Abstract
Context: The prevalence of infectious bursal disease has brought about enormous financial losses to the world poultry industry. Chinese herb medicines can provide valuable materials for discovery and development of new drugs.
Objective: To screen constituents derived from Chinese herb medicines for their antiviral activity against infectious bursal disease virus (IBDV) in vitro.
Materials and methods: Twenty constituents derived from Chinese herb medicines and B87 strain of IBDV were used. The 50% cytotoxic concentration (CC50) and 50% effective concentration (EC50) were determined by visualization of cytopathologic effect (CPE) and 3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) test on chicken embryo fibroblast. Selectivity index (SI) and inhibition ratio (%I) were calculated from the data obtained from the MTT test.
Results: Antiviral assays showed dipotassium glycyrrhizinate and ligustrazine hydrochloride among the 20 constituents tested exhibited significant inhibitory activity against IBDV in a dose-dependent manner. EC50 of dipotassium glycyrrhizinate and ligustrazine hydrochloride were 663.2 ± 268.4 and 92.52 ± 21.13 µg/mL, and SI were >4.52 and >21.62, respectively. The time-of-addition and virucidal assay indicated that anti-IBDV activity of the two constituents could be due to their inhibiting virus replication and/or inactivating virus directly. The inhibition of virus attachment was not observed in the adsorption inhibition assay. Dipotassium glycyrrhizinate and ligustrazine hydrochloride exhibited more than 70% and 80% inhibition of IBDV, respectively, at the maximum safe concentration.
Discussion and conclusion: We believe that dipotassium glycyrrhizinate and ligustrazine hydrochloride can be used to develop a new anti-IBDV compound, and it is worth applying the constituents in clinical practice.
Introduction
Infectious bursal disease (IBD) is one of the most important avian acquired immunosuppressive diseases (Luo et al., Citation2009). The challenge of infectious bursal disease virus (IBDV) can lead to lymphoid depletion in the bursa of Fabricius, which can cause immunodepression and immune failure in poultry (Sharma et al., Citation2000). The prevalence of IBD has brought about enormous financial losses to the world poultry industry. At present, the prevention of IBD is mainly dependent on vaccination that does not completely prevent the disease. Vaccine-derived immunity is significantly compromised when vaccine strains are not well matched with the circulating strains as a result of strain variation or inaccurate epidemiological predictions. Moreover, the pandemic of IBD presents some new characteristics in recent years. It is not only contracted by chickens aged 3–6 weeks but also those aged 7–20 weeks, even layer chicken. Thus, with the limited range of current treatments and the threat of a new pandemic, it is necessary to develop new drugs so as to eradicate IBD.
It has been proved that Chinese herb medicines can provide valuable materials for the discovery and development of new drugs, and clinical trials have shown that some Chinese herb medicines and their ingredients have strong antiviral activity in human and animal viral infection (Fan, Citation2011; Iwata, Citation2010). In this study, we investigated the anti-IBDV activity of 20 constituents derived from Chinese herb medicines as an attempt to search for the most promising anti-IBDV drugs. These constituents have been proven to possess multipharmacological effects including antivirus, antitumor activities, anti-inflammation and immunity-regulation.
Materials and methods
Constituents and reagents
Twenty constituents and ribavirin (R) were purchased from National Institute for Food and Drug Control (Beijing, China) (). Dulbecco’s Modified Eagle Medium (DMEM) (Sigma, St. Louis, MO) supplemented with 100 units/mL penicillin G/streptomycin and 8% fetal bovine serum was used for the growth medium of cells. For diluting the constituents and maintaining the cells, fetal bovine serum (FBS) concentration in the maintenance medium (MM) was reduced to 2% and other constituents were the same as the cell growth medium. Trypsin (Amresco, Solon, OH) was dissolved with PBS (pH 7.2) at a concentration of 0.25%. 3-(4,5-Dimethyithiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Amresco, Solon, OH) was dissolved at 5 mg/mL with PBS (pH 7.4). These reagents were filtered through a 0.22 µm millipore membrane filter and distributed into small portions for future use. DMEM and MM were stored at 4 °C; MTT and trypsin were stored at −20 °C with opaque paper.
Cell and virus
Chicken embryo fibroblasts (CEFs) were harvested according to the method described by Zhao et al. (Citation2011). Briefly, CEFs were prepared with 10-day-old SPF chicken embryo (SYXK (Jin) 2010–0007, Shanxi Longker Biopharmaceutical Co., Ltd, China). The cells were diluted to 1 × 106 mL−1 with 8% DMEM and then inoculated in 96-well plates at 37 °C in a 5% CO2 atmosphere prior to use.
IBDV vaccine (B87 strain, No.110572026, Zhejiang Yibang Biologic Co., Ltd, Hangzhou, China) was propagated with 10-day-old SPF chicken embryo. The tissue culture infectious dose 50 (TCID50) of the virus liquid was 1 × 10−3.5 tested by the Reed–Muench assay. It was diluted to 1 × 10−1.5 (100TCID50) with MM and stored at −80 °C for future use.
Cytotoxicity
The cytotoxicity of the constituents was microscopically assessed via changes in cellular morphology and was confirmed and measured by the colorimetric method based on the reduction of MTT by mitochondrial enzymes (Mosmann, Citation1983). The constituents and ribavirin were diluted two-fold serially with DMEM containing 2% FBS and matching lytic agent to eight gradients, respectively. CEF cells were seeded into 96-well plates at a density of 1 × 106 cells/well and incubated at 37 °C in a 5% CO2 atmosphere until 90% or greater confluency of the monolayer was reached. Then, the medium was removed and the cells were further incubated with different concentrations of each constituent and ribavirin at 37 °C in a 5% CO2 humidified atmosphere for 72 h. After 72 h incubation, the media were discarded and 20 µL of MTT (5 mg/mL in PBS, pH7.2) solution was added to each well. The plates were then further incubated at 37 °C for 4 h. Subsequently, the supernatant was removed and 100 µL of DMSO was added into each well to solubilize the dark blue formazan crystals. The optical density (OD) at 490 nm was measured by an automatic plate reader (Bio Tek®, EL × 808, Gene Co., Ltd., Hong Kong, China) with absorbance at 630 nm as a reference (Pang et al., Citation2010). The 50% cytotoxic concentrations (CC50) were determined as the concentration of the constituents that reduced the cell viability to 50% of cell control (cells without addition of constituents) (Li et al., Citation2005). The maximum safe concentration was calculated as the concentration required to retain cell viability by 90% (Chen et al., Citation2010).
Antiviral activity
The maximum safe concentrations of the constituents and 100 TCID50 IBDV were used for the determination of anti-IBDV activity with the MTT test (Li et al., Citation2005). Briefly, monolayer of CEF was grown in 96-well plates, and IBDV and 20 constituents were added into each well, respectively, and incubated together under 5% CO2 at 37 °C. CEF control, IBDV negative control and ribavirin positive control were set up simultaneously. When CPE of IBDV negative control reached 80–90% compared with CEF control, MTT test was carried out as described above. The inhibition ratio (%I) was expressed as %I = (ODtest − ODvirus)/(ODcell − ODvirus). The constituents whose inhibition ratio was more than 50% were selected and diluted two-fold serially with 2% FBS/DMEM to eight gradients, respectively, and the procedures as described above were repeated and 50% effective concentration (EC50) of the constituents were determined as 50% cyto-protection against IBDV infection was achieved by the constituents. The selectivity index (SI) was the ratio of CC50 to EC50.
Time-of-addition
The dynamic trail was conducted according to previous methods with some modifications (Alvarez et al., Citation2010). The maximum safe concentrations of the constituents and 100 TCID50 IBDV were used in the assay. CEF cells in 96-well plates were pre-incubated with the constituents at 37 °C in a 5% CO2 humidified atmosphere for 2 h, then the constituents medium was removed and washed twice with PBS, and then the cells were challenged with IBDV (2 h). The CEF cells that were pre-incubated with IBDV for 2, 4, 6, 8, 10, 12, 14 and 16 h, respectively, and then the medium was removed and replaced by the constituents, or co-incubated with the mixture of the constituents and IBDV 0 h, were also performed simultaneously. CPE was observed at a time interval of 12 h under microscope. When CPE of IBDV negative control reached 80–90% compared with CEF control, the anti-IBDV activity of all phases was assessed by the MTT test and viral inhibition rate as described above.
Adsorption inhibition
Two methods were performed in this assay. In the first method, CEF cells grown in 96-well plates were pre-chilled at 4 °C for 15 min, and then the medium was discarded. Subsequently, the mixture of the constituents with concentration gradients and IBDV with 100 TCID50 were added to each well, and the plates were further incubated at 4 °C for 3 h to allow IBDV adsorption. At this time, the cell monolayer was gently washed with PBS and the medium was replaced by MM for further incubation at 37 °C in a 5% CO2 humidified atmosphere until CPE of the cells in IBDV negative control reached 80–90% compared with CEF control, and MTT test and viral inhibition rate were determined as above. In the second method, CEF cells grown in 96-well plates was incubated with the constituents at 37 °C in a 5% CO2 humidified atmosphere for 1, 2, 4 and 6 h, respectively, and then the plates were incubated at 4 °C for 15 min. Subsequently, the medium was removed and 100 TCID50 IBDV were added to each well, and then the procedures described above were followed.
Virucidal
Each constituent of its maximum safe concentration and 100 TCID50 IBDV were mixed and interacted at 37 °C for 15 min, 30 min, 1 h and 2 h, respectively. At specific time intervals, 100 µL of each virus/constituent suspension was added to the already adherent and plated cells and incubated at 37 °C in a 5% CO2 humidified atmosphere and observed under a microscope daily until the CPE of the IBDV negative control reached 80–90% compared with the CEF control, and the MTT test was carried out.
Statistical analysis
All data represent the means from three replicates of three separate experiments and were expressed at mean ± SD. Statistical analyses were performed using SPSS17.0 software (SPSS Inc., Chicago, IL). Student’s t-test and one-way ANOVA were used. A value of p < 0.05 was considered statistically significant. CC50 was calculated by regression analysis of the dose-response curves for the MTT assay and EC50 values were performed using PrismTM version 5 software (Graphpad Software, Inc., La Jolla, CA).
Results
Cytotoxicity
The maximum safe concentration (MSC) and CC50 of the tested constituents are listed in . CC50 values of the tested 20 constituents against CEF ranged from 5.6 ± 0.79 to >3000 µg/mL. According to the values of MSC, aesculin, geniposide, polydatin, liquiritin, ligustrazine hydrochloride and dipotassium glycyrrhizinate had no cytotoxic effect within the concentration employed in this assay. The other constituents showed cytotoxicity in a dose-dependent manner. Microscopically, the morphological changes in the cells included the loss of monolayer, lysis, granulation, pyknosis, condensation, vacuolization in the cytoplasm and darkening of cell boundaries.
Table 1. Data obtained in cytotoxicity and antiviral assays.
Antiviral
Dipotassium glycyrrhizinate and ligustrazine hydrochloride in the tested 20 constituents exhibited significant inhibitory activity against IBDV in a dose-dependent manner. Dipotassium glycyrrhizinate had higher EC50 and significantly lower SI (p < 0.05) than those of ligustrazine hydrochloride, while these two constituents shared a similar inhibition ratio (p > 0.05) (). Compared with dipotassium glycyrrhizinate, ligustrazine hydrochloride had much higher anti-IBDV activity and a wider safety concentration (p < 0.05) ().
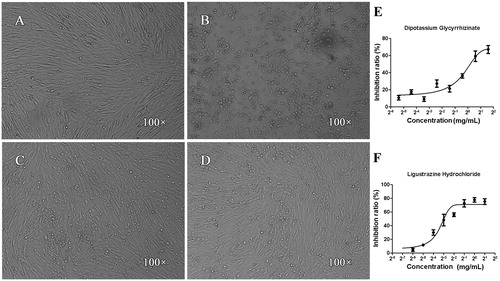
Figure 2. Effects of dipotassium glycyrrhizinate and ligustrazine hydrochloride on CEF cells infected by IBDV. (A) CEF control, the cells displayed normal shape, satisfactory refractivity and confluent monolayer; (B) IBDV negative control, after 72 h incubation with IBDV, the cells presented some morphological changes including monolayer, lyses, granulation, pyknosis, condensation, vacuolization in the cytoplasm and darkening of cell boundaries; (C and D) after 72 h co-incubation with IBDV and dipotassium glycyrrhizinata (C) or ligustrazine hydrochloride (D), the apoptotic morphological changes were significantly weaker, the number of living cells was more numerous and the cell monolayer was more confluent compared with that in (B, E and F): the data indicated that dipotassium glycyrrhizinate (E) and ligustrazine hydrochloride (F) had significant inhibitory effects with dose-dependent patterns.
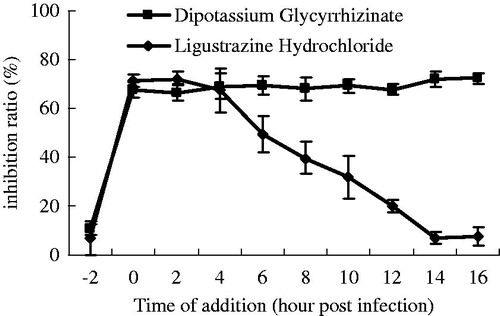
Time-of-addition
The results of this assay demonstrated that pre-incubating the cells with the two constituents, dipotassium glycyrrhizinata and ligustrazine hydrochloride, had no effect on the infection and replication of IBDV. Dipotassium glycyrrhizinate exhibited a consistent inhibiting effect during a single replication cycle from 0 to 16 h and the highest inhibition ratio reached 72.30%. But the inhibition ratio of ligustrazine hydrochloride declined from about 70.31% at 0, 2 and 4 h to 10% at 16 h ().
Adsorption inhibition
The results of pre-incubating the cells with dipotassium glycyrrhizinate and ligustrazine hydrochloride, respectively, or co-incubating the cells with the constituents and IBDV simultaneously, both failed to prove that the two constituents could prevent the adsorption of IBDV to the cells (data not shown).
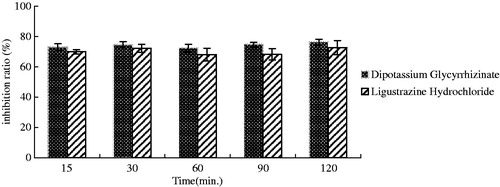
Virucidal
When dipotassium glycyrrhizinate or ligustrazine hydrochloride was mixed with IBDV and interacted for 15, 30, 60, 90 and 120 min, respectively, at room temperature, the inhibition ratio of the two constituents at each time point was larger than 70%, and had no significant difference (p > 0.05). The highest inhibition ratios of dipotassium glycyrrhizinate and ligustrazine hydrochloride were 76.32 and 72.63%, respectively ().
Discussion
The prevalence of IBD has brought about enormous financial losses to the world poultry industry by inducing severe immunosuppression and high mortality in chicken flocks, and there are limited kind of treatments to prevent and cure the disease (Gao et al., Citation2008). Hence, there is an urgent need for the development or extension of lifespan of novel anti-IBDV agents. Nowadays, people pay close attention to Chinese herb medicines for combating diseases including antiviral activity. It has been reported that the extracts of Kumazasa, Solanum nigrum, Myrothamnus flabellifolia Welw and Alpinia katsumadai had anti-infection properties against Pseudorabies virus, hepatitis C virus, herpes simplex virus type 1 and rotaviruses, respectively (Gescher et al., Citation2011; Iwata et al., Citation2010; Javed et al., Citation2011; Kim et al., Citation2012), but these crude extracts from Chinese herb medicine possessed complex or undefined constituents which hampered the development of new antiviral drugs. In this experiment, we challenged the CEF cells with IBDV and observed the anti-IBDV activity of 20 constituents derived from Chinese herb medicines. These constituents have known constituents, and this is beneficial to be used in clinical practice and/or as a lead compound for developing new antivirus drugs.
The results obtained in this study clearly demonstrated the anti-IBDV activity of dipotassium glycyrrhizinate and ligustrazine hydrochloride in vitro with EC50 values of 663.2 ± 268.4 and 92.52 ± 21.13 µg/mL, and inhibition ratios of 70 and 80%, respectively. The cytotoxicity assay proved that these two constituents posed no significant toxicity to cells. Compared with ribavirin, a similar inhibition ratio, higher EC50 and lower SI of the two constituents indicated that higher dosage of the two constituents would be needed in antiviral practice and they might have a contracted safety concentration range.
Glycyrrhizin is the most important bioactive compound of licorice root (Glycyrrhiza uralensis Fish). Pompei et al. (Citation2009) documented the antiviral properties of glycyrrhizic acid and its semisynthetic derivatives and led to the conclusion that new synthetic derivatives of glycyrrhizic acid are even more active than the parent molecule, and glycyrrhizic acid can alter the expression of viral genes involved in cell transformation, thus opening a new window for speculating on viral carcinogenesis. It was reported that glycyrrhizin was active against herpes viruses such as varicella zoster virus (VZV), SARS coronavirus (SARS-CoV), Epstein-Barr virus (EBV) and influenza A virus (IAV) (Baba & Shigeta, Citation1987; Hoever et al., Citation2005; Lin, Citation2003; Wolkerstorfer et al., Citation2009) and diammonium glycyrrhizin, a salt from glycyrrhizinate, possessed strong inhibitory effect on pseudorabies herpesvirus (PrV) and infectious bronchitis virus (IBV) (Li et al., Citation2009; Sui et al., Citation2010). Dipotassium glycyrrhizinate also is a salt from glycyrrhizinate and showed significant activity against IBDV in vitro in our study. However, Lin et al. (Citation2008) reported that the introduction of potassium or ammonium salt to glycyrrhizic acid reduced the antiviral activity with no significant effect on cytotoxicity, and Li et al. (Citation2007) proved that diammonium glycyrrhizinate had no antiviral activity on patients with HBeAg-positive chronic hepatitis B.
Ligustrazine is one of the major effective components of Ligusticum chuanxiong Hort. It has been widely used, especially in the treatment of patients with cardiovascular diseases in China, and also has properties of antibacterial. Ligustrazine hydrochloride has the same pharmacologic and pharmacodynamic properties as ligustrazine but better physicochemical properties (Li et al., Citation2006). There are very few reports on the antiviral activity of ligustrazine hydrochloride. Our results demonstrated that ligustrazine hydrochloride possessed stronger inhibitory activity against IBDV than dipotassium glycyrrhizinate.
In time-of-addition assay, both of the two constituents could inhibit viral replication. Dipotassium glycyrrhizinate played a part in the whole stage of IBDV replication cycle, but ligustrazine hydrochloride only disturbed the early stage of the IBDV replication cycle. Pre-treating the cells with the constituents could not stop the challenge of IBDV. Meanwhile, to assess the possibility of direct virus inactivation, we pre-treated the cells with the two constituents at different time intervals and then challenged the cells with IBDV in the adsorption inhibition assay. Either co-incubating the cells with the mixture of the constituent and IBDV or pre-treating the cells with the constituent failed to prevent the attachment of IBDV to the cells. The results indicated that the antiviral activity of the two constituents probably involves restricted, virus-specific events occurring inside the cell, rather than non-specific host cell-virus interaction at the membrane level, such as virus attachment, entry or release. Lin (Citation2003) reported that glycyrrhizic acid interfered with an early step of EBV replication cycle, and neither had an effect on viral adsorption, nor did it inactivate EBV particles. It was also found that the anti-IAV effect of glycyrrhizin was not only limited to an early step in the virus replication cycle, but was also mediated by an interaction with the cell membrane which most likely resulted in reduced endocytotic activity and hence reduced virus uptake (Wolkerstorfer et al., Citation2009). However, Li et al. (Citation2009) confirmed that glycyrrhizin diammonium had a direct anti-IBV activity and the inhibitory effect was a viral factor, rather than a cellular factor.
In our study, dipotassium glycyrrhizinate and ligustrazine hydrochloride showed positive results in the virucidal activity test with no time-dependence, and their inhibition ratios were over 70%. It was suggested that they could inactivate IBDV directly. However, Wolkerstorfer et al. (Citation2009) found that glycyrrhizin had no direct inhibitory action on IAV particles and did not interact with virus receptor binding.
Conclusions
Dipotassium glycyrrhizinate and ligustrazine hydrochloride can inhibit IBDV infection effectively in vitro, and their antiviral activity is attributed to directly inactivating and/or disturbing the replication of IBDV. We believe that dipotassium glycyrrhizinate and ligustrazine hydrochloride can be used to develop new anti-IBDV compounds, and it is worth applying the constituents in clinical practice.
Declaration of interest
This research was sponsored by key scientific and technological project of Shanxi Province (Grant No. 2010311047 and 20120311022-1). These experiments complied with the current laws of P.R. China. The authors report no declarations of interest.
References
- Alvarez AL, Habtemariam S, Juan-Badaturuge M, et al. (2010). In vitro antiHSV-1 and HSV-2 activity of Tanacetum vulgare extracts and isolated compounds: An approach to their mechanisms of action. Phytother Res 25:296–301
- Baba M, Shigeta S. (1987). Antiviral activity of glycyrrhizin against varicella-zoster virus in vitro. Antiviral Res 7:99–107
- Chen MZ, Xie HG, Yang LW, et al. (2010). In vitro anti-influenza virus activities of sulfated polysaccharide fractions from Gracilaria lemaneiformis. Virol Sin 25:341–51
- Fan Y, Liu J, Wang D, et al. (2011). Epimedium polysaccharide and propolis flavone can synergistically inhibit the cellular infectivity of NDV and improve the curative effect of ND in chicken. Int J Biol Macromol 48:439–44
- Gao Y, Liu W, Gao H, et al. (2008). Effective inhibition of infectious bursal disease virus replication in in vitro by DNA vector-based RNA interference. Antiviral Res 79:87–94
- Gescher K, Kühn J, Hafezi W, et al. (2011). Inhibition of viral adsorption and penetration by an aqueous extract from Rhododendron ferrugineum L. as antiviral principle against herpes simplex virus type-1. Fitoterapia 82:408–13
- Hoever G, Baltina L, Michaelis M, et al. (2005). Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J Med Chem 48:1256–9
- Iwata K, Naito E, Yamashita K, et al. (2010). Anti-Pseudorabies virus activity of Kumazasa extract. Biocontrol Sci 15:123–8
- Javed T, Ashfaq UA, Riaz S, et al. (2011). In vitro antiviral activity of Solanum nigrum against hepatitis C virus. Virology J 8:26--32
- Kim HH, Kwon HJ, Ryu YB, et al. (2012). Antiviral activity of Alpinia katsumadai extracts against rotaviruses. Res Vet Sci 92:320–3
- Li SY, Chen C, Zhang HQ, et al. (2005). Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res 67:18–23
- Li J, Yin J, Sui X, et al. (2009). Comparative analysis of the effect of glycyrrhizin diammonium and lithium chloride on infectious bronchitis virus infection in vitro. Avian Pathol 38:215–21
- Li LL, Zhang ZR, Gong T, et al. (2006). Simultaneous determination of gastrodin and ligustrazine hydrochloride in dog plasma by gradient high-performance liquid chromatography. J Pharmaceut Biomed 41:1083–7
- Li J, Zhao H, Si CW, et al. (2007). Diammonium glycyrrhizinate does not affect efficacy of adefovir dipivoxil therapy in patients with HBeAg-positive chronic hepatitis B. Chinese J Exper Clin Virol 21:270–2
- Lin JC. (2003). Mechanism of action of glycyrrhizic acid in inhibition of Epstein–Barr virus replication in vitro. Antiviral Res 59:41–7
- Lin JC, Cherng JM, Hung MS, et al. (2008). Inhibitory effects of some derivatives of glycyrrhizic acid against Epstein-Barr virus infection: Structure–activity relationships. Antiviral Res 79:6–11
- Luo J, Zhang GP, Fan JM, et al. (2009). Infectivity and propagation of attenuated infectious bursal disease virus in the chicken B-lymphocyte cell line DT40. Arch Virol 154:513–17
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
- Pang R, Tao JY, Zhang SL, et al. (2010). In vitro antiviral activity of lutein against hepatitis B virus. Phytother Res 24:1627–30
- Pompei R, Laconi S, Ingianni A. (2009). Antiviral properties of glycyrrhizic acid and its semisynthetic derivatives. Mini Rev Med Chem 9:996–1001
- Sharma JM, Kim IJ, Rautenschlein S, Yeh HY. (2000). Infectious bursal disease virus of chickens: Pathogenesis and immunosuppression. Dev Comp Immunol 24:223–35
- Sui X, Yin J, Ren X. (2010). Antiviral effect of diammonium glycyrrhizinate and lithium chloride on cell infection by pseudorabies herpesvirus. Antiviral Res 85:346–53
- Wolkerstorfer A, Kurz H, Bachhofner N, Szolar OH. (2009). Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Res 83:171–8
- Zhao X, Hu Y, Wang D, et al. (2011). Optimization of sulfated modification conditions of tremella polysaccharide and effects of modifiers on cellular infectivity of NDV. Int J Biol Macromol 49:44–9