Abstract
Context: Few methods have been reported for the quantification of ligustilide (LIG) in biosamples: the pretreatment of the biological samples were laborious and time-consuming.
Objective: A high-performance liquid chromatographic method with fluorescence detection (HPLC-FLD) for the determination of LIG in rat plasma was developed and validated. Pharmacokinetics and bioavailability of LIG were determined by systematic investigation in Sprague-Dawley rats.
Materials and methods: LIG was isolated from the volatile oil of Radix Angelica sinensis and further purified by silica gel column chromatography. Podophyllotoxin was used as an internal standard. The analytes were detected by using fluorescence detection at an excitation and emission wavelength of 290 and 395 nm during 0–4 min, and 336 and 453 nm during 4–14 min, respectively. LIG pharmacokinetics was studied in rats after oral and intravenous administration of 12.5, 25 and 50 mg/kg doses.
Results: Two calibration curves (Y = 133.49 X − 14.27 (r = 0.9995), Y = 145.61 X + 13.76 (r = 0.9996)) were constructed in the range of 2.44–10 000 ng/mL for LIG with a lower limit of quantitation of 2.44 ng/mL. Both intra-day and inter-day precision were less than 6%. Accuracy ranged from 88.93 to 99.52%. The recovery ranged from 89.07 to 99.71%. The absolute bioavailability values were 71.36, 68.26 and 75.44% for oral doses of 12.5, 25 and 50 mg/kg, respectively.
Conclusion: The present HPLC-FLD method was rapid, sensitive and reliable. LIG was absorbed and eliminated rapidly in rat.
Introduction
Ligustilide [LIG, 3-butylidene-4,5-dihydro-1(3H)-isobenzofuranone], a phthalide derivative, is the main lipophilic component of the essential oil constituents in Ligusticum chuanxiong Hort. (Chuanxiong in Chinese) and Angelica sinensis (Oliv.) Diels (Danggui in Chinese) of the Umbelliferae family, two of the most widely used Traditional Chinese Medicines (TCMs) (Asuming et al., Citation2005; Gijbels et al., Citation1982; Naito et al., Citation1992; Shi et al., Citation1995; Takashi, Citation1982). Ligustilide is also considered to be the main active ingredient of the aroma of celery (Apium graveolens Linn.) (Tang et al., Citation1990), lovage (Levisticum officinale Linn.) (Gijbels et al., Citation1982) and North America oshá (Ligusticum portieri Coult. & Rose) (Mei et al., Citation1991). Ligustilide, including Z-ligustilide and E-ligustilide, has been found to be an unstable compound, decomposing rapidly at high temperature to form other phthalides through oxidation, isomerization, dimerization, etc. (Li et al., Citation2000; Li & Wang, Citation2003; Schinkovitz et al., Citation2008; Zhou & Li, Citation2001). Ligustilide has been reported to have a wide range of pharmacological activities, including vasodilatation (Cao et al., Citation2006), neuroprotective effect (Kuang et al., Citation2006; Wang et al., Citation2010; Wu et al., Citation2011), analgesic effect (Du et al., Citation2007), antiviral activity (Kwon et al., Citation1997), memory-improving property (Cheng et al., Citation2011), a decrease of platelet aggregation (Zhang et al., Citation2009), anticancer effects (Chen et al., Citation2007; Kan et al., Citation2008; Tsai et al., Citation2006; Yu et al., Citation2008), attenuation of endotoxic shock (Shao et al., Citation2011), antiproliferation of smooth muscle cells (Lu et al., Citation2006), inhibition of spontaneous and agonists- or K+ depolarization-induced contraction of rat uterus (Du et al., Citation2006), prevention of LPS-induced iNOS expression (Su et al., Citation2011), alleviation of brain damage and improvement of cognitive function (Feng et al., Citation2012; Kuang et al., Citation2008), regulation of hormesis (Qi et al., Citation2012), protective effect of amyloid β-induced neurotoxicity (Kuang et al., Citation2009), antiinflammatory effect (Chao et al., Citation2007, Citation2009a,Citationb, 2010; Chen et al., Citation2010; Chung et al., Citation2012; Ma & Bai, Citation2013) and antihepatotoxic effect (Dietz et al., Citation2008). With the growing significance of a potential beneficial role of LIG in human health, there is an increasing demand for analyzing it in vivo and systematic researching on its pharmacokinetics. However, data on pharmacokinetics of LIG are scanty. Shi et al. (Citation2006) studied pharmacokinetics of LIG after p.o. dose, which was not designed to estimate absolute bioavailability, therefore, the potential problem with absorption was not addressed. Yan et al. (Citation2008) reported that the oral bioavailability of LIG was only 2.6% at the 500 mg/kg dose in rat, which was partly because of extensive first-pass metabolism in the liver, but the article did not study the pharmacokinetic properties of LIG after intravenous and oral administration. It would be detected only up to 1.5 h; because plasma concentrations of LIG were below the limit of detection after 1.5 h. In another pharmacokinetic study of LIG, it was detected in rat brain after 5–20 min of nasal and oral administration, indicating that LIG has a rapid onset of action to enter the central nervous system by permeating blood-brain barrier (Guo et al., Citation2011). Studies suggested that conjugated with glutathione, cysteine, glucuronic acid and sulphuric acid may be the main metabolic reactions of some phthalides (Zuo et al., Citation2011). It is well known that pharmacokinetic studies play an increasingly important role in drug discovery and development processes, not only further supporting toxicity or clinical studies but also optimizing drug candidates (Sun et al., Citation2009). The pharmacokinetic study of a bioactive component could help us understand its in vivo actions and explain a variety of events related to efficacy and toxicity of the relevant herbs or herbal preparations in which this constituent is involved (Lv et al., Citation2011). Therefore, it is of clinical importance to explore the in vivo pharmacokinetic profiles of LIG for a better understanding of the mechanism of action and facilitating further research and development of LIG.
However, developing effective methods for the quantitative analysis of LIG is very important. To date, there have been some reports on the quantitative analysis of LIG in different plant materials or their extracts by gas chromatography (GC)-flame ionization detection (FID) (Fang et al., Citation1979; Li et al., Citation2001), capillary zone electrophoresis (CZE) (Ji et al., Citation1999), gas chromatography-mass spectrometry (GC-MS) (Chen et al., Citation2010; Cho et al., Citation2007; Deng et al., Citation2005; Hu & Ding, Citation2006; Kim et al., Citation2006a,Citationb; Lao et al., Citation2004; Li et al., 2006a), liquid chromatography-mass spectrometry (LC-MS) (Lin et al., Citation1998; Liu et al., Citation2009; Lu et al., Citation2004; Qi et al., Citation2008; Yi et al., Citation2007; Zschocke et al., Citation1998), liquid chromatography (LC) (Chao & Chao, Citation2004; Cui et al., Citation2006; Hu et al., 2005a,b; Li et al., 2006b; Lu et al., Citation2005a,Citationb, Citation2009), bioactivity-guided fractionation method combining countercurrent chromatography (CCC), the MTS cell viability assay and gas chromatography (GC) (Yeh et al., Citation2012). Although few methods have been reported for the quantification of LIG in biosamples (Chen et al., Citation2011; Guo et al., Citation2009, Citation2011; Li et al., Citation2009, Citation2011; Shi et al., Citation2006; Yan et al., Citation2008; Zuo et al., Citation2011), which are commonly measured as biomarkers. As for extraction of LIG from biological samples, the analytical methods involved multiple steps of liquid--liquid extraction, which used relatively toxic agents such as n-hexane-ether, followed by evaporation to prepare and concentrate the sample prior to injection to an LC system, the pretreatment of the biological samples were laborious and time-consuming. Due to the multiple steps of sample pretreatment and in order to maximize the sensitivity of the assay, a relatively large volume of blood (0.16–0.8 mL) was needed. Capillary gas chromatography/mass spectrometry was used to measure the processed sample (Yan et al., Citation2008), which may not be sensitive for the determination of trace samples. Additionally, MS equipment might not be available in most laboratories because of the high analysis cost; consequently, in the present paper, a liquid chromatographic (LC) analytical method equipped with fluorescence detection (FLD) has been reported to determine LIG in rat plasma.
To the authors’ best knowledge there was no entirely validated HPLC-FLD reported in the literature for quantification of LIG in biological sample and so the aim of the current study was to develop a rapid and sensitive HPLC-FLD for the determination of LIG concentration in plasma and its validation and to apply the established method to preliminarily study the pharmacokinetics of LIG. Herein, we report an HPLC method with FLD for the analysis of LIG in biological samples using podophyllotoxin as an internal standard that outperformed both sensitivity and reproducibility. This method had been comprehensively validated, offering the advantage of simplicity with adequate sensitivity, selectivity, precision and accuracy for the determination of LIG, while small volume plasma was needed. The assay was also successfully applied in the pharmacokinetic studies of LIG in rats (intravenous and oral administration at doses of 12.5, 25 and 50 mg/kg, respectively). The development of the method would facilitate the ease of adaptability of LIG assay in other biological samples such as urine, human plasma and so on. This work helps fill some of the gaps in overall knowledge of LIG.
Materials and methods
Chemicals and reagents
The volatile oil of Radix Angelica sinensis (VOAS) was purchased from Shaanxi margin biological technology Co., Ltd (Xi’an, China). The concentration of ligustilide in VOAS was determined by gas chromatography (GC) to be 47.14%. Liguistilide was isolated from VOAS and further purified by silica gel column chromatography (Bohrmann et al., Citation1967; Kanamori & Sakamoto, Citation1992; Li et al., Citation2001; Lin et al., Citation1998). The purity of the liguistilide was 97.26%. Podophyllotoxin, the internal standard (IS), was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Acetonitrile and methanol were purchased from Shandong Yuwang reagent limited liability company (Shandong, China) were of chromatographic grade. Dichloromethane of chromatographic grade was purchased from Tianjin City Kay Chemical Co., Ltd (Tianjin, China). Distilled water was further purified by Mill-Q (Millipore, Billerica, MA). Other chemicals were of analytical grade.
HPLC chromatographic conditions
Quantitative analysis of LIG in rat plasma was performed using an HPLC-FLD analytical system. The separation of compounds was carried out using an Agilent 1200 HPLC (Boeblingen, Germany) system consisting of G1322A Vacuum Degasser, G1311A Quat Gradient Pump, G1316A Thermostatted Column Compartment, G1329A Autosampler, LC 3D instrument ChemStation and G1321A fluorescence detector. Separations were achieved on a YMC-packed ODS-A C18 column, 150 × 4.6 mm, 5 µm (YMC Co. Ltd., Kyoto, Japan) preceded by an Agilent Zorbax Reliance Cartridge guard column (Eclipse XDB-C18, 12.5 × 4.6 mm, 5-µm) with a column temperature of 30 °C. The fluorescence intensity was measured on a PerkinElmer luminescence spectrometer equipped with a xenon lamp and a Dell model 110 L. All the measurements were performed in a 10 mm path length quartz cell thermo stated at 50 °C, with 5 nm band-widths both emission and excitation monochromators. The mobile phase was composed of acetonitrile and water in a volume ratio of 50:50. The mobile phases were delivered at a constant 1 mL/min flow. FLD was performed at an excitation wavelength of 290 nm and emission wavelength of 395 nm for IS during 0–4 min and an excitation and emission wavelength of 336 and 453 nm for LIG during 4–14 min.
Animals
Male Sprague-Dawley (SD) rats (220 ± 20 g), obtained from the Laboratory Animal Center of Lanzhou University (Lanzhou, China), were used for pharmacokinetic studies. They were kept in an environmentally controlled breeding room for at least one week before starting the experiments and fed with standard laboratory food and water ad libitum. Prior to each experiment, the rats were fasted for 12 h with free access to water. All protocols and procedures were approved by Lanzhou University Animal Care and Use Committee.
Drug administration and sampling
For pharmacokinetic study, 36 rats were randomly assigned to six groups. Each group contained six rats. LIG was dissolved in medicinal ethanol-Tween 80–0.9% sodium chloride saline (25:20:55, v/v/v) to the concentrations of 5 mg/mL for oral administration (p.o.) and intravenous injection (i.v.). The LIG solution was injected in rats (n = 6) via a tail vein injection at a dose of 12.5, 25 and 50 mg/kg to groups 1, 2 and 3, respectively, and administered orally at the doses to groups 4, 5 and 6, respectively. Then an approximately 0.3 mL aliquot of blood samples were collected by retro-orbital venous plexus puncture at time intervals of 0.08, 0.25, 0.5, 0.75, 1, 2, 3, 5, 8, 12 and 24 h after the injection, and 0.08, 0.17, 0.25, 0.5, 0.75, 1, 2, 3, 5, 8, 12 and 24 h after p.o. administration. Plasma was obtained by centrifugation at 4500 × g for 10 min and stored at −80 °C before analysis. Blank plasma was collected by the same method before the rats were treated.
Sample pretreatment
A 100 μL volume of plasma standard or sample was transferred to a 1.5 mL centrifuge tube, and then 25 μL of IS working solution (10 μg/mL) was spiked and vortex-mixed for several seconds. Next 1 mL of dichloromethane and some sodium chloride were added and the sample was vortex-mixed for 5 min. The mixture was centrifuged at 12 000 × g for 5 min at a low temperature (4 °C). The supernatant (0.5 mL) was transferred to a 1.5 mL centrifuge tube and evaporated to dryness under a nitrogen gas stream in a 30 °C water bath. The residue was dissolved in 100 μL mobile phase (acetonitrile-water = 50:50, v/v) and the sample was vortex-mixed for 2 min. After vortex mixing, the mixture was centrifuged at 12 000 × g for 10 min at a low temperature (4 °C). After centrifuging 50 μL of the supernatant was injected into the HPLC system. The ratio of LIG peak area over the internal standard was used for quantitative analysis.
Calibration standards and quality control samples
Primary standard stock solutions of LIG (1 mg/mL) and podophyllotoxin (1 mg/mL) were prepared in acetonitrile. Working solutions of podophyllotoxin were prepared by appropriate dilution with the mixture of acetonitrile-water just before use, using amber glass volumetric flasks in order to avoid photo degradation. The IS working solutions of 10 μg/mL were prepared in the same manner. All solutions were stored in darkness at 4 °C. Aliquots of LIG (25 μL) working solutions were added to 100 μL drug-free rat plasma to obtain LIG calibration standard (2.44, 4.88, 9.76, 19.5, 39, 78, 156 ng/mL and 0.156, 0.313, 0.625, 1.25, 2.5, 5 and 10 μg/mL) in plasma samples for two calibration curves. Quality control (QC) samples were separately prepared in the similar manner for two calibration curves; the range of the calibration curves were 2.44–156 ng/mL and 0.156–10 μg/mL. For calibration curve one, 4.88, 39 and 156 ng/mL plasma samples were corresponding to the low QC, medium QC and high QC, respectively. For another calibration curve, 0.156, 1.25 and 5 μg/mL plasma samples were corresponding to the low QC, medium QC and high QC, respectively.
Bioanalytical method validation
Specificity
Specificity of the method was assessed by analyzing five independent sources of blank rat plasma or plasma samples spiked with ligustilide and podophyllotoxin, observing the extent to which interferents from plasma may interfere with the analyte or the internal standard.
Calibration curves and linearity
Each calibration standard concentration was tested in triplicate. After injecting all the processed calibration standard samples of various concentrations covering the working range of the assay, two calibration curves were established in the range of 2.44–156 ng/mL and 0.156–10 μg/mL. The calibration curves were generated by plotting peak area ratios of analytes to IS against the respective standard concentrations. The acceptance criteria for a calibration curve was correlation coefficient (r) greater than 0.99 and each back-calculated standard concentration must be within 15% deviation from the nominal value except for concentrations at the LLOQ, where 20% was accepted.
Determination of the lower limit of quantitation (LLOQ) and limit of detection (LOD)
Batches of blank rat plasma were spiked with two different concentrations (2 and 3 ng/mL) of ligustilide and measured to determine the LLOQ, which is defined as a lowest concentration of analyte that can be determined with acceptable precision and accuracy under stated experimental conditions. LLOQ was identified based on the two criteria (FDA, Citation2001): (1) the lowest concentration of the analyte that produced S/N ratio of greater than 10; (2) the analyte response that can be determined with sufficient precision and accuracy, i.e., the percentage deviation and RSD are to be less than 20%. The lowest concentration that meets both the mentioned criteria was accepted as LLOQ. The limit of detection (LOD) for the determination of LIG in the proposed assay was established by the analysis of signal-to noise ratio (S/N ratio) which was obtained by serial extraction of plasma samples spiked with decreasing concentrations of LIG. The analyte concentration that produced S/N ratio greater than 3 was accepted as LOD.
Extraction recoveries
The recovery of LIG from plasma was determined by injecting the processed QC samples at three concentrations of low QC, medium QC and high QC for two calibrations. Recovery was evaluated by comparing the analyte peak areas, obtained from the QC samples (n = 5) after extraction, with those obtained from the corresponding unextracted reference standards prepared at the same concentrations, respectively.
Accuracy and precision
Intra-day accuracy and precision evaluations were performed by repeated analysis of LIG in rat plasma on the same day. The run consisted of a calibration curve plus five replicates of each low, medium and high QC samples. Inter-day accuracy and precision were assessed by the analysis of samples consisting of a calibration curve and five replicates of three concentrations samples for LIG on three consecutive days.
Accuracy was calculated as the percentage of the concentration of drug measured from calibration curve to the theoretical concentration value of drug added to the blank plasma. Precision was expressed as the percentage coefficient variation (CV, %), of measured concentrations for each QC samples. The values within ± 15% for accuracy and precision were considered acceptable, except for concentrations at the LLOQ, where 20% was accepted.
Stability
LIG stability in plasma was assessed by analyzing QC samples at concentrations of 4.88, 19.5 and 156 ng/mL, 1.25 and 5 μg/mL, respectively (n = 5), for three freeze-thaws, short-term, long-term and post-preparative stabilities. For the short-term stability, the plasma samples were kept at room temperature (about 25 °C) for 6 h before sample preparation. The freeze-thaw stability of LIG was determined over three freeze-thaw cycles within 3 days. In each freeze-thaw cycle, the spiked plasma samples were frozen at −80 °C for 24 h and thawed at room temperature. When completely thawed, the samples were refrozen for 12–24 h under the same conditions. After three cycles, the percent loss of the analyte was determined by comparing the concentrations with those obtained before freezing. The long-term stability was evaluated after keeping the plasma samples frozen at −80 °C for 30 days. The stability of the prepared plasma samples was tested after keeping the samples in an autosampler at about 25 °C for 24 h. The samples were analyzed and the results compared with those obtained from the freshly prepared samples.
Pharmacokinetic study of LIG
Compartmental and non-compartmental pharmacokinetic analysis of LIG concentrations versus time data were performed using DAS 2.1 (Drug and Statistics 2.1, the Committee of the Mathematic Pharmacology, the Chinese Society of Pharmacology, Hefei, China) to estimate the pharmacokinetic parameters. Pharmacokinetic parameters were estimated using model-independent methods (Gibaldi & Perrier, Citation1982). The terminal elimination rate constant (λn) was estimated by linear regression analysis of the terminal portion of the log-linear blood concentration--time profile of a drug. The terminal elimination half-life (t1/2) was calculated from the terminal elimination rate constant using the formula t1/2 = 0.693/λn. Maximum plasma concentration (Cmax) and time of maximum concentration (Tmax), were obtained directly from the plasma concentration--time plots. The volume of distribution (V) was calculated using standard equations. The area under concentration--time curve (AUC) and area under the first moment curve (AUMC) were calculated by the method of trapezoids, and extrapolation to infinity was performed. The total body clearance was calculated as Cl = Dose/AUC. Mean residence time (MRT) was given by the AUMC0−∞ to AUC0−∞ ratio. Absolute bioavailability (F) was calculated as (AUCp.o. × Div)/(AUCi.v. × Dp.o.) × 100%, where p.o. and i.v., respectively, express oral and intravenous administration, and D was the dose of administration. The F value was calculated based on the AUC after intravenous administration of LIG at a dose of 25 mg/kg.
Statistical analysis
The pharmacokinetic model and the parameters were calculated by the DAS 2.1 (Drug and Statistics 2.1) edited by the Committee of the Mathematic Pharmacology, the Chinese Society of Pharmacology. The data were presented as mean ± SD (standard deviation). Comparisons between groups were performed by one-way ANOVA. A p-value of 0.05 or less was considered significant.
Results and discussion
Optimization of the chromatographic conditions
Methanol and water along with acetonitrile and water systems are often used as mobile phase in the reverse chromatography. LIG is a neutral molecule; we chose acetonitrile and water without any additive as mobile phase instead of methanol and water. Several trials to screen the ratios of acetonitrile to water were carried out in order to obtain good peak shape and high theoretical plates, and reasonable retention time was also needed to considerate. Sometimes the change of the ratio may significantly affect the response of the analytes. Most important, the analyte and IS should be separate from the interferences of biomatrix components such as plasma proteins at baseline. When the ratio of acetonitrile to water was 50:50, two sharp and symmetrical peaks of LIG and podophyllotoxin were obtained. The effective separation was achieved between the analyte and IS which were also completely separated from the interferences. A short run time is obtained because the retention time of LIG and podophyllotoxin were 12.02 and 3.25 min, respectively, and the total run was 15 min, and thus we can analyze more samples in a working day. Finally, the ratio of acetonitrile to water was set to 50:50. The injection volume of the sample was also investigated. The values of 20, 50 and 100 μL volume of processed sample were injected into the HPLC system sequentially. It was found that when injection volume increased from 20 to 50 μL, no significant reducing column performance was observed and the peak shape was still sharp and symmetrical, but the plate number obviously decreased with bad peak shape when injection volume increased from 50 to 100 μL. In order to improve the sensitivity and prolong the life of the column, 50 μL injection volume was chosen.
Sample pretreatment
First, the selection of the IS must be solved. We had used the structural analogue of LIG such as levofloxacin, pazufloxacin and fluconazol as IS, neither good peak shape nor proper retention time was obtained. Podophyllotoxin was selected as an IS because of relatively high and reproducible recovery and suitable retention time. In addition, the use of podophyllotoxin offered satisfactory validation results of the developed method. The present study was the first report to apply podophyllotoxin as an internal standard for HPLC analysis of LIG. Conventional extraction procedures including protein precipitation and liquid--liquid extraction were studied. Ethyl acetate, dichloromethane and chloroform were used to extract LIG and podophyllotoxin from the plasma. After the extraction, the organic layer was transferred to a 1.5 mL Eppendorf tube and evaporated to dryness under a stream of nitrogen in a water bath at 30 °C. The residues were reconstituted in proper volume mobile phase. If these solvents were directly injected into the HPLC system, high background noise would affect the baseline; besides, chronically using dichloromethane and chloroform would damage the system and be harmful to human health. Finally, we chose dichloromethane as the reagent for plasma sample pretreatment. Extraction time and volume of dichloromethane were tested, and the results indicated that extraction recoveries were not improved when the shaking time was longer than 5 min and volumes larger than 1 mL.
Method validation
Specificity
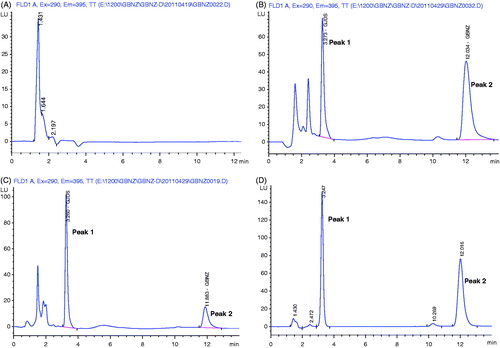
shows the representative chromatograms of blank plasma, blank plasma samples spiked with LIG, plasma sample obtained from a rat following an injection 25 mg/kg dose of LIG and standard solution of LIG spiked with IS. The retention time of IS and LIG were 3.25 and 12.02 min, respectively. As described above, good resolution was achieved between analyte and IS and no interference from different sources of rat plasma was observed interfering the separation and quantitation of LIG.
Figure 1. Typical chromatograms of LIG from rat plasma: (A) blank plasma sample; (B) blank plasma sample spiked with LIG; (C) plasma sample obtained at 1 h from rat after an i.v. administration of LIG (12.5 mg/kg); (D) standard solution of LIG spiked with IS. Peak 1, podophyllotoxin (internal standard); Peak 2, LIG.
Linearity, limit of detection and lower limit of quantization
The calibration model for the two calibration curves was selected based on the analysis of the data by linear regression and with weighting factors (1/x, 1/x2 and 1). For the calibration curves, the weighted factor was set to 1. The peak area ratio of LIG to IS in rat plasma was linear with respect to the analyte concentration over the range of 2.44–156 ng/mL and 0.156–10 μg/mL. The regression equation for calibration one was Y = 133.49 X − 14.27 (correlation coefficient, r = 0.9995), and Y = 145.61 X + 13.76 (correlation coefficient, r = 0.9996) was the regression equation for another calibration curve in the range of 0.156–10 μg/mL, where Y is the mean peak area ratio of the analyte to the IS and X is the concentration of the analyte. Each back-calculated standard concentration was within 10% deviation from the nominal value. The LOD for LIG was found to be 0.5 ng/mL (S/N ≥ 3) and LLOQ was 2.44 ng/mL with acceptable precise increased by using more volume of plasma or concentrating the processed sample, but the presented method was sensitive enough for the pharmacokinetic study.
Accuracy and precision
The within-batch and between-batch accuracy and precision values of the method are presented in . The within-batch coefficient of variation (RSD) for LIG ranged from 2.69 to 4.83% and the accuracy from 88.93 to 99.35%. Coefficient of variation of the between-batch was from 2.18 to 5.72% and the accuracy from 90.37 to 99.52%. The results indicated that the assay was reproducible, accurate and reliable.
Table 1. Precision and accuracy data for LIG in rat plasma (n = 5).
Extraction efficiency
The recovery of LIG was higher than 89.07% at all the five concentrations studied () and it was found to be excellent in plasma samples. From the obtained results, it could be concluded that the volume of the dichloromethane was sufficient to extract the analyte.
Table 2. Extraction recoveries of LIG from rat plasma (n = 5).
Stability
The stabilities of samples are summarized in . The study results demonstrated that no significant degradation of LIG in plasma occurred under experimental conditions. The mean recoveries following the storage period ranged from 91.37 to 99.71% in plasma.
Table 3. Summary of stability studies of LIG in rat plasma under various storage conditions (n = 5).
Pharmacokinetics of LIG after intravenous administration in rats
The mean plasma concentration--time profiles of LIG after intravenous administration at doses of 12.5, 25 and 50 mg/kg in rats are shown in , and some relevant pharmacokinetic parameters are listed in . Using one-way ANOVA, statistical comparisons of individual pharmacokinetic parameters such as t1/2, clearance (Cl) and volume of distribution (V) demonstrated no significant (p > 0.05) differences when each parameter was compared for the three test doses. Note that the AUC values of LIG were proportional to intravenous doses studied. For example, the dose-normalized (based of 12.5 mg/kg) AUC values were 235.74 ± 2.85, 246.76 ± 6.36 and 216.59 ± 4.36 ng*h/mL for 12.5, 25 and 50 mg/kg, respectively. The slope between log AUC and log dose of LIG was close to 1.0 (the value was 0.9389). depicts the plot of Cmax and AUC as a function of dose. Comparisons of AUC normalized to 12.5 mg/kg dose did not result in any significant differences among the three doses studied. The peak plasma concentrations (Cmax) and the AUC increased in proportion with increasing doses of LIG. Also, MRT was not changed over the dose range studied. The volume of distribution was larger than the total water (60–70% body-weight) (Granero & Amidon, Citation2008), suggesting that LIG appeared to be widely distributed throughout the body.
Figure 2. Plasma concentration--time curves of LIG in rats after oral and intravenous administration (n = 6). (A): intravenous administration of 12.5, 25 and 50 mg/kg, b.w. LIG; (B): oral administration of 12.5, 25 and 50 mg/kg, b.w. LIG.
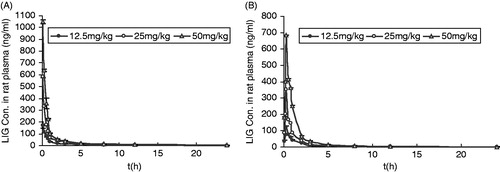
Figure 3. Relationship between Cmax and AUC vs. dose in rats receiving single 12.5, 25 and 50 mg/kg, b.w. intravenous dose of LIG.
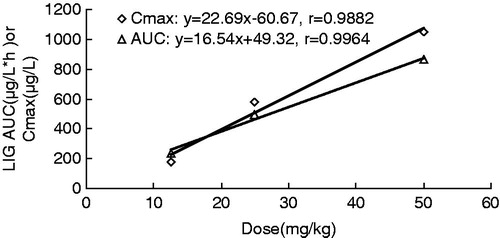
Table 4. Pharmacokinetic parameters of LIG in rats after oral and intravenous administration at three different doses (n = 6, mean ± SD).
The compartment model was established by the methods of the survival square sum (SUM), the Akaike’s information criterion (AIC) and the fitted degree (r2). In our study, a two compartment open model (weight = 1) after i.v. injection gave the best fit to the plasma concentration time curves obtained in rats. After i.v. administration of LIG at doses of 12.5–50 mg/kg in rats, LIG is rapidly eliminated with an elimination half life (t1/2) of 3.33–5.60 h and the terminal elimination rate constants (λn) of 0.11–0.23 h−1, which are consistent with that reported by Yan et al. (Citation2008) in rats (3.43 h).
The apparent volumes of distribution at steady-state (V) obtained after i.v. administration at doses of 12.5, 25 and 50 mg/kg in our study indicate that LIG is widely distributed (). LIG is highly lipophilic, and would be expected to exhibit extensive tissue distribution (Shi et al., Citation2006). Our values (220.85–572.62 L/kg) were clearly higher than the values obtained by Yan et al. (Citation2008) in rats (3.76 L/kg).
After intravenous administration of LIG at doses of 12.5–50 mg/kg in rats, the total body clearance (Cl) of 50.69–57.74 L/h/kg () were considerably higher than the reported cardiac output in rats, 17.76 L/h/kg, based on blood data (Davies & Morris, Citation1993). This suggested that the first pass effects of LIG in the lung and heart could be considered in rats. The Cl value was higher than that of hepatic blood flow in rat of 24–36 L/h/kg, suggesting hepatic elimination is involved. However, it is not certain as to how cardiac and hepatic elimination is involved. More study is required to elucidate this. In our study, the Cl value significantly differs from that reported by Yan et al. (Citation2008) after single i.v. administration in rats (9.14 L/h/kg).
The discrepancies between the values calculated for pharmacokinetic parameters may be attributed to the animal species, the drug formulation employed, the age, size or sex of the animals, to differences in fatty tissue deposits between animal species or breeds, or even to inter-individual variations.
Pharmacokinetics of LIG after oral administration in rats
After oral administration of LIG at doses of 12.5, 25 and 50 mg/kg to rats, the mean plasma concentration--time profiles of LIG are shown in , and some relevant pharmacokinetic parameters are listed in . After oral administration of LIG, the absorption of the drug from the rat gastrointestinal tract was rapid, and LIG was detected in plasma from the first blood sampling time (5 min) and rapidly reached Tmax (15 min) for all three oral doses studied. Note that the dose-normalized (based on 12.5 mg/kg) AUC values of LIG were also comparable (not significantly different) among the three doses studied. For example, the dose-normalized (based on 12.5 mg/kg) AUC values were 171.94 ± 8.69, 168.45 ± 1.37 and 186.15 ± 1.92 ng * h/mL for 12.5, 25 and 50 mg/kg, respectively. The F values were 71.36, 68.26 and 75.44% for oral doses of 12.5, 25 and 50 mg/kg, respectively. Moreover, other pharmacokinetic parameters of LIG listed in were also not significantly different among three oral doses studied, except Cmax and AUC, indicating that the pharmacokinetic parameters of LIG are also independent of three oral doses studied.
LIG pharmacokinetics after p.o. administration was best described in rats by a two-compartment open model, which is in accordance with the previous study in rats (Shi et al., Citation2006). The absorption process was rapid with time to reach maximum concentration (Cmax) of 0.25 h, which was shorter than that reported by Shi et al. (Citation2006) (0.65 h) and Yan et al. (Citation2008) (0.36 h) in rats.
Following oral administration of LIG at doses of 12.5–50 mg/kg in rats, LIG is quickly eliminated with an elimination half life (t1/2) of 3.33–5.60 h, the values are consistent with those reported by Yan et al. (Citation2008) in rats (3.43 h).
There are few previous data available describing LIG p.o. bioavailability in rats, which was only 2.6% in the rat at a dose of 500 mg/kg, partly because of extensive first-pass metabolism in the liver (Yan et al., Citation2008). In our study, the absolute bioavailability values were 71.36, 68.26 and 75.44% for oral doses of 12.5, 25 and 50 mg/kg, respectively. The differences might be due to the pharmacokinetic curve-fitting routines or the analytical techniques used, the drug formulation administered, and the health status of the animals. The reason for the incomplete F value needs to be elucidated by more studies.
Conclusion
A novel sensitive, specific and reproducible fluorimetric HPLC method for the determination of LIG in rat plasma was developed in this study. The method was comprehensive validated over a concentration range of 2.44–156 ng/mL and 0.156–10 μg/mL (r > 0.999) and it offered good accuracy and precision. The advantages of the method are summarized as follows. First, the analysis was rapid with a short time run of 15 min and sensitive with the LLOQ of 2.44 ng/mL. Second, podophyllotoxin was used for the first time as an internal standard for LIG analysis. Lastly, the method was successfully applied in the LIG pharmacokinetic study and could meet the current requirements for bioanalytical methods specified by the Pharmacopoeia Committee of China.
Declaration of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors are grateful to Xue Hu and Zhao Yunfeng for the dedicated animal care.
References
- Asuming WA, Beauchamp PS, Descalzo JT, et al. (2005). Essential oil composition of four Lomatium Raf. species and their chemotaxonomy. Biochem Syst Ecol 33:17–26
- Bohrmann H, Stahl E, Mitsmhashi H. (1967). Constituents of umbelliferous plants. XIII Chromatographic studies on the constituents of Cnidium officinale. Chem Pharm Bull 15:1606–8
- Cao YX, Zhang W, He JY, et al. (2006). Ligustilide induces vasodilatation via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Vasc Pharmacol 45:171–6
- Chao WW, Kuo YH, Lin BF. (2007). Construction of promoters based immunity screening system and its application on the study of traditional Chinese medicine herbs. Taiwanese J Agric Chem Food Sci 45:193–205
- Chao WW, Kuo YH, Hsieh SL, et al. (2009a). Inhibitory effects of ethyl acetate extract of Andrographis paniculata on NF-κB trans-activation activity and LPS-induced acute inflammation in mice. Evid Based Compl Alt Med 18:206–15
- Chao WW, Kuo YH, Li WC, et al. (2009b). The production of nitric oxide and prostaglandin E2 in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morus alba ethyl acetate fractions. J Ethnopharmacol 122:68–75
- Chao WW, Hong YH, Chen ML, et al. (2010). Inhibitory effects of Angelica sinensis ethyl acetate extract and major compounds on NF-κB trans-activation activity and LPS-induced inflammation. J Ethnopharmacol 129:244–9
- Chao ZZ, Chao RB. (2004). Determination of ligustilide content in Chuanxiong by HPLC. West Chin J Pharm Sci 19:197–8
- Chen LL, Wang YH, Qi J, et al. (2011). Identification and determination of absorbed components of Danggui-Shaoyao-San in rat plasma. Chin J Nat Med 9:0363–8
- Chen QC, Lee JP, Jin WY, et al. (2007). Cytotoxic constituents from Angelica sinensis radix. Arch Pharm Res 30:565–9
- Chen QH, Li P, Li B, et al. (2010). A GC-MS-SIM simultaneous determination of ligustilide and butylidenephthalide from Ligusticum chuanxiong using SFE. Chromatographia 72:963–7
- Cheng LL, Chen XN, Wang Y, et al. (2011). Z-Ligustilide isolated from Radix Angelicae sinensis ameliorates the memory impairment induced by scopolamine in mice. Fitoterapia 82:1128–32
- Cho SK, Abd El-Aty AM, Choi JH, et al. (2007). Optimized conditions for the extraction of secondary volatile metabolites in Angelica roots by accelerated solvent extraction. J Pharm Biomed Anal 44:1154–8
- Chung JW, Choi RJ, Seo EK, et al. (2012). Antiinflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch Pharm Res 35:723–32
- Cui F, Feng L, Hu J. (2006). Factors affecting stability of Z-ligustilide in the volatile oil of Radix Angelicae sinensis and Ligusticum chuanxiong and its stability prediction. Drug Dev Ind Pharm 32:747–55
- Davies B, Morris T. (1993). Physiological parameters in laboratory animals and humans. Pharm Res 10:1009–95
- Deng CH, Ji J, Wang XC, et al. (2005). Development of pressurized hot water extraction followed by headspace solid-phase microextraction and gas chromatography-mass spectrometry for determination of ligustilides in Ligusticum chuanxiong and Angelica sinensis. J Sep Sci 28:1237–43
- Dietz BM, Liu D, Hagos GK, et al. (2008). Angelica sinensis and its alkylphthalides induce the detoxification enzyme NAD (P) H: Quinine oxidoreductase 1 by alkylating Keap1. Chem Res Toxicol 21:1939–48
- Du JR, Bai B, Kuang X, et al. (2006). Ligustilide inhibits spontaneous and agonists- or K+ depolarization-induced contraction of rat uterus. J Ethnopharmacol 108:54–8
- Du JR, Yu Y, Ke Y, et al. (2007). Ligustilide attenuates pain behavior induced by acetic acid or formalin. J Ethnopharmacol 112:211–14
- Fang HJ, Lu RM, Liu GS, et al. (1979). Studies on the components of essential oils. II. Comparison of the major constituents of the essential oil from two species of Danggui Angelica sinensis (Oliv.) Diels and Levisticum officinale Koch. Yao Xue Xue Bao 14:617–23
- FDA. (2001). U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM), Guidance for Industry, Bioanalytical Method Validation
- Feng ZB, Lu YP, Wu XM, et al. (2012). Ligustilide alleviates brain damage and improves cognitive function in rats of chronic cerebral hypoperfusion. J Ethnopharmacol 144:313–21
- Gibaldi M, Perrier D. (1982). Non-Compartmental Analysis Based on Statistical Moment Theory. Pharmacokinetics, 2nd ed. New York: Marcel Dekker, 409–17
- Gijbels MJM, Scheffer JJC, Svendsen AB. (1982). Phthalides in the essential oil from roots of Levisticum officinale. Planta Med 44:207–11
- Granero GE, Amidon GL. (2008). Possibility of enterohepatic recycling of ketoprofen in dogs. Int J Pharm 349:166–71
- Guo JM, Duan JA, Shang EX, et al. (2009). Determination of ligustilide in rat brain after nasal administration of essential oil from Rhizoma chuanxiong. Fitoterapia 80:168–72
- Guo JM, Shang EX, Duan JA, et al. (2011). Determination of ligustilide in the brains of freely moving rats using microdialysis coupled with ultra performance liquid chromatography/mass spectrometry. Fitoterapia 82:441–5
- Hu CY, Ding XL. (2006). Two methods for determining ligustilide content of Angelica oil. J Chin Cereal Oil Assoc 21:152–5
- Hu LH, Chen XG, Kong L, et al. (2005a). Improved performance of comprehensive two-dimensional HPLC separation of traditional Chinese medicines by using a silica monolithic column and normalization of peak heights. J Chromatogr A 1092:191–8
- Hu J, Feng LL, Liu Y, et al. (2005b). Study on the determination and extraction of ligustilide in Radix Angelicae sinensis and Rhizoma chuanxiong. J Shenyang Pharm Univ 22:67–70
- Ji SG, Chai YF, Wu YT, et al. (1999). Determination of ferulic acid in Angelica sinensis and Chuanxiong by capillary zone electrophoresis. Biomed Chromatogr 13:333–4
- Kanamori H, Sakamoto I. (1992). Evaluation of Angelicae radix. Purification method and stability of ligustilide. Hiroshima-ken Eisei Kenkyusho Kenkyu Hokoku, 39:23–6
- Kan WLT, Cho CH, Rudd JA, et al. (2008). Study of the antiproliferative effects and synergy of phthalides from Angelica sinensis on colon cancer cells. J Ethnopharmacol 120:36–43
- Kim MR, Abd EI-Aty AM, Choi JH, et al. (2006a). Identification of volatile components in Angelica species using supercritical-CO2 fluid extraction and solid phase microextraction coupled to gas chromatography-mass spectrometry. Biomed Chromatogr 20:1267–73
- Kim MR, Abd EI-Aty AM, Kim IS, et al. (2006b). Determination of volatile flavor components in danggui cultivars by solvent free injection and hydrodistillation followed by gas chromatographic-mass spectrometric analysis. J Chromatogr A 1116:259–64
- Kuang X, Yao Y, Du JR, et al. (2006). Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res 102:145–53
- Kuang X, Du JR, Chen YS, et al. (2009). Protective effect of Z-ligustilide against amyloid β-induced neurotoxicity is associated with decreased pro-inflammatory markers in rat brains. Pharmacol Biochem Behav 92:635–41
- Kuang X, Du JR, Liu YX, et al. (2008). Postischemic administration of Z-Ligustilide ameliorates cognitive dysfunction and brain damage induced by permanent forebrain ischemia in rats. Pharmacol Biochem Behav 88:213–21
- Kwon YS, Kobayashi A, Kajiyama S, et al. (1997). Antimicrobial constituents of Angelica dahurica roots. Phytochemistry 44:887–9
- Lao SC, Li SP, Kan KKW, et al. (2004). Identification and quantification of 13 components in Angelica sinensis (Danggui) by gas chromatography-mass spectrometry coupled with pressurized liquid extraction. Anal Chim Acta 526:131–7
- Li CY, Qi LW, Li P. (2011). Correlative analysis of metabolite profiling of Danggui Buxue Tang in rat biological fluids by rapid resolution LC-TOF/MS. J Pharm Biomed Anal 55:146–60
- Li CY, Qi LW, Li P, et al. (2009). Identification of metabolites of Danggui Buxue Tang in rat urine by liquid chromatography coupled with electrospray ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 23:1977–88
- Li G, Ma C, Li X, et al. (2000). Studies on the stability of ligustilide and the analysis of its isomerized products by GC-MS. Chin Tradit Herb Drug 31:405–7
- Li GS, Ma CJ, Lim ZF. (2001). Extraction of essential oil from Angelica sinensis by supercritical-CO2 fluid in comparison with that by steam distillation. Chin Tradit Herb Drug 32:581–3
- Li H, Wang YT. (2003). Influencing factor of the stability of ligustilide and means of stabilization. J Jiangxi Coll Tradit Chin Med 15:56–59
- Li P, Li SP, Lao SC, et al. (2006a). Optimization of pressurized liquid extraction for Z-ligustilide, Z-butylidenephthalide and ferulic acid in Angelica sinensis. J Pharm Biomed Anal 40:1073–9
- Li XR, Liang YZ, Guo FQ. (2006b). Analysis of volatile oil in Rhizoma Ligustici Chuanxiong--Radix Paeoniae Rubra by gas chromatography-mass spectrometry and chemometric resolution. Acta Pharmacol Sin 27:491–8
- Lin LZ, He XG, Lian LZ, et al. (1998). Liquid chromatographic-electro-spray mass spectrometric study of the phthalides of Angelica sinensis and chemical changes of Z-ligustilide. J Chromatogr A 810:71–79
- Liu EH, Qi LW, Peng YB, et al. (2009). Rapid separation and identification of 54 major constituents in Buyang Huanwu decoction by ultra-fast HPLC system coupled with DAD-TOF/MS. Biomed Chromatogr 23:828–42
- Lu GH, Chan K, Chan CL, et al. (2004). Quantification of ligustilides in the roots of Angelica sinensis and related umbelliferous medicinal plants by high-performance liquid chromatography and liquid chromatography-mass spectrometry. J Chromatogr A 1046:101–7
- Lu GH, Chan K, Leung K, et al. (2005a). Assay of free ferulic acid and total ferulic acid for quality assessment of Angelica sinensis. J Chromatogr A 1068:209–19
- Lu GH, Chan K, Liang YZ, et al. (2005b). Development of high-performance liquid chromatographic fingerprints for distinguishing Chinese Angelica from related umbelliferae herbs. J Chromatogr A 1073:383–92
- Lu Q, Qiu TQ, Yang H. (2006). Ligustilide inhibits vascular smooth muscle cells proliferation. EurJ Pharmacol 542:136–40
- Lu JL, Zhao J, Duan JA, et al. (2009). Quality evaluation of Angelica sinensis by simultaneous determination of ten compounds using LC-PDA. Chromatographia 70:455–65
- Lv Y, Lou ZH, Chen SH, et al. (2011). Pharmacokinetics and tissue distribution of 2,3,5,4-tetrahydroxystilbene-2-O-β-d-glucoside from traditional Chinese medicine Polygonum multi florum following oral administration to rats. J Ethnopharmacol 137:449–456
- Ma ZJ, Bai LH. (2013). Antiinflammatory effects of Z-Ligustilide nanoemulsion. Inflammation 36:294–99
- Mei QB, Tao JY, Cui B. (1991). Advances in the pharmacological studies of radix Angelica sinensis (Oliv) Diels (Chinese danggui). Chin Med J 104:776–81
- Naito T, Katsuhara T, Niitsu K, et al. (1992). Two phthalides from Ligusticum chuangxiong. Phytochemistry 31:639–42
- Qi HY, Han YF, Rong JH. (2012). Potential roles of PI3K/Akt and Nrf2eKeap1 pathways in regulating hormesis of Z-ligustilide in PC12 cells against oxygen and glucose deprivation. Neuropharmacology 62:1659–70
- Qi LW, Wen XD, Cao J, et al. (2008). Rapid and sensitive screening and characterization of phenolic acids, phthalides, saponins and isoflavonoids in Danggui Buxue Tang by rapid resolution liquid chromatography/diode-array detection coupled with time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 22:2493–509
- Schinkovitz A, Pro SM, Main M, et al. (2008). Dynamic nature of the ligustilide complex. J Nat Prod 71:1604–11
- Shao M, Qu K, Liu K, et al. (2011). Effects of ligustilide on lipopolysaccharide-induced endotoxic shock in rabbits. Planta Med 77:809–16
- Shi LF, Zheng XM, Cai Z, et al. (1995). Comparison of influence of essential oil from Ligusticum chuanxiong Hort on microcirculation in rabbit conjunctiva bulbar before and after decomposition of ligustilide. J Chin Pharmacol Toxicol 9:157–8
- Shi YF, He LC, Wang SC. (2006). Determination of ligustilide in rat blood and tissues by capillary gas chromatography/mass spectrometry. Biomed Chromatogr 20:993–8
- Sun XH, Niu LJ, Li XQ, et al. (2009). Characterization of metabolic profile of mosapride citrate in rat identification of two new metabolites: Mosapride N-oxide and morpho linering-opened mosapride by UPLC-ESI-MS/MS. J Pharm Biomed Anal 50:27–34
- Su YW, Chiou WF, Chao SH, et al. (2011). Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol 11:1166–72
- Takashi Y. (1982). Quantitative determination of ligustilide and butylidenephthalide in touki (Angelica radix) and senkyu (Cnidii rhizome) by high-performance liquid chromatography. Hokkaidoritsu Eisei Kenkyusyoho 32:12–16
- Tang J, Zhang Y, Hartman TG, et al. (1990). Free and glycosidically bond volatile compounds in fresh celery (Apium graveolens L.). J Agri Food Chem 38:1937–40
- Tsai NM, Chen YL, Lee CC, et al. (2006). The natural compound n-butylidenephthaliude derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo. J Neurochem 99:1251–62
- Wang J, Du JR, Wang Y, et al. (2010). Zligustilide attenuates lipopolysaccharide induced proinflammatory response via inhibiting NF-κB pathway in primary rat microglia. Acta Pharmacol Sin 31:791–7
- Wu XM, Qian ZM, Zhu L, et al. (2011). Neuroprotective effect of ligustilide against ischaemia--reperfusion injury via up-regulation of erythropoietin and down regulation of RTP801. Brit J Pharmacol 164:332–43
- Yan R, Ko NL, Li SL, et al. (2008). Pharmacokinetics and metabolism of ligustilide, a major bioactive component in Rhizoma chuanxiong in the rat. Drug Metab Dispos 36:400–8
- Yeh JC, Garrard IJ, Cho CWC, et al. (2012). Bioactivity-guided fractionation of the volatile oil of Angelica sinensis radix designed to preserve the synergistic effects of the mixture followed by identification of the active principles. J Chromatogr A 1236:132–8
- Yi T, Leung KSY, Lu GH, et al. (2007). Comparative analysis of Ligusticum chuanxiong and related umbelliferous medicinal plants by high performance liquid chromatography-electrospray ionization mass spectrometry. Planta Med 73:392–8
- Yu Y, Du JR, Wang CY, et al. (2008). Protection against hydrogen peroxide induced injury by Z-ligustilide in PC12. Exp Brain Res 184:307–12
- Zhang L, Du JR, Wang J, et al. (2009). Z-Ligustilide extracted from radix Angelica sinensis decreased platelet aggregation induced by adp ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi 129:855–9
- Zhou C, Li X. (2001). Studies on the stability of ligustilide with solvent effect. Acta Pharmacol Sin 36:793–5
- Zschocke S, Liu JH, Stuppner H, et al. (1998). Comparative study of roots of Angelica sinensis and related umbelliferous drugs by thin layer chromatography, high-performance liquid chromatography, and liquid chromatography-mass spectrometry. Phytochem Anal 9:283–90
- Zuo AH, Wang L, Xiao HB, et al. (2011). Identification of the absorbed components and metabolites in rat plasma after oral administration of Rhizoma chuanxiong decoction by HPLC-ESI-MS/MS. J Pharm Biomed Anal 56:1046–56
