Abstract
Context. Conyza filaginoides (D.C.) Hieron (Asteraceae) is a medicinal Mexican plant highly prized in contemporary Mexico for the treatment of upset stomach and diabetes.
Objective: This work was undertaken to develop a suitable high performance liquid chromatography (HPLC)-diode array detection (DAD) method for quantifying rutin (1), the main active principle from the aerial parts of C. filaginoides.
Materials and methods: The method was performed using a LiChrospher 100 RP-18 column. The mobile phase was water (containing 0.1% phosphoric acid)-methanol-acetonitrile (80:5:15, v/v) at a flow rate of 1.2 mL min−1.
Results: Limits of detection and quantification were 7.5 and 22.8 μg mL−1, respectively. The main recoveries measured at three concentrations were higher than 98%, with RSD <2%. Quantitative analysis of a few samples showed the presence of high concentrations of 1 (3.6 ± 0.2 g/100 g of dry plant material). The volatile components were extracted by hydrodistillation or head space solid-phase microextraction (HS-SPME), and thereafter analyzed by gas chromatography coupled to mass spectrometry (GC-MS). Forty-three chemical constituents representing 90% of the total content of the oil were identified. The major light volatile compounds obtained by HS-SPME revealed a high content of monoterpene hydrocarbons.
Conclusions: A precise, reliable, and accurate analytical HPLC method to detect and quantify 1 in the crude drug and some preparations were developed and fully validated. The volatile components of the plant are described for the first time. The proposed method would be useful for quality control assurance of this important Mexican plant.
Introduction
Conyza filaginoides (D.C.) Hieron (Asteraceae), popularly known as “simonillo”, is a medicinal Mexican plant highly prized in contemporary Mexico for treating upset stomach, painful complaints, and diabetes (Andrade-Cetto & Heinrich, Citation2005; Martínez, Citation1989; Mendoza-Castelán et al., Citation1997). The plant can be consumed alone or in combination with other herbs such as Marrubium vulgare L. (Lamiaceae), Peumus boldus Molina (Monimiaceae), and Quassia amara L. (Simaroubaceae) (Monroy-Ortíz & Castillo España, Citation2007).
Chemical analyses carried out on C. filaginoides resulted in the isolation of a few aromatic acids including caffeic and chlorogenic acids (Tada et al., Citation2001), triterpenoids, and some flavonoids, namely, rutin (1), isoquercitrin (2) and quercetin (3) (Calzada et al., Citation2001; Dominguez et al., Citation1972; Mata et al., Citation1997). The crude extract of the plant as well as some of the isolates showed noted antimicrobial, smooth-muscle relaxant, and antiprotozoal activities (Calzada et al., Citation1998; Rojas et al., 1995).
Even though C. filaginoides is nowadays widely commercialized in Mexico as a crude drug for preparing infusions and other phytomedicines (extracts, capsules or tablets), its quality control parameters have not been established. Therefore, this work was undertaken to develop a suitable high performance liquid chromatography (HPLC)-diode array detection (DAD) method for quantifying rutin (1), the main active principle from the aerial parts of C. filaginoides and to identify the volatile components of the plant obtained by hydrodistillation and head space solid-phase microextraction (HS-SPME) analysis using gas chromatography-mass spectrometry (GC-MS).
Materials and methods
Instrumentation and chromatographic conditions
HPLC-DAD analysis
A Waters liquid chromatography (LC) system equipped with a 2487 Dual detector (DAD) (Waters Corp., Milford, MA), a quaternary pump and a manual injector was used. Waters Empower 2 software was used for data handling. Analytical chromatography of the organic extract (20 μL) was carried out on a C-18 Hibar RT LiChrospher 100 column (250 mm × 4.0 mm; film thickness 5 μm; Merck, Darmstadt, Germany). The mobile phase consisted of a gradient water (containing 0.1% phosphoric acid) (A) and methanol-acetonitrile (5:15) (B) of 80:5:15 (0 min) to 55:5:40 (15 min) at a flow rate of 1.2 mL min−1 and detection was carried out at 254 and 280 nm.
Gas chromatography-mass spectrometry (GC-MS) analysis
The essential oil was analyzed by GC-MS on an Agilent 6890N series gas chromatograph coupled with a LECO time of flight mass spectrometer detector (MS-TOF; Agilent Technology, Santa Clara, CA) with ionization voltage of 70 eV and equipped with fused silica non-polar DB-5 capillary column (10 m × 0.18 mm; film thickness 0.18 μm; Agilent HP). The operating conditions were as follows: the injector operated in split mode (ratio 20:1) and mass spectrometer transfer line temperatures were set at 200 and 300 °C, respectively; the oven temperature gradually rose from 40 to 260 °C, at warming rate of 4 °C min−1, kept at 260 °C during 20 min, and finally up to 340 °C, at a warming rate of 4 °C min−1 for 20 min isothermally; the injector temperature was set at 300 °C. The carrier gas (He) was set to 1 mL min−1 flow. Compounds were identified by co-injection of the sample with standard references when available, and by comparison of their mass spectral data with those of NIST Mass Spectral Library 98 and equipment Libraries as well as by comparison of their retention indices (RIs) relative to a homologous series of C8–C20 n-alkanes calculated according to Van Den Dool and Kratz (Citation1963) and with literature values (Adams, Citation2007). Relative amounts of individual components were calculated based on GC peak areas without response factor correction. The analyses were performed in triplicate and the relative amounts of individual components were calculated based on GC peak areas without response factor correction.
Solid-phase microextraction (SPME) analysis
Volatile compounds in C. filaginoides were separated and identified using SPME (Pawliszyn, Citation1997). One centimeter long PDMS, CAR/PDMS, and CAR/DVB/PDMS-coated fibers were used for this analysis. The fibers were conditioned in a gas chromatogram injection port at 250 °C for 2 h, prior to use. The extraction procedure was conducted as follows: the sample phases containing dried material (30 mg), sodium chloride (7.5 mg) and distilled water (5 mL) were placed in suitable vials; then, the fiber-containing needles of the SPME device were pricked through the septum of the vials to the headspace shaking (80 rpm) for 1.5 min at room temperature. After sampling, the analytes were thermally desorbed by subsequent chromatographic analysis using the GC conditions describe above. All analyses were performed in triplicate.
Chemicals and plant samples
All HPLC and AR grade solvents were purchased from Honeywell B & J (Morristown, NJ). Phosphoric acid (AR grade), α-terpinene, α-phellandrene, α-pinene, β-pinene, o-cymene, p-cymene, carvone, limonene, β-caryophyllene oxide, rutin, quercetin, isoquercitrin, n-alkanes [C8–C20] standards and sodium chloride were acquired from Sigma-Aldrich (St. Louis, MO). Manual SPME fiber holder and carboxen/polydimethylsiloxane (CAR/PDMS; 75 μm), carboxen/divinylbenzene/polydimethylsiloxane (CAR/DVB/PDMS; 50/30 μm) and polydimethylsiloxane (PDMS, 100 μm)-coated fibers were purchased from Supelco (Bellefonte, PA).
Aerial parts of C. filaginoides were purchased in the Sonora Market, Mexico City in September 2010 and identi-fied by Sol Cristians. A voucher specimen (no. 130770) was deposited at the Herbarium of The School of Science (FCME), Universidad Nacional Autonoma de Mexico in Mexico City.
Sample preparation
The air-dried aerial parts of the plant (1.5 kg) were ground into powder (mesh 2 mm) and exhaustively extracted with dichloromethane-methanol (1:1) by maceration at room temperature, without stirring during a week. After filtration, the extract was concentrated under reduced pressure to yield 137 g of a green residue. This extract (10 mg) was dissolved in a mixture 2:8 of dichloromethane-methanol (1 mL). After filtration through a 0.45 μm GHP (Port Washington, NY) filter, the samples were analyzed by HPLC. The essential oil from the plant was extracted from the air-dried and ground aerial parts (50 g) by hydrodistillation for 8 h using a modified Clevenger-type apparatus. The distilled material was exhaustively partitioned with dichloromethane (3 × 250 mL). The combined organic fractions were dried over anhydrous sodium sulfate and concentrated in vacuo to yield ∼50 mg of colorless oil.
Method validation
The developed HPLC method was subjected to an analytical validation process according to ICH guidelines (ICH, Citation2005), using the organic extract of C. filaginoides. Limits of detection (LOD) and quantification (LOQ) were assessed at signal-to-noise (S/N) ratios of 3.3 and 10, respectively. Linearity of the system was performed through the calibration curves of the stock standard solution of rutin (1) prepared by stepwise dilution with methanol at five concentration levels within the range of 50–750 μg mL−1. Three analyses were accomplished and the least square line and the correlation coefficient were calculated from the calibration curves using the Origin 8 software (Origin Labs, Wheeling, IL). The accuracy of the method was evaluated by recovery experiments, assaying independently three amounts equivalents to 50% (50 μg mL−1), 100% (500 μg mL−1) or 150% (750 μg mL−1) of 1. At each level, 1 was added simultaneously to the organic extract of C. filaginoides. The mean percentage recovery and coefficient of variation (RSD%) were calculated.
The precision was tested by repeatability and reproducibility parameters repeating (×6) independently samples of the organic extract of C. filaginoides, on the same day. The standard deviation (SD) and coefficient of variation (RSD%) were calculated for each different analysis.
Results
Volatile chemical composition analysis
Gas chromatographic coupled to mass spectrometry (GC-MS) is widely used for identifying characteristic volatile compounds present in herbal drugs and to construct representative chromatographic profiles useful for their quality control (Drew et al., Citation2012). In the present investigation, the volatile constituents of C. filaginoides were initially established through the analysis of the essential oil obtained by hydrodistillation as required in most pharmacopeias (European, United States and Mexican Pharmacopeias, among others). The examination of the oil (0.1% w/w of the dry plant material) allowed the identification of 43 chemical constituents representing about 90% of the total content (, ). As shown in the oil contained a high percentage of oxygenated monoterpenoids derivatives, mainly trans-pinocarveol (11.5%), cis-sabinol (9.9%), caryophyllene oxide (8.7%), (+)-pulegone (7.1%), isoeugenol (6.8%), o-cymene (5.1%), perillaldehyde (3.0%), linalool (2.7%), α-terpineol (2.6%), cis-p-ment-2-en-1-ol (2.5%) and carvone (2.1%). In addition, considerable amounts of (Z)-3-hexen-1-ol (11.6%) were detected.
Figure 1. Total ion chromatogram of the light volatile components from C. filaginoides obtained by hydrodistillation. Peaks: (Z)-3-hexen-1-ol (a), o-cymene (b), linalool (c), trans-pinocarveol (d), α-terpineol (e), cis-sabinol (f), pulegone (g), thuja-2,4(10)-diene (h), and caryophyllene oxide (i).
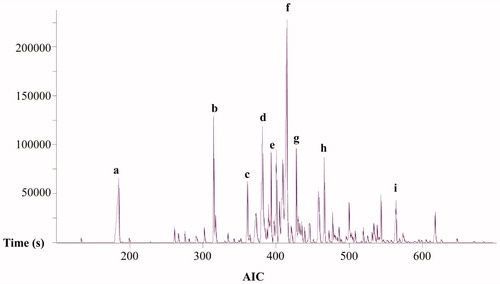
Table 1. Volatile components identified in the essential oil of C. filaginoides using GC-MS analysis.
Next, head space solid-phase microextraction (HS-SPME) was applied to complete the analysis of the volatile compounds of the plant. This is a highly sensitive, time saving and solvent-free valuable technique used nowadays for the extraction of the aroma components from plants (Pawliszyn, Citation1997). HS-SPME is also coupled to GC-MS for identifying components of interest. SPME can be tuned to a given application by the choice of appropriate coating; therefore, three different coated-fibers were tested PDMS, CAR/PDMS and CAR/DVB/PDMS. The three fibers contained PDMS, a liquid phase favoring the absorption of non-polar analytes; two contained CAR, a porous solid that absorbs polar components, and one has DVB. The best results (; ) were obtained with the PDMS and CAR/DVB/PDMS-coated fibers and the most relevant components identified with both fibers were α-phellandrene, γ-terpinene, m-cymene, β-phellandrene, and α-terpinene. On the other hand, with CAR/PDMS p-cymene and β-pinene were the main light volatiles identified. Finally, good amounts of limonene and α-phellandrene were detected with CAR/DVB/PDMS.
Figure 2. Total ion chromatogram of the light volatile components from C. filaginoides obtained by HS-SPME. (A) PDMS-fiber and (B) CAR/DVB/PDMS-fiber. Peaks: (±)-α-pinene (a), β-pinene (b), α-phellandrene (c), α-terpinene (d), m-cymene (e), β-phellandrene (f), γ-terpinene (g), terpinolene (h), p-cymene (i), and limonene (j).
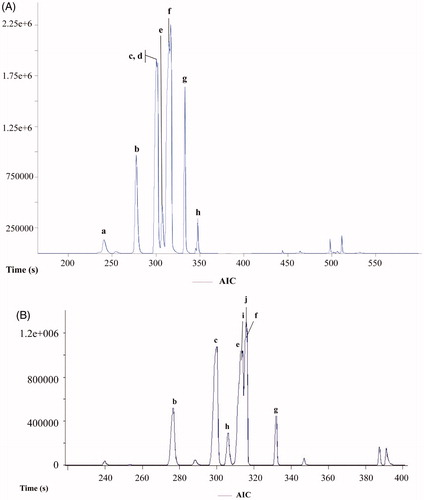
Table 2. Volatile constituents identified in C. filaginoides by HS-SPME using GC-MS analysis.
Quantitative HPLC analysis
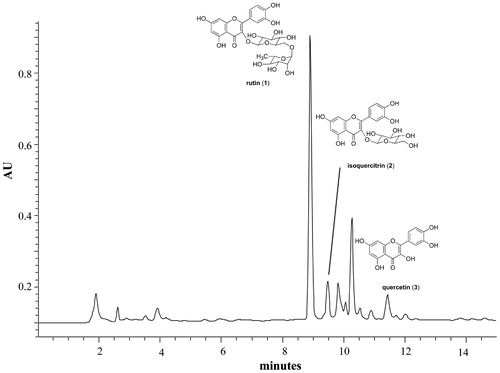
In the present study, rutin (1) was selected as the active marker of C. filaginoides to develop a suitable quantification procedure using a HPLC method. LC was chosen, as it is one of the key methods for estimation of marker compounds in herbal drugs. For this endeavor several mobile and stationary phases were examined; the columns tested included a Synergi 4 μ Hydro-RP (250 mm × 4.60 mm, 4 μm), Nucleosil C18 (250 mm × 3.2 mm, 5 μm), and LiChrospher 100 RP-18 (250 mm × 4.0 mm, 5 μm) the latter, being the most appropriated. Regarding the mobile phase, different mixtures of water (0.1% phosphoric acid, A) and methanol–acetonitrile (5:15; solvent B) were employed. A typical chromatogram of the organic extract of C. filaginoides is shown in , which indicated that the major active component present in the analyzed samples was rutin (1) along with its hydrolysis products, isoquercitrin (2) and quercetin (3).
Validation of the HPLC method
The HPLC method developed was fully validated according to the International Conference on Harmonization guidelines (ICH, Citation2005). The overall results are summarized in .
Table 3. Regression parameters, LOD, LOQ, accuracy and precision for rutin (1) in C. filaginoides crude drug.
Linearity
The linearity of the system was tested by analyzing a series of different concentrations of 1 ranging between 50 and 750 μg mL−1. All calibration curves showed good linearity within the test ranges (r2 ≥ 0.9963). The RSD value was less than 2.0% at each level tested.
Accuracy
The accuracy was assessed by the method of standard addition. The resulting mixtures were analyzed in the range from 50% to 150% of the working concentration, and the mean recoveries and their SD for the proposed method for three replicates were calculated. The linear regression equation was expressed as y = 0.9969 × −0.0221 (r2 ≥0.9995) and the mean recoveries was found to be between 98.1% and 101.8% (). According to the obtained results, good accuracy was observed for this method.
Reproducibility and repeatability
In order to evaluate precision of the method, reproducibility and repeatability were performed in terms of the intermediate precision throughout the analysis of three replicates of six samples of stock solution (750 μg mL−1); the precision was good with RSD value of 1.8%, which confirmed that the method was precise.
Limits of detection (LOD) and quantification (LOQ)
The approach based on the residual SD of the regression line and the slope in the concentration range from 1 to 25% was used for determining LOD and LOQ. The results are given in .
Analysis of plant samples
The proposed method was applied to the quantification of the main flavonol rutin (1) in eight different samples of C. filaginoides within the same batch. Every sample was analyzed in triplicate and the results indicated that 1 was present in 3563.3 ± 198.1 mg/100 g of dry plant material.
Discussion
In order to promote the rational use of herbal medicines according to the World Health Organization (WHO) guidelines (WHO, Citation1991) the Mexican Herbal Pharmacopeia (FHEUM) was created and first published in 2001. Unfortunately, the monographs of the most widely used medicinal plants in contemporary Mexico were incomplete or missing. The lack of suitable protocols for quality control assessment of these herbs is now a major concern for health authorities who are requesting to Official Research Centers to develop these official documents. Thus, as part of our systematic studies to generate pharmacopeia and WHO monographs of selected Mexican medicinal plants we have developed suitable identity and composition tests useful for quality control of the crude drug Conyza filaginoides.
To our knowledge, this is the first report on the chemical composition of C. filaginoides essential oil, and the results showed a few similarities, albeit partial, to those described for other species belonging to the genus Conyza collected in Africa and Europe. Thus, Barbosa and coworkers (Citation2005) found that C. bonarensis contains matricaria methyl ether and manool, along with limonene and carvone as the main volatiles metabolites. Hrutfiord et al. (1988) and Lis et al. (Citation2002) described the presence of trans-α-bergamotene, cis-α-bergamotene, (Z)-β-farnesene and R-(+)-limonene, together with β-pinene and myrcene for C. canadensis. Finally, Ndiege et al. (Citation2005) identified five major components including limonene, 2-methyl-5-(methylethyl)-2-cyclohexen-1-ol, 1,8-cineol, perillaldehyde and perillalcohol in the oil of C. newii.
Altogether, the analysis by HS-SPME of the crude drug of C. filaginoides revealed a high content of monoterpene hydrocarbons in the plant. In contrast, the major components of the oil were oxygenated monoterpenoids and sesquiterpenoids. Comparison of the compounds identified in the essential oil and those extracted by HS-SPME ( and ) suggested that significant chemical modifications could occur during hydrodistillation. The same differences have been reported during the analysis of other plants using both methods, which in turn raised the question about the true nature of the volatile compounds of the analyzed herbs (Drew et al., Citation2012). Dawidowicz et al. (Citation2008) attributed the differences in the components of the essential oil of Thymus vulgaris obtained by hydrodistillation and those found by HS-SPME were due to the nature of the fiber. In our case this explanation can be ruled out because different types of fibers were employed. For Rhaponticum acaule the dissimilarities found in the components of the hydrodistilled oil and those obtained using HS-SPME were explained considering that the former technique is based on the liquid quasi-total extraction of plant volatiles while the latter is controlled by a solid/gas equilibrium step (Benyelles et al., Citation2011). In spite of the differences found between the two techniques and the advantages that offer HS-SPME, most pharmacopeias require the analysis of the essential oils prepared by hydrodistillation for quality control purposes.
The crude drug of C. filaginoides possesses high amounts of rutin (1) and its hydrolysis products (2 and 3), which are multi-target constituents with antidiabetic (Kamalakkannan & Mainzen, Citation2006; Kwon et al., Citation2007; Rauter et al., Citation2010), analgesic (Awaad et al., Citation2011; Lapa et al., Citation2009; Rad et al., Citation2008), anti-inflammatory (Yildizoglu-Ari et al., Citation1991) and smooth muscle relaxant properties (Mata et al., Citation1997), among others.
Conclusion
A precise, reliable and accurate analytical HPLC method to detect and quantify 1 in the crude drug and some preparations was developed and fully validated. The volatile profile of the plant was established using two different approaches. The proposed methods would be useful for quality control assurance for this very Mexican plant and would be included in the second edition of the FHEUM to be published.
Declaration of interest
The authors report no declaration of interest. This work was supported by grants of DGAPA IN212913 and CONACyT No. 150966.
Acknowledgements
The authors wish to thank for the technical assistance of Araceli Pérez-Vásquez, Georgina Duarte and Rosa Isela del Villar. B.O. acknowledges a fellowship from CONACyT to pursue graduate studies.
Notes
*Taken in part from the MS thesis of B. Ovalle.
References
- Adams RP. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream: Allured Publishing
- Andrade-Cetto A, Heinrich M. (2005). Mexican plants with hypoglycaemic effect used in treatment of diabetes. J Ethnopharmacol 99:325–48
- Awaad AS, El-meligy RM, Qenawy SA, et al. (2011). Anti-inflammatory, antinociceptive and antipyretic effects of some desert plants. J Saudi Chem Soc 15:367–73
- Barbosa LC, Paul V, Azevedo AS, et al. (2005). Essential oil composition from some plant parts of Conyza bonariensis (L.) Cronquist. Flavour Fragr J 20:39–41
- Benyelles B, Allali H, El Amine Dib M, et al. (2011). Essential oil from Rhaponticum acaule L. roots: comparative study using HS-SPME/GC/GC–MS and hydrodistillation techniques. J Saudi Chem Soc. Available from: http://www.sciencedirect.com/science/article/pii/S131961031100247X/dx. doi:10.1016/j.jscs.2011.12.001. [last accessed 8 Dec 2011]
- Calzada F, Cedillo-Rivera R, Mata R. (2001). Antiprotozoal activity of the constituents of Conyza filaginoides. J Nat Prod 64:671–3
- Calzada F, Meckes M, Cedillo-Rivera R, et al. (1998). Screening of Mexican medicinal plants for antiprotozoal activity. Pharm Biol 36:305–9
- Dawidowicz AL, Rado E, Wianowska D, et al. (2008). Application of PLE for the determination of essential oil components from Thymus vulgaris L. Talanta 76:878–84
- Drew DP, Rasmussen SK, Avato P, Simonsen HT. (2012). A comparison of headspace solid-phase microextraction and classic hydrodistillation for the identification of volatile constituents from Thapsia spp. provides insights into guaianolide biosynthesis in Apiaceae. Phytochem Anal 23:44–51, and references cited therein
- Dominguez XA, Quintero G, Butruille D. (1972). Triterpenoids and triacontane from Conyza filaginoides. Phytochemistry 11:1855–6
- Hrutfiord BF, Hatheway WH, Smith DB. (1988). Essential oil of Conyza canadensis. Phytochemistry 27:1858--60
- International Conference of Harmonisation of Technical Requirements for registration of Pharmaceuticals for Human Use. (2005). ICH Harmonised Tripartite Guideline, Validation of Analytical Procedures: Text and Methodology Q2 (R1). Step 4 version, Genève, Switzerland
- Kamalakkannan N, Mainzen Prince PS. (2006). Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic Wistar rats. Basic Clin Pharmacol Toxicol 98:97–103
- Kwon O, Eck P, Chen S, et al. (2007). Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J 21:366–77
- Lapa F, Gadotti VM, Missau FC, Pizzolatti M. (2009). Antinociceptive properties of the hydroalcoholic extract and the flavonoid rutin obtained from Polygala paniculata L. in mice. Basic Clin Pharmacol Toxicol 104:306–15
- Lis A, Piggott JR, Góra J. (2002). Chemical composition variability of the essential oil of Conyza canadensis Cronq. Flavour Fragr J 18:364–7
- Martínez M. (1989). Las Plantas Medicinales de México. Botas, Mexico, p. 90
- Mata R, Rojas A, Acevedo L, et al. (1997). Smooth muscle relaxing flavonoids and terpenoids from Conyza filaginoides. Planta Med 63:31–5
- Mendoza-Castelán G, García-Pérez J, Estrada-Lugo E. (1997). Catálogo y usos Terapéuticos de Plantas Medicinales que se Comercializan en Fresco en el Mercado de Sonora. Universidad Autónoma Chapingo, Carretera México-Texcoco
- Monroy-Ortíz C, Castillo España P. (2007). Plantas medicinales utilizadas en el estado de Morelos. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. 2nd ed. Universidad Autónoma del Estado de Morelos, México
- Ndiege O, Omolo MO, Okinyo D, et al. (2005). Fumigant toxicity of the essential oils of some African plants against Anopheles gambiae sensu stricto. Phytomedicine 12:241–6
- Pawliszyn J. (1997). Solid Phase Microextraction: Theory and Practice. New York, NY: Wiley-VCH
- Rad SS, Asl MN, Zaman Soltani Z. (2008). Anticonvulsive effects of rutin in a rat model of absence seizure: A novel compound to treat seizure. Arch Gen Psychiatry 7:S219
- Rauter A, Martins A, Borges C, et al. (2010). Antihyperglycaemic and protective effects of flavonoids on streptozotocin-induced diabetic rats. Phytother Res 24:S133–8
- Rojas A, Cruz S, Rauch V, et al. (1995). Spasmolytic potential of some plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. Phytomedicine 2:51--5
- Tada T, Tezuka Y, Shimomura K, et al. (2001). Effect of depigmentation for 3,4-di-O-caffeoylquinic acid guided by tyrosinase inhibitory activity from Conyza filaginoides. J Oleo Sci 50:211–15
- Van Den Dool H, Kratz PD. (1963). A generalization of Retention Index System including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11:463–71
- World Health Organization. (1991) Guidelines for the Assessment of Herbal Medicines. Programme on Traditional Medicines. Genève, Switzerland
- Yildizoglu-Ari N, Melih Altan V, Altinkurt O, Ozturk Y. (1991). Pharmacological effects of rutin. Phytother Res 5:19–23