relatively comparable to the control and vitamin C-treated groups.
Discussion and conclusion: These results clearly show that the artichoke extract in lead-poisoned rats has suitable chelating properties for the reduction of blood lead levels.
Introduction
Lead (Pb) and its compounds are known to be major environmental pollutants adversely affecting human health (Hernberg, Citation2000). Lead rapidly accumulates in liver, kidney and other human organs after intestinal absorption (Mansouri & Cauli, Citation2009; Navarro-Moreno et al., Citation2009). Many previous studies have suggested that Pb might induce undesirable complications in laboratory animals and humans, such as physiological, biochemical, neurological, behavioral and reproductive dysfunctions (Abdallah et al., Citation2010; Patrick, Citation2006). Several mechanisms have been suggested to describe lead-induced toxicities (Tian & Lawrence, Citation1995). Oxidative stress due to Pb toxicity has been considered one of the major mechanisms of injury in the liver, kidneys, brain and other organs. Therefore, antioxidants may increase the efficacy of treatment designed to reduce lead intoxication (Mansouri & Cauli, Citation2009). Treatment of Pb toxicity in humans needs to increase the excretion of Pb from human body by chelation (Ahamed & Siddiqui, Citation2007). The current therapeutic drugs, such as meso-2,3-dimercaptosuccinic acid (DMSA), are unsuitable for administration in a long period of time (Flora et al., Citation2007). Some published studies have shown that some ethnomedicines have therapeutic effects on Pb toxicity in animals by improving the histological pictures of organs and the biochemical parameters towards the normal values (El-Nekeety et al., Citation2009; Queiroz et al., Citation2011; Sharma et al., Citation2010). Therefore, the search for natural compounds, with low or without adverse effects, to reduce Pb toxicity, is warranted.
Artichoke, Cynara scolymus L. (Asteraceae), leaves have been used in folk medicine for the treatment of diseases such as hyperlipidemia (Heidarian et al., Citation2011a; Heidarian & Soofiniya, Citation2011; Joy & Haber, Citation2007). Some researchers have isolated and identified several bioactive components of artichoke, i.e., caffeic acid, chlorogenic acid, cynarin and luteolin. These bioactive components decrease the production of reactive oxygen species (ROS), lipid peroxidation and the oxidation of low-density lipoproteins in vitro experiments (Juzyszyn et al., Citation2008; Zapolska-Downar et al., Citation2002). On the other hand, several studies have reported that vitamin C has some protective activity against Pb intoxication (Patra et al., Citation2001; Shalan et al., Citation2005). The treatment of Pb toxicity with vitamin C is due to its antioxidant efficacy that inhibits lipid peroxidation enhanced by Pb (Upasani et al., Citation2001). Therefore, considering the antioxidant properties of artichoke, this study was undertaken to evaluate the protective effect of artichoke extract against Pb toxicity in rat.
Materials and methods
Animals
The study was done on 32 adult male Wistar rats weighing 150–200 g. The animals were acclimated to laboratory conditions with regular temperature control (23 ± 2 °C), a 12:12 h light–dark cycle, and relative humidity of 55 ± 5%. All animal procedures were performed with regard to Iranian Animal Ethics Society and local university rules.
Chemicals and reagents
Alanine aminotransferase (ALT), aspartate aminotranferase (AST) and alkaline phosphatase (ALP) kits were purchased from Pars Azmon Co. (Tehran, Iran). Lead acetate, 2,4,6-tripyridyl-s-triazine (TPTZ) were obtained from Sigma (St. Louis, MO). Vitamin C, 2-thiobarbituric acid (TBA), ferric chloride (FeCl3 ċ 6H2O) and sodium acetate were provided by Merck Company (Darmstadt, Germany). All other chemicals used were of analytical grade.
Plant material and extraction preparation
The artichoke was purchased from Isfahan Agricultural Research Center. The artichoke was air-dried, ground and extracted two-times with ethanol:water (70:30, v/v) at room temperature for 2 d. The hydroethanolic extract was filtered through Whatman No. 1 filter paper. The solvent was then removed under vacuum and the extract was stored in 5 °C until used. In this study, we used a single dose of the artichoke extract (300 mg/kg b.w.) based on a previously published study (Heidarian & Soofiniya, Citation2011).
Determination of the total phenol, flavonoid and antioxidant capacity
The total flavonoids content in the artichoke extract was measured as described by Chang et al. (Citation2002). Total flavonoids were expressed in terms of rutin equivalents (in mg/g). Total phenols were determined by the colorimetry method with the Folin–Ciocalteu reagent (McDonald et al., Citation2001). The standard curve was prepared using 12.5, 25, 50, 62.5, 100 and 125 mg/L solutions of gallic acid in methanol and water (60:40, v/v). Total phenol values were expressed in terms of gallic acid equivalent (mg/g), as a common reference compound. The antioxidant activity of the artichoke extract was determined using a β-carotene-linoleate model system, as described by Singh et al. (Citation2011).
Experimental design
Animals were randomly divided into four groups (n = 8/ group) as follows. The first (control) group received standard pellet chow (Pars Dam, Tehran, Iran). This group received 0.5 mL distilled water by stomach tube to produce injection shock similar to other groups. The second group, lead acetate intoxicated without treatment, received lead acetate (500 mg lead acetate/kg diet) daily and water ad libitum for 6 weeks (Shalan et al., Citation2005). The third group received 500 mg lead acetate/kg diet and 300 mg/kg b.w. artichoke extract, daily for 6 weeks by stomach tube. The fourth group, vitamin C-protected group as a positive herbal antioxidant group (Acharya et al., Citation2003; Vij et al., Citation1998) received 500 mg lead acetate/kg diet and 1 mg vitamin C/100 g b.w., daily for 6 weeks by stomach tube (Shalan et al., Citation2005). At the end of the experimental period, fasted animals were anesthetized with chloroform and their blood samples were collected in test tubes. All serum specimens were separated and stored at −80 °C until they were analyzed.
Biochemical analysis
Serum total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), ALT, AST and ALP levels were determined by enzymatic methods (Pars Azmun kit, Tehran, Iran) using autoanalyzer (BT 3000, Biotecnica Instruments, SpA Rome, Italy). Pb concentration was determined in serum by atomic absorption (Varian, model spectr AA 240, Mulgrave, Australia).
Measurement of serum MDA
Lipid peroxidation in the serum was assessed by measuring MDA level after reacting MDA with thiobarbituric acid according to the method of Ohkawa et al. (Citation1979). The serum samples were mixed with thiobarbituric acid solution and incubated for 1 h at 95 °C with thiobarbituric acid. The reaction color product was measured spectrophotometrically at 532 nm using a visible spectrophotometer (Unico, model 1200 UV, Portland, OR). The measurements were done in duplicates and the results were expressed in μM. MDA standards were prepared from 1,1,3,3-tetraethoxypropane (TEP).
Ferric reducing/antioxidant power assay
The ferric reducing/antioxidant power (FRAP) assay of each sample was measured according to the procedure described by Benzie and Strain (Citation1996). In this method, the complex between Fe2+ and TPTZ gives a blue color with absorbance at 593 nm. FeSO4 ċ 7H2O was used as a standard of FRAP assay at a concentration range between 100 to 1000 μM.
Histopathological studies
Immediately after sacrificing rats, the livers were dissected out and fixed in 10% formalin. After processing (dehydrating in gradual ethanol (50–100%) and clearing in xylene), the livers were embedded in paraffin wax and sectioned into 5 µm thickness using a microtome. The sections were stained with hematoxylin and eosin (H and E) (Drury & Wallington, Citation1980) for photomicroscopic observation, including inflammatory activity and stage of fibrosis.
Statistical analysis
The results were expressed as mean ± S.D. All statistical analyses were performed using SPSS 17.0 statistical software (Chicago, IL). Comparison of means was carried out using One-way Analysis of Variance (ANOVA) and Tukey’s tests. p Values less than 0.05 were taken as statistically significant.
Results
Total phenol, flavonoid components and antioxidant capacity of the artichoke extract
The total phenolic content of the artichoke extract was 113.58 ± 9.18 mg of gallic acid equivalent/g dried extract. The total flavonoid content of the artichoke extract was 39.04 ± 2.31 mg of rutin equivalent/g dried extract. The antioxidant activity of the artichoke extract was 51.40 ± 5.37% inhibition of β-carotene.
Biochemical results
shows the effects of artichoke extract and vitamin C on some lead sensitive biochemical variables, as well as liver function related enzymes activities in serum of lead exposed rats. Administration of lead resulted in a significant increase (p < 0.001) in serum ALP, ALT and AST activities of the second group (lead exposed group without treatment) compared to the control group (). In group three (lead exposed group treated with artichoke extract) the serum ALP, ALT and AST activities significantly decreased (26.86, 21.90 and 12.81%, respectively) (p < 0.05) compared to the second group. Also, in group four (lead exposed group treated with vitamin C) the serum ALP, ALT and AST activities significantly decreased (13.76, 18.34 and 14.38%, respectively) (p < 0.05) compared to the second group. No significant change was observed in ALP and ALT between artichoke- and vitamin C-treated groups (p > 0.05). In group four, the serum AST activity significantly elevated compared to group three, whereas serum ALP and ALT activities had no remarkable change (p > 0.05) in comparison with group three.
Table 1. Lead induced changes in serum ALT, AST, ALP, MAD and FRAP in different animal groups.
summarizes the mean levels of TG, TC, HDL-C, LDL-C and VLDL-C in the serum of lead-exposed rats. The levels of serum TG and VLDL-C in the second group (lead-exposed group without treatment) significantly increased (27.16 and 27.40%, respectively) (p < 0.05) compared to the control group. However, LDL-C and TC remarkably decreased in the second group compared to the control group. The serum HDL-C between all groups had no significant change (p > 0.05). In group three, the serum level of TG, TC and VLDL-C significantly decreased (38.26, 13.15 and 38.38%, respectively) (p < 0.001) in comparison with the second group. In group four (lead exposed group treated with vitamin C), the serum levels of TG, TC and VLDL-C were significantly elevated compared to group three (lead exposed group treated with artichoke extract). In group four, the serum levels of TG, TC, LDL-C and VLDL-C had no remarkable change (p > 0.05) in comparison with the first group (control group). Also, in the second group, a significant elevation (p < 0.05) in serum MDA (48.43%) and a significant reduction (p < 0.05) in serum FRAP (37.87%) was observed after lead intake with respect to the control group (). In group three, the consumption of artichoke extract caused a suitable reduction (p < 0.05) of serum MDA (46.91%) in comparison with the second group (lead-exposed group without treatment). On the other hand, supplementation with vitamin C (group four) resulted in a significant reduction (p < 0.001) of serum MDA (57.30%) in comparison with group three. In group three, supplementation with the artichoke extract caused a significant elevation in serum FRAP compared to group four. No significant change was observed in the serum level of antioxidant power between the first and the third groups (p > 0.05).
Table 2. Lead induced changes in serum TC, TG, LDL-C, HDL-C and VLDL-C lipoprotein levels in different animal groups.
The efficacy of artichoke extract and vitamin C on serum lead concentration
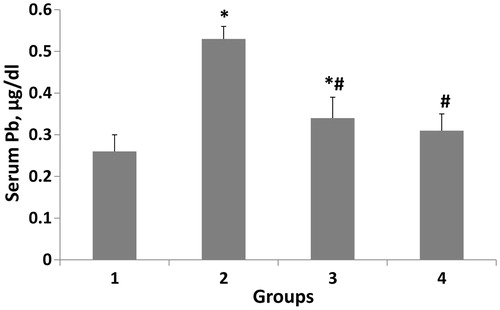
shows the serum lead level in the experimental groups. In the second group (lead-intoxicated group without treatment) administration of lead resulted in a significant (p < 0.001) elevation (96.29%) in the serum lead compared to the first group. In groups three and four consumption of artichoke extract or vitamin C resulted in a remarkable reduction (p < 0.001) of the serum lead in comparison with the second group (35.84 and 41.50%, respectively). On the other hand, no significant change was observed in serum lead between artichoke and vitamin C-treated groups.
Figure 1. Therapeutic efficacy of artichoke extract and vitamin C on serum lead concentration. The data are expressed in mean ± S.D and n = 8 in each group. Normal diet (1); lead-intoxicated rats without treatment (2); lead-intoxicated rats supplemented with artichoke extract (3); lead-intoxicated rats supplemented with vitamin C (4) groups. *p < 0.001 compared with the corresponding value in the first group (normal control animals). #p < 0.001 compared with the corresponding value in the second group (lead-intoxicated without treatment).
Histopathological findings
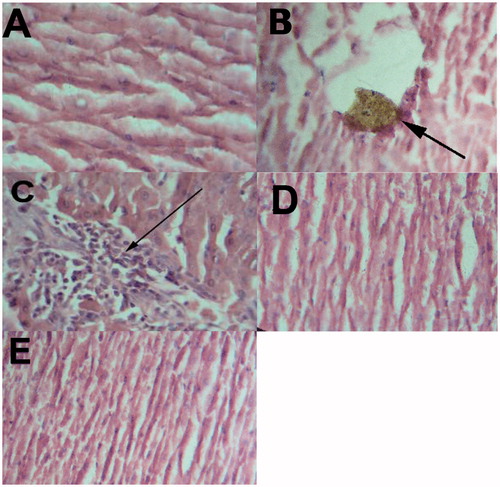
Histology of the liver sections of control animals (the first group) showed normal hepatic cells with well-preserved cytoplasm and prominent nucleus (). Histopathological findings in rats without treatment showed a broad infiltration of the lymphocytes accompanied with necrosis (). Also, showed a macrophage-laden with yellowish brown pigment in rats without treatment. On the other hand, rats treated with the artichoke extract showed a mild degree of lymphocyte infiltration (), almost comparable to the control and rats treated with vitamin C (group four). Also, in rats treated with vitamin C (group four), a remarkable reduction of the histological inflammatory exudate was observed () compared with rats without treatment (group two).
Figure 2. Photomicrography of rat liver sections in experimental groups. (A) Normal control (the first group); (B and C) lead-exposed rats without treatment (the second group); (D) lead-exposed rats supplemented with artichoke extract (group three); (E) lead-exposed rats supplemented with vitamin C (group four) (H&E stain). Figure 2B Shows a macrophage-laden with yellowish brown pigment in rats without treatment. (C) Shows infiltrated mononuclear cells (arrow) in rats without treatment.
Discussion
Lead (Pb) is a dangerous heavy metal for human health. Therefore, the detection and prevention of lead toxicity is a main object for international public health (Ahamed & Siddiqui, Citation2007). There are several effective chelators of lead such as DMSA for treating lead toxicity. Nevertheless, these chelating agents have side effects such as hemolytic anemia (Flora & Pachauri, Citation2010). Nowadays, using traditional and complementary medicine is developing for the treatment of many diseases because herbal drugs have less toxicity and side effects compared to synthetic drugs (Heidarian et al., Citation2011b). Artichoke has long been used effectively for treating a variety of diseases in the world (Heidarian & Soofiniya, Citation2011). The studies carried out on artichoke have shown the presence of natural antioxidant components such as caffeoylquinic acid derivatives, luteolin and apigenin (Wang et al., Citation2003). Our results showed that the ethanol extract of artichoke has an inhibitory activity against lead-induced toxicity. The administration of vitamin C to lead-intoxicated rats caused a reduction in serum lead concentration. Previous studies have reported protective effects of vitamin C against intoxication with lead (Houston & Johnson, Citation2000; Shalan et al., Citation2005). Studies in rats have demonstrated that vitamin C could reduce the gastrointestinal lead absorption (Morton et al., Citation1985) and promotes the renal excretion of lead (Niazi et al., Citation1982). In addition, there is evidence that vitamin C in the duodenum results in the reduction of ferric iron to the ferrous state, which competes with lead for intestinal transporter (Morton et al., Citation1985; Suzuki & Yoshida, Citation1979). Nevertheless, shows that artichoke extract has a remarkable reduction effect on serum lead concentration in lead-intoxicated rats compared to vitamin C treatment. This effect of artichoke extract might be depended on its flavonoids because, in a study, it was demonstrated that phenolics and flavonoids compounds are chelating agents which significantly reduce blood and tissue lead burden (Skoczynska et al., Citation1993). It is worth mentioning here that in our study the used artichoke extract concentration and duration time of experiment were limited. Therefore, we suggest that future studies focus on other possible mechanisms of the serum lead concentration-lowering action of the artichoke extract due to the bioactive components of artichoke, especially, on glutathione S-transferase, catalase, glutathione peroxidase (GPx) and glucose 6-phosphate dehydrogenase (G6PD).
In group two, lead administration resulted in liver damage and increased ALT, AST, ALP and MDA compared to the control group (). The same results have been reported by other investigators in experimental animals (Shalan et al., Citation2005). On the other hand, in groups three and four the administration of the artichoke leaf extract and vitamin C caused a reduction in the activity of these enzymes that indicates the protective effects of the artichoke leaf extract and vitamin C against liver damage. However, it seems that artichoke leaf extract is more effective compared to vitamin C. The liver protection power of artichoke leaf extract against liver lead damage is seen in . The artichoke leaf extract prevented the increase of blood lead level and decreased hepatocyte proliferation. Therefore, elevated serum ALT, AST, ALP and MDA were lowered to that of normal control after supplementation of intoxicated rats with artichoke leaf extract. The efficiency of artichoke is possibly due to its antioxidant and flavonoids such as cynarin, chlorogenic acid and caffeoylquinic acid. These biologically active compounds might have chelated lead and result in reduced lead in the serum and liver tissue. There is evidence that serum MDA levels were strongly associated with lead concentration in the tissues (Aykin-Burns et al., Citation2005). Lead induces oxidative stress in the body by the generation of reactive oxygen species (ROS) and decreasing the cell antioxidant defense system. On the other hand, previous studies showed that flavonoids are scavengers of peroxyl radicals and inhibit lipid peroxidation (Polovka et al., Citation2003). Therefore, the reduction of serum MDA level in group three is due to the effects of the artichoke extract flavonoids (). Nevertheless, in this study, it seems that vitamin C has a strong potential to reduce serum MDA level compared to artichoke extract (). However, the ability of artichoke extract for increasing serum antioxidant level was greater than vitamin C through its flavonoids (). The high potential of vitamin C to reduce serum MDA level resulted from inhibition of the lipid peroxidation by very rapid electron transfer to aqueous ROS (Jones et al., Citation1995).
In this study, lead consumption in group two (lead exposed group without treatment) created a significant reduction in the serum LDL-C and cholesterol compared to the control group, which is in accordance with findings of other investigators (Shalan et al., Citation2005; Skoczynska et al., Citation1993). On the other hand, in this study lead increased the serum triglyceride concentration in parallel with the reduction in serum cholesterol level. In group three, supplementation with artichoke extract caused a reduction in the serum TG and VLDL concentration compared to group two (lead exposed group without treatment). The reduction in serum TG and VLDL levels may be caused by chlorogenic acid that is a bioactive component of artichoke as a lipid-lowering agent (Joy & Haber, Citation2007; Wider et al., Citation2009). However, supplementation of lead-intoxicated rats with vitamin C showed no significant changes in serum TG and VLDL levels in comparison with group two (lead exposed group without treatment). Therefore, artichoke extract can be more effective compared to vitamin C for controlling lipid abnormalities and amelioration of lead toxicity in lead-intoxicated situation.
Conclusion
The chelating effects of oral ascorbic acid and the ethanol extract of artichoke in lead-poisoned rats showed equivalent chelating properties. The beneficial effects of artichoke extract can be due to its ability to complex with lead and result in a remarkable reduction of blood-lead levels.
Declaration of interest
The authors declare that there is no conflict of interest.
This study was funded by Shahrekord University of Medical Sciences (grant no. 800), Shahrekord, Iran.
Acknowledgements
The authors are grateful to Dr. Hossein Zeinali for his help in providing and identifying the artichoke from Isfahan Agricultural Research Center (Iran).
References
- Abdallah GM, El-Sayed el SM, Abo-Salem OM. (2010). Effect of lead toxicity on coenzyme Q levels in rat tissues. Food Chem Toxicol 48:1753–56
- Ahamed M, Siddiqui MK. (2007). Environmental lead toxicity and nutritional factors. Clin Nutr 26:400–8
- Acharya UR, Rathore RM, Mishra M. (2003). Role of vitamin C on lead acetate induced spermatogenesis in swiss mice. Environ Toxicol Pharmacol 13:9–14
- Aykin-Burns N, Franklin EA, Ercal N. (2005). Effects of N-acetylcysteine on lead-exposed PC-12 cells. Arch Environ Contam Toxicol 49:119–23
- Benzie IF, Strain JJ. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 239:70–6
- Chang C, Yang M, Wen H, Chern J. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–82
- Drury RAB, Wallington EA. (1980). Carlton’s Histological Technique. 5th ed. Oxford: Oxford University Press
- El-Nekeety AA, El-Kady AA, Soliman MS, et al. (2009). Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem Toxicol 47:2209–15
- Flora SJ, Pachauri V. (2010). Chelation in metal intoxication. Int J Environ Res Public Health 7:2745–88
- Flora SJ, Saxena G, Gautam P, et al. (2007). Response of lead-induced oxidative stress and alterations in biogenic amines in different rat brain regions to combined administration of DMSA and MiADMSA. Chem Biol Interact 170:209–20
- Heidarian E, Jafari-Dehkordi E, Seidkhani-Nahal A. (2011a). Lipid-lowering effect of artichoke on liver phosphatidate phosphohydrolase and plasma lipids in hyperlipidemic rats. J Med Plant Res 5:4918–24
- Heidarian E, Jafari-Dehkordi E, Seidkhani-Nahal A. (2011b). Effect of garlic on liver phosphatidate phosphohydrolase and plasma lipid levels in hyperlipidemic rats. Food Chem Toxicol 49:1110–14
- Heidarian E, Soofiniya Y. (2011). Hypolipidemic and hypoglycemic effects of aerial part of Cynara scolymus in streptozotocin-induced diabetic rats. J Med Plant Res 5:2717–23
- Hernberg S. (2000). Lead poisoning in a historical perspective. Am J Ind Med 38:244–54
- Houston DK, Johnson MA. (2000). Does vitamin C intake protect against lead toxicity? Nutr Rev 58:73–5
- Jones DP, Kagan VE, Aust SD, et al. (1995). Impact of nutrients on cellular lipid peroxidation and antioxidant defense system. Fundam Appl Toxicol 26:1–7
- Joy JF, Haber SL. (2007). Clinical uses of artichoke leaf extract. Am J Health Syst Pharm 64:1904–09
- Juzyszyn Z, Czerny B, Pawlik A, Drozdzik M. (2008). The effect of artichoke (Cynara scolymus L.) extract on ROS generation in HUVEC cells. Phytother Res 22:1159–61
- Mansouri MT, Cauli O. (2009). Motor alterations induced by chronic lead exposure. Environ Toxicol Pharmacol 27:307–13
- McDonald S, Prenzler PD, Antolovich M, Robards K. (2001). Phenolic content and antioxidant activity of olive extracts. Food Chemistry 73:73–84
- Morton A, Partridge S, Blair J. (1985). The intestinal uptake of lead. Chem Br 15:923–7
- Navarro-Moreno LG, Quintanar-Escorza MA, Gonzalez S, et al. (2009). Effects of lead intoxication on intercellular junctions and biochemical alterations of the renal proximal tubule cells. Toxicol In Vitro 23:1298–304
- Niazi S, Lim J, Bederka JP. (1982). Effect of ascorbic acid on renal excretion of lead in the rat. J Pharm Sci 71:1189–90
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Patra RC, Swarup D, Dwivedi SK. (2001). Antioxidant effects of alpha tocopherol, ascorbic acid and l-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 162:81–8
- Patrick L. (2006). Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev 11:2–22
- Polovka M, Brezova V, Stasko A. (2003). Antioxidant properties of tea investigated by EPR spectroscopy. Biophys Chem 106:39–56
- Queiroz ML, da Rocha MC, Torello CO, et al. (2011). Chlorella vulgaris restores bone marrow cellularity and cytokine production in lead-exposed mice. Food Chem Toxicol 49:2934–41
- Shalan MG, Mostafa MS, Hassouna MM, et al. (2005). Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicology 206:1–15
- Sharma V, Sharma A, Kansal L. (2010). The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem Toxicol 48:928–36
- Singh RP, Chidambara Murthy KN, Jayaprakasha GK. (2011). Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem 50:81–6
- Skoczynska A, Smolik R, Jelen M. (1993). Lipid abnormalities in rats given small doses of lead. Arch Toxicol 67:200–4
- Suzuki T, Yoshida A. (1979). Effectiveness of dietary iron and ascorbic acid in the prevention and cure of moderately long-term lead toxicity in rats. J Nutr 109:1974–8
- Tian L, Lawrence DA. (1995). Lead inhibits nitric oxide production in vitro by murine splenic macrophages. Toxicol Appl Pharmacol 132:156–63
- Upasani CD, Khera A, Balaraman R. (2001). Effect of lead with vitamin E, C, or Spirulina on malondialdehyde, conjugated dienes and hydroperoxides in rats. Indian J Exp Biol 39:70–4
- Vij AG, Satija NK, Flora SJ. (1998). Lead induced disorders in hematopoietic and drug metabolizing enzyme system and their protection by ascorbic acid supplementation. Biomed Environ Sci 11:7–14
- Wang M, Simon JE, Aviles IF, et al. (2003). Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J Agric Food Chem 51:601–8
- Wider B, Pittler MH, Thompson-Coon J, Ernst E. (2009). Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst Rev 7:CD003335
- Zapolska-Downar D, Zapolski-Downar A, et al. (2002). Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci 71:2897–908