Abstract
Context: The diethyl ether extract of the stems of Schisandra pubescens Hemsl. et Wils. (Schisandraceae) was found to exhibit cytotoxic activity in vitro. However, investigations of the bioactive constituents of this plant have been very limited.
Objective: Elucidation of the cytotoxic constituents of S. pubescens was performed.
Methods: Repeated silica gel column chromatography and preparative TLC were used for the chemical investigation of the diethyl ether extract of S. pubescens stems. All isolates were evaluated for their in vitro cytotoxicity against A549, PC-3, KB and KBvin human cancer cell lines.
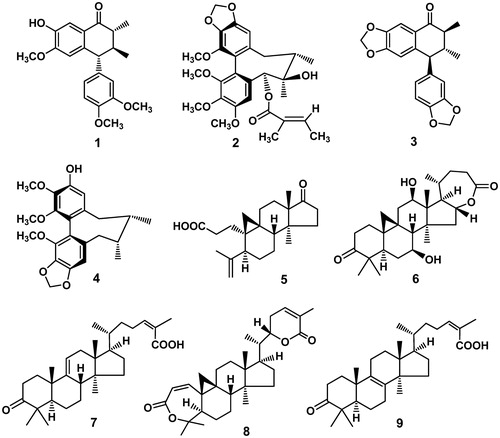
Results: Nine known compounds were obtained, including four lignans, epischisandrone (1), tigloylgomisin P (2), cagayanone (3) and (−)-gomisin L2 (4), together with five triterpenoids, micranoic acid B (5), lancifodilactone H (6), coccinic acid (7), schisanlactone B (8) and anwuweizonic acid (9). Compounds 2–6 and 8 showed moderate to marginal cytotoxicity, with GI50 values of 11.83–35.65 μM.
Conclusion: The isolation of 1–9 from S. pubescens and the cytotoxicities of 3–6 are first reported. Compounds 2–6 and 8 could be the active principles responsible for the anticancer effects of S. pubescens.
Introduction
Lignans and triterpenoids from plants of the Schisandraceae family have attracted much attention due to their diverse structures and beneficial bioactivities, such as hepatoprotective (Bao et al., Citation1979), antioxidant (Peng et al., Citation1996), anti-HBV (Ma et al., Citation2009), anti-HIV (Chen et al., Citation1997; Sun et al., Citation1996) and anticancer (He et al., Citation2010; Huang et al., Citation2011) effects. Schisandra pubescens Hemsl. et Wils. (Schisandraceae) is a climbing plant distributed in western Hubei Province and Sichuan Province, China. Its fruits are used in Sichuan as a substitute for the traditional Chinese medicine Wuweizi. Six lignans and one triterpenoid have been isolated from the fruits of this plant (Xu et al., Citation2009). Various sesquiterpenoids have been found in the leaves and stems of a related plant, S. pubescens var. pubinervis (Huang et al., 2006). As an extension of our investigations of anticancer agents from Chinese herbs, the diethyl ether extract of the stems of S. pubescens was found to exhibit cytotoxic activity in vitro. Subsequent investigation of the diethyl ether extract of S. pubescens led to the isolation of four lignans, epischisandrone (1), tigloylgomisin P (2), cagayanone (3) and (−)-gomisin L2 (4), together with five triterpenoids, micranoic acid B (5), lancifodilactone H (6), coccinic acid (7), schisanlactone B (8) and anwuweizonic acid (9). This article details the isolation of these compounds and their evaluation for cytotoxicity against human tumor cell lines.
Materials and methods
Plant material
The stems of S. pubescens were collected in Hubei Province, P.R. China, in August 2006 and were identified by one of the authors, Prof. Daofeng Chen, based on the macroscopic characters of the fruits and leaves of the original plant specimen. A voucher specimen (DFC-WWZ-20060803) has been deposited in the Herbarium of Materia Medica, Department of Pharmacognosy, School of Pharmacy, Fudan University.
General experimental procedures
The 1H and 13C NMR spectra were recorded on a Bruker DRX 400 spectrometer in CDCl3, using TMS as an internal standard. ESI-MS was measured using an Agilent SL G1946D single quadrupole mass spectrometer equipped with an ESI source. Silica gel (200–300 and 300–400 mesh, Qingdao Marine Chemical Factory, China) was used for column chromatography. Analytical and preparative TLC were performed on precoated silica-gel plates (GF254, Yantai Institute of Chemical Technology, China). The spots were visualized under UV light and spraying with 10% H2SO4 in EtOH, followed by heating.
Extraction and isolation
Air-dried and powdered stems of S. pubescens (5 kg) were extracted exhaustively with 95% EtOH at room temperature. The EtOH extract was partitioned between diethyl ether (4 × 2 L) and water (1 L). The organic layer was evaporated under reduced pressure to give a residue (190 g), which was subjected to silica gel column chromatography, eluted with a gradient of petroleum ether/EtOAc (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 3:7 and 0:10), to afford eight fractions (Fr. 1–Fr. 8). Fr. 4 was subjected to repeated silica gel column chromatography, eluted with chloroform/acetone (10:1), to yield 1 (10 mg). Compounds 2 (11 mg) and 3 (13 mg) were obtained from Fr. 5 after silica gel column chromatography, eluted with petroleum ether/acetone (3:1). Fr. 3 was chromatographed over silica gel with a gradient of petroleum ether/EtOAc (10:1 to 3:1) and further purified by preparative TLC to afford 4 (11 mg) and 5 (11 mg). Fr. 2 was separated by repeated silica gel column chromatography with petroleum ether/EtOAc (10:1) to give 6 (11 mg), 7 (18 mg), 8 (15 mg) and 9 (13 mg).
Cytotoxicity assay
Four different human cancer cell lines, lung carcinoma (A549), prostate carcinoma (PC-3), epidermoid carcinoma of the nasopharynx (KB) and vincristine-resistant nasopharyngeal (KBvin), were cultured and maintained at 37 °C in a humidified 5% CO2 atmosphere. The test compounds were dissolved in DMSO as stock solutions and stored in the dark at −20 °C. Upon dilution into culture medium, the final DMSO concentration was <0.5% (v/v) DMSO.
The cytotoxicity of the compounds against the cancer cell lines was determined by the sulforhodamine B (SRB) colorimetric aassay (Skehan et al., Citation1990). In brief, the cells (3−5 × 103 cells/well) were seeded in 96-well plates filled with culture medium (0.1 mL/well) containing various concentrations of samples and were incubated for 72 h. At the end of the exposure period, the attached cells were fixed with cold 50% trichloroacetic acid for 30 min followed by staining with 0.04% SRB for 30 min. The bound SRB was solubilized in 10 mM Tris-base and the absorbance was measured at 515 nm on a Microplate Reader ELx800 (Bio-Tek Instruments, Winooski, VT) with Gen5 software. Growth inhibition was assessed by GI50 that was defined as the concentration of test compound causing 50% inhibition of cell growth, as compared to the untreated control. All results were representative of three experiments and were reported as mean ± SD. Paclitaxel was used as a positive control.
Results and discussion
Based on the comparison of the spectroscopic data with those reported, compounds 1–9 were identified as four known lignans: epischisandrone (1) (Liu et al., Citation1988b), tigloylgomisin P (2) (Ikeya et al., Citation1980), cagayanone (3) (Kuo et al., Citation1989) and (−)-gomisin L2 (4) (Ikeya et al., Citation1982), as well as five known triterpenoids: micranoic acid B (5) (Li et al., Citation2003), lancifodilactone H (6) (Xiao et al., Citation2006), coccinic acid (7) (Li & Xue, Citation1986), schisanlactone B (8) (Liu et al., Citation1983) and anwuweizonic acid (9) (Liu et al., Citation1988a). Their structures are shown in . None of the nine compounds had been isolated previously from S. pubescens.
All isolates were evaluated for their in vitro cytotoxicity against A549, PC-3, KB and KBvin. As seen in , compounds 3, 5 and 8 exhibited moderate cytotoxic activity against all of the four human cancer cell lines, with GI50 values of 11.83–18.09 μM. In general, compounds 2, 4 and 6 displayed weak cytotoxicity against two (A549, KB), three (PC-3, KB, KBvin) and four cell lines, respectively. It is noteworthy that compounds 3–6 and 8 were almost equally active against KB and KBvin, while paclitaxel, the positive control, was almost 570-fold less active against the latter cell line.
Table 1. Cytotoxicity data for 1–9.
It is well known that some 4-aryltetralin lignans, especially podophyllotoxin and its analogs, show proven antitumor activities (Canel et al., Citation2000) and that a methylenedioxy group on the tetralin ring can be important for the cytotoxic activities of these compounds (Sun et al., Citation2011). Thus, because of the different substitution on their tetralin rings, it was not unexpected that 3 showed cytotoxic activity, while 1 was inactive against the cancer cell lines tested. Among the five lanostane triterpenoids, 5 and 8 possessed 3,4-seco structures and showed moderate cytotoxicity, while 6, 7 and 9 exhibited weaker effects or were inactive against the cancer cell lines. Therefore, the ring A cleavage may play an important role in enhancing cytotoxicity for lanostane-type triterpenoids.
Conclusion
In this investigation on anticancer constituents from the stems of S. pubescens, four lignans and five triterpenoids were isolated for the first time from the plant and evaluated for their cytotoxicity against human cancer cell lines (A549, PC-3, KB and KBvin). Previously, tigloylgomisin P (2) had been reported to possess cytotoxicity against the Nicotiana BY-2 cell line (Smejkal et al., Citation2010) and schisanlactone B (8) was found to inhibit human cancer cell lines Bel-7402 and HL-60 (Wang et al., Citation2006). No prior literature had addressed the cytotoxicities of cagayanone (3), (−)-gomisin L2 (4), micranoic acid B (5) and lancifodilactone H (6).
Declaration of interest
The authors declare no conflict of interest. This work was supported financially by grants from the Shanghai Educational Development Foundation (05SG06), the National Natural Science Foundation of China (30271586, 30925042), and the State Key Program (2009ZX09502-013) from the Ministry of Science and Technology, P.R. China, and by grant CA-17625 from the National Cancer Institute (NIH), USA.
References
- Bao TT, Xu GF, Liu GT, et al. (1979). A comparison of the pharmacological actions of seven constituents isolated from fructus schizandrae. Yao Xue Xue Bao 14:1–7
- Canel C, Moraes RM, Dayan FE, Ferreira D. (2000). Podophyllotoxin. Phytochemistry 54:115–20
- Chen DF, Zhang SX, Xie L, et al. (1997). Anti-AIDS agents. 26. Structure--activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg Med Chem 5:1715–23
- He F, Pu JX, Huang SX. (2010). Schinalactone A, a new cytotoxic triterpenoid from Schisandra sphenanthera. Org Lett 12:1208–11
- Huang SX, Yang J, Xiao WL, et al. (2006). Three novel terpenoids from Schisandra pubescens var. pubinervis. Helv Chim Acta 89:1169–75
- Huang ZH, Lu Y, Liu Y, et al. (2011). Kadsufolins A--D and related cytotoxic lignans from Kadsura oblongifolia. Helv Chim Acta 94:519–27
- Ikeya Y, Taguchi H, Yosioka I. (1982). The constituents of Schisandra chinensis Baill. X. The structures of γ-schizanrin and four new lignans, (−)-gomisins L1 and L2, (±)-gomisin M1 and (+)-gomisin M2. Chem Pharm Bull 30:132–9
- Ikeya Y, Taguchi H, Yosioka I, Kobayashi H. (1980). The constituents of Schisandra chinensis Baill. VIII. The structures of two new lignans, tigloylgomisin P and angeloylgomisin P. Chem Pharm Bull 28:3357–61
- Kuo YH, Lin ST, Wu RE. (1989). Three new lignans from the nutmeg of Myristica cagayanesis. Chem Pharm Bull 37:2310–12
- Li LN, Xue H. (1986). Triterpenoids from roots and stems of Kadsura coccinea. Planta Med 52:492–3
- Li RT, Han QB, Zhao AH, Sun HD. (2003). Micranoic acids A and B: Two new octanortriterpenoids from Schisandra micrantha. Chem Pharm Bull 51:1174–6
- Liu JS, Huang MF, Ayer WA, Bigam G. (1983). Schisanlactone B, a new triterpenoid from a Schisandra sp. Tetrahedron Lett 24:2355–8
- Liu JS, Huang MF, Tao Y. (1988a). Anwuweizonic acid and manwuweizic acid, the putative anticancer principle of Schisandra propinqua. Can J Chem 66:414–15
- Liu JS, Tao Y, Huang MF. (1988b). Studies on the constituents of Schisandra henryi. 5. The structures of wulignan A1, A2, epiwulignan A1 and epischisandrone. Acta Chim Sin 46:483–8
- Ma WH, Lu Y, Huang H, et al. (2009). Schisanwilsonins A--G and related anti-HBV lignans from the fruits of Schisandra wilsoniana. Bioorg Med Chem 19:4958–62
- Peng LH, Chen DF, Lan HX, et al. (1996). Anti-lipid peroxidation of gomisin J on liver mitochondria and cultured myocardial cells. Acta Pharmacol Sin 17:538–41
- Skehan P, Storeng R, Scudiero D, et al. (1990). New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–12
- Smejkal K, Slapetova T, Krmencik P, et al. (2010). Evaluation of cytotoxic activity of Schisandra chinensis lignans. Planta Med 76:1672–7
- Sun HD, Qiu SX, Lin LZ. (1996). Nigranoic acid, a triterpenoid from Schisandra sphaerandra that inhibits HIV-1 reverse transcriptase. J Nat Prod 59:525–7
- Sun YJ, Li ZL, Chen H, et al. (2011). Three new cytotoxic aryltetralin lignans from Sinopodophyllum emodi. Bioorg Med Chem Lett 21:3794–7
- Wang W, Liu JZ, Han J, et al. (2006). New triterpenoids from Kadsura heteroclita and their cytotoxic activity. Planta Med 72:450–7
- Xiao WL, Tian RR, Pu JX, et al. (2006). Triterpenoids from Schisandra lancifolia with anti-HIV-1 activity. J Nat Prod 69:277–9
- Xu XM, Li L, Chen M. (2009). Chemical constituents of Schisandra pubescens. Zhongyaocai 32:1399–401