Abstract
Context: Asparagus adscendens Roxb (Liliaceae) has a promising role in modulation of various disorders such as leucorrhea, diarrhea, dysentery, diabetes, senile pruritus, asthma, fatigue antifilarial, antifungal, spermatorrhea, and sexual debility/seminal weakness.
Objective: To investigate dose-dependent effects of Asparagus adscendens root (AARR) extract on anabolic, reproductive, and sexual behavioral activities with a view to emphasize the pharmacological basis.
Materials and methods: Rats were divided into five groups: Group I (control), Groups II–IV (AARR treated, 100, 200, and 300 mg/kg body weight, respectively, orally for 30 d) and Group V (standard control treated with sildenafil citrate, 5 mg/kg body weight). On day 31, copulatory and potency tests were carried out and an autopsy was done to study the reproductive function, namely, organ weights, spermatogenesis, daily sperm production rate (DSP), and epididymal sperm counts (ESC).
Results: AARR extract (200 and 300 mg/kg doses) caused a significant increase in body (p < 0.02 and p < 0.001) and testes (p < 0.01 and p < 0.001, control versus treated) weights. Reproductive activity showed significant a increase in testicular tubular diameter (p < 0.005–0.001), the number of round/elongated spermatids (p < 0.02–0.001), DSP, and ESC (p < 0.05–0.001). The sexual behavioral parameters including mounting/intromission frequency (13.0 ± 0.32/11.8 ± 0.37 and 18.2 ± 2.12/14.8 ± 1.15 versus 11.2 ± 0.66/8.2 ± 1.16), ejaculation latency (187.4 ± 1.91 and 191.4 ± 1.72 versus 180.0 ± 3.47), and penile erections (13.5 ± 0.3 and 14.5 ± 0.5 versus 8.5 ± 0.2) showed a significant increase at 200 and 300 mg/kg doses (ED50 300 mg/kg), but less than a standard control. In contrast, 100 mg/kg dose caused an increase (p < 0.005) in mounting latency only.
Conclusion: These results indicate increased anabolic, reproductive, and sexual activities by AARR treatment. Thus, the data provide scientific rationale for its traditional use as an aphrodisiac or for sexual disorders.
Introduction
Asparagus adscendens Roxb. (Liliaceae), commonly known as “Safed Musali” or “Sataver” in Hindi, has been one of the chief ingredients in Ayurveda and other local folklore medicines for ages. The plant is a sub-erect, prickly shrub with fusiform white tuberous roots (rhizome), generally found in oak forests up to 1800 m in North West Himalaya, Punjab, at higher altitudes up to 5300 ft, Gujarat, parts of Maharashtra, Rohilkhand, Central Mussori hills, Madhya Pradesh, and Uttar Pradesh, India, as well as in Afghanistan (Bhatt & Negi, 2005; Gaur, Citation1999; Mehta & Subramanian, Citation2005). It has been traditionally used in the preparation of health tonic for general weakness/debility, in case of apathies of mouth and throat, for the treatment of spermatorrhea, chronic leucorrhea, diarrhea, dysentery, diabetes, senile pruritus, asthma, and fatigue in Indian Unani medicine (Asolkar et al., Citation1992; Gaur, Citation1999; Negi et al., Citation2010). Different parts of this plant possess multifarious medicinal properties such as insulinotropic, insulin-enhancing, inhibitory effects on starch digestion, acetylcholinesterase/butyrylcholinesterase activity, antifungal, antifilarial activity, and beneficial in the management of stress and inflammatory conditions, memory impairments, and the symptoms of AIDS (Kanwar & Bhutani, Citation2010; Khan et al., Citation2010; Mathews et al., Citation2006; Singh & Rai, Citation2000; Singh et al., Citation1997; Trivedi & Upadhyay, Citation1993). The phytochemical studies on A. adscendens roots (AARR) extract identified several important steroids, saponins and sapogenins, triterpenoids, glycosides, oligospirostanosides and oligofurostanosides, essential oil, and phytoecdysteroids that are analogues of invertebrate steroid hormones (Dinan et al., Citation2001; Huang & Kong, Citation2006; Jadhav & Bhutani, Citation2006; Negi et al., Citation2010; Singh et al., Citation1997; Tandon & Shukla, Citation1995). The plant contains significant amounts of saponin, which has been shown to inhibit DNA and RNA synthesis and cell proliferation of HL60 and tumor cells (Chin Rutgers, Citation2003).
The availability of the large number of sexual function improving drugs in the traditional Unani System of Medicine is a unique and distinctive feature of this system. Nowadays, the plant-based remedies have been used as the most popular alternative methods besides having many specific drugs for enhancing sexual functions and for the treatment of sexual dysfunction, infertility, and male disorders. There are several plants/plant products such as Butea frondosa Koen ex Roxb. (Papillionaceae) (Ramachandran et al., Citation2004), Turnera aphrodisiaca Ward (Turneraceae) (Kumar & Sharma, Citation2005), Terminalia catappa Linn. (Combretaceae) (Ratnasooriya & Dharmasiri, Citation2000), Eurycoma longifolia Jack (Simaroubaceace) (Ang et al., Citation2001), Tribulus terrestris L. (Zygophyllaceae) (Gauthaman & Adaikan, Citation2005), Myristica fragrans Houtt. (Myristicaceae) (Tajuddin et al., Citation2005), Fadogia agrestis Schweinf. Ex Hiern (Rubiaceae) (Yakubu et al., Citation2005), Abutilon indicum Linn. SW (Malvaceae), Withania somnifera Dunal (Solanaceae) (Ganu et al., Citation2010), including the most commonly used spices (Tajuddin et al., Citation2003, Citation2005) have been demonstrated to show aphrodisiac activity that stimulate the mounting behavior, increases anxiety, and the mating performances. Some of these are empirically used as promising aphrodisiacs in traditional medicine practice in cases of sexual debility or depressed desire. In an indigenous system of medicine, the dried roots of A. adscendens have been used as an aphrodisiac and are very effective in increasing male potency (Kapoor, Citation2001). Reported studies on Asparagus recemous Wild. (Liliaceae) and several other plant products have been demonstrated to increase sexual and anabolic activities in normal as well as in induced-hyperglycemic rats (Chauhan & Dixit, Citation2008; Esfandiari & Dehghani, Citation2010; Thakur et al., Citation2009a–c). However, there is little information available about the aphrodisiac potential of AARR, although it has been claimed traditionally as an aphrodisiac. Therefore, the present study was conducted to determine the dose-dependent effects of AARR extract on the anabolic activity, reproductive organ function (namely organ weights, testicular spermatogenesis, DSP, and ESC), and sexual behavioral performance so as to emphasize the pharmacological basis and mechanism of action in adult male rats.
Materials and methods
Chemicals
All chemicals used in the present study were purchased from Sigma Chemical Company, St. Louis, MO.
Plant collection and extraction
Asparagus adscendens was collected in months of September–October 2010 locally from Almora district of Uttarakhand State, India, identified by Dr. Kamal Ram Arya from Botany Division, and authenticated in Institutional Herbarium (Voucher no. KRA-23986). The roots of these plants were shade-dried for 3 d and milled into fine powder using an electric laboratory grinder. For preparation of the ethanol extract, the powdered roots (3 kg) were macerated in 3000 ml ethanol (96% w/v) for 48 h and, subsequently, the mixture was filtered and semi-dried under reduced pressure (Rotor Vapors, Buchi, Germany) at 35 °C (yield: 3.4–3.6% w/w; Panda, Citation2000). The reconstituted extract was administered orally using a metal oropharyngeal cannula with the animals in treatment groups.
Animals used
Adult Sprague–Dawley rats of either sex (males, 180–200 g and females, 170–180 g) were used in the present study and were obtained from the Institute’s Breeding Colony. They were housed in stainless steel cages and acclimatized to housing conditions at ambient temperature of 22 ± 3°C and relative humidity 45% under a light-dark (12 h light/dark) cycle for 1 week before the commencement of the experiment, and fed with standard pellets’ diet (Hindustan Lever Ltd., Bangalore, India) and free access to water. This study was carried out under the guidelines laid down by Institutional Animal Ethics Committee (IAEC) and the experimental protocol was approved by IAEC (Approval no. 126/10/Endo/IAEC dated 4.11.2010).
Experimental design
There were three sets of experiments conducted and a total of 84 males and 90 female rats were used. In the 1st set of experiment, 24 adult male rats were randomized into four groups (Groups I–IV) of six rats in each group. Group I served as a control and received distilled water (10 ml/kg body weight) only. Rats in Groups II, III, and IV were administered ethanol extract of A. adscendens roots (AARR) at doses of 100, 200, and 300 mg/kg body weight, respectively, for 30 d. The animals were allowed free access to food and drinking water ad libitum during the entire treatment period. The physical appearance/activity of the rats was observed daily for 1–2 h for (if any) toxic effect. On day 31 (10:00 AM), the body weights of control and treated animals were recorded and the autopsy was done by anaesthetizing with anesthetic ether. The reproductive organs (namely testes, epididymis, ventral prostate, seminal vesicle, vas deferens, and penis) were dissected, rinsed in chilled saline, freed from connective tissues and blood clots, and then weighed. The testes and epididymis from either side (left) were fixed in Bouin’s fluid (24 h) for histology purpose. The tissues from the other side (right) were processed further for daily sperm production (DSP) rate and epididymal sperm counting as per the methods described previously (Amann et al., Citation1976; Bansode et al., Citation1998).
In the 2nd and 3rd set of experiments, 30 animals were divided into five groups (Groups I–V) having six rats each. Group I served as a control and received distilled water (10 ml/kg) only. Rats in Groups II, III, and IV administered ethanol extract of AARR at the doses 100, 200, and 300 mg/kg body weight, respectively, for 30 d. An additional group (Group V) of six animals was used as a standard control and received oral suspension of sildenafil citrate (5 mg/kg body weight) 1 h prior to the commencement of the copulatory and potency tests.
Histomorphometry/quantitation of spermatogenesis
In hematoxylin- and eosin-stained testicular cross sections (5 µm thick), the measurements of tubular diameter of round seminiferous tubules (100 tubules in each group) and a nuclear diameter of Leydig cells (100 Leydig cells nuclei/group) in 3–5 cross sections form each group were carried out by using Biovis Image Plus Software for image analysis and processing (Expert Vision Labs Pvt. Ltd., Mumbai, India) at ×100 and ×1000 magnification under the Olympus Trinocular microscope (BX51, Olympus, Tokyo, Japan).
Quantitative analysis of testicular spermatogenesis was done on the basis of nuclear morphology of the germ and Sertoli cells. The number of germ cells and Sertoli cell nuclei was counted in at least 20 round seminiferous tubules selected randomly from at least 2–3 cross sections in each rat at ×400 magnification under an Olympus Trinocular microscope (Olympus, BX 51, Tokyo, Japan). Cell counts were corrected by Abercrombie’s formula and values expressed as an average (mean) cell counts per Sertoli cell ratio in each group of animals as per the method described earlier (Bansode et al., Citation1998; Srivastav et al., Citation2010).
Estimation of DSP
Measurement of DSP in testicular homogenates was carried out as per the method of Amann et al. (Citation1976). Briefly, testicular parenchyma was cut into small pieces, placed in 0·25 mol/l sucrose solution (pH 7.5), and homogenized in a fluid containing 150 mM NaCl/l, 3.8 mM NaN3/l, and 0.05% (v/v) Triton X-100 using an Ultra Turrax® (Janke and Kunkel, Staufen, Germany). The homogenates were further diluted with the same medium, and homogenization-resistant spermatid nuclei in steps 17–19 of stages IV–VIII of the spermatogenic cycle were counted in triplicate under the Olympus microscope using a Neubauer’s hemocytometer (Bio-Rad, Hercules, CA). DSP per gram testicular parenchyma was calculated by dividing the number of spermatids nuclei with the product of weight of parenchyma and a time divisor of 6.3 d was reported for a rat (Johnson et al., Citation1980). The values are represented as mean ± SE for five animals in each group.
Epididymal sperm counts (ESC)
Cauda epididymis was excised surgically, cut into small pieces in suspension medium containing 140 mmol NaCl, 0.3 mmol KCl, 0.8 mmol Na2HPO4, 0.2 mmol KH2PO4, and 1.5 mmol d-glucose (pH adjusted to 7.3 by adding 0.1 N NaOH), and sperms were collected by centrifugation at 100 × g for 2 min. The resultant precipitate was resuspended in fresh suspension medium, diluted, and placed on both the sides of Neubauer’s hemocytometer. The counting of the number of sperms was carried out in four chambers of a hemocytometer in duplicate in each rat under Olympus Trinocular microscope at ×200 magnification. The values were expressed as an average of total counts of sperms per ml of suspension (Amann et al., Citation1976).
Copulatory test
The copulatory test was conducted in the 2nd experimental group of animals as per the method described previously (Lucio et al., Citation2001; Mercier et al., Citation1987). The behavior test was performed by placing the male in a Plexiglas cylindrical arena (55 cm in diameter) for a 5-min habituation period before the females are brought into the estrous by the subcutaneous injection of estradiol benzoate (10 µg) and progesterone (2 mg) for 48 and 4 h before the test, respectively. A total of 90 females were used (in the ratio of 1 male:3 females) to permit each observation to be made using fresh females and to avoid possible effects of repeated copulation. Test session was ended when the male displayed the ejaculatory pattern. The mated female rats were sacrificed next day morning, seminal plugs were obtained surgically and weighed. Copulatory parameters studied, included the mounting latency (ML), mounting frequency (MF), intromission latency (IL), intromission frequency (IF), ejaculation latency (EL), post-ejaculatory interval time (PEI), and seminal plug weights (SPW), were recorded after 30 d treatment of AARR extract. Hit rate [number of intromissions/number of mounts + number of intromissions] was calculated from these parameters.
Potency test
In the 3rd experimental group of animals, the spinal cord was transected in the midthoracic region of castrated male rats, as described previously (Tajuddin et al., Citation2005). The animals were divided into five groups (Groups I–V) of six rats in each group. Control (Group I) rats received distilled water (10 ml/kg) only. Rats in Groups II, III, and IV were administered AARR extract orally at 100, 200, and 300 mg/kg doses, respectively, for 30 d. Rats in Group V were treated with oral suspension of Sildenafil citrate (5 mg/kg) 1 h prior to the commencement of the potency test and used as a standard control. Following the next day, each animal was placed on its back in a glass cylinder for partial restraint. The preputial sheath was pushed behind the glans penis by using the thumb and index finger for a period of 30 min. During this period, a cluster of genital responses consisting of erections, quick flips, and long flips of the glans penis were recorded.
Toxicity study
Rats were observed daily for 1–2 h after the administration of AARR extract for physical appearance, behavioral activities, and death (if any). The gross behavioral aspects included the salivation, lachrymation, ptosis, exopthalmos, arousal response, spontaneous motor activity, posture/position, gait, ataxia, tremor, convulsions, straub’s tail, abnormal stereotypy, catalepsy, righting reflex, defecation, and urination.
Statistical analysis
Statistical analysis for significance of differences among body and reproductive organ weights, spermatogenic germ cell analysis, DSP, epididymal sperm counts, and copulatory and potency test parameters of control and treated rats was done by applying Student’s “t”-test and a one-way ANOVA (one-factor analysis of variance) test followed by the Newman–Keuls multiple comparison test wherever applicable. Values with p < 0.05 were considered as significant and expressed as mean ± SEM.
Results
Body and reproductive organ weights
No significant changes were observed in the final body weights as well as in the reproductive organ weights (namely testis, epididymis, ventral prostate, seminal vesicle, vas deferens, and penis) in AARR extract-treated rats at the 100 mg/kg dose as compared with controls. However, there was a significant increase in body weights and weights of testes, epididymis, ventral prostate, and seminal vesicles in rats treated with 200 and 300 mg/kg doses of root extract as compared with controls. The weights of vas deferens and penis were similar to controls at 200 mg/kg dose, but exhibited an increase in weight of vas deferens at 300 mg/kg dose of extract ().
Table 1. Effects of ethanol extract of Asparagus adscendens Roxb. roots on the body and reproductive organ weights in adult male rats.
Histomorphometry/quantitation of spermatogenesis/DSP/ESC
In control rats, the testes showed normal spermatogenesis depicting all the spermatogenic germ cell types namely spermatogonia, primary spermatocytes, spermatids, and spermatozoa (). Cauda epididymal tubular lumen was filled with a large number of sperms (). In treated rats, at 100 mg/kg dose, there was no change in testicular and epididymal histologies as compared with control ( and ). But they showed increased density of round and elongated spermatids in the seminiferous epithelium, and sperms in epididymal lumen in treated groups with 200 and 300 mg/kg body weight of AARR extract ( and ). The morphometric measurements of testicular tubular diameter showed a significant increase (p < 0.005–0.001) in rats treated with 200 and 300 mg/kg doses of extract, but showed no significant change at its lower dose of 100 mg/kg as compared with controls. The nuclear diameter of Leydig cells did not show any significant change in treated rats (), but showed insignificant increase at 300 mg/kg dose of extract. Quantitative analysis of the germ cells/Sertoli cell ratio showed a significant increase (p < 0.02–0.001) in the number of round and elongated spermatids in treated rats at doses of 200 and 300 mg/kg body weight as compared with control. However, the number of A-spermatogonia, and primary spermatocytes (pachytene and non-pachytene) as well as Sertoli cell number did not change significantly in treated rats. Similarly, treatment of 100 mg/kg AARR extract did not cause any significant change in testicular germ cell population dynamics and Sertoli cell number (). DSP rate in testicular homogenates and ESC was also increased significantly in treated rats at 200 and 300 mg/kg doses than that of control group rats. However, the 100 mg/kg dose of AARR extract did not produce any significant change in DSP and ESC in treated rats ().
Figure 1. Testis histology of control (A) and Asparagus adscendens root extract treated rats at 100 (B), 200 (C), and 300 (D) mg/kg doses for 30 d. A, spermatogonia; P, pachytene spermatocytes; R, round spermatids; E, elongated spermatids; LC, Leydig cells; SC, Sertoli cells. Note the increased density of round and elongated spermatids in treated rats with 200 mg/kg (C) and 300 mg/kg (D) dose level. Magnification: ×1000 (under oil immersion); H–E staining.
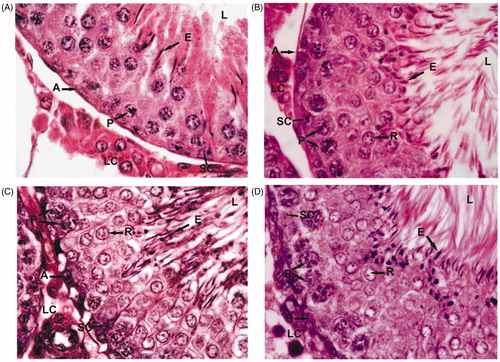
Figure 2. Cauda epididymal cross sections from control (A) and Asparagus adscendens root extract treated rats with 100 (B), 200 (C), and 300 (D) mg/kg doses for 30 d. Note the increased sperm density in tubular lumen at 200 (C) and 300 (D) mg/kg than in 100 mg/kg (B) and control (A) rats. EP, epididymal epithelium; ES, epididymal stroma; S, luminal sperms. Magnification: ×1000 (under oil immersion); H–E staining.
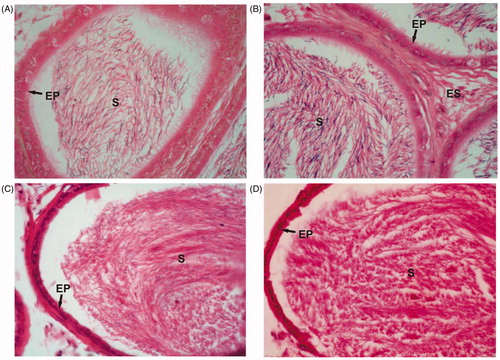
Figure 3. Effects of Asparagus adscendens Roxb root extract on testicular daily sperm production/g of testicular parenchyma and cauda epididymal sperm counts (×106)/ml.
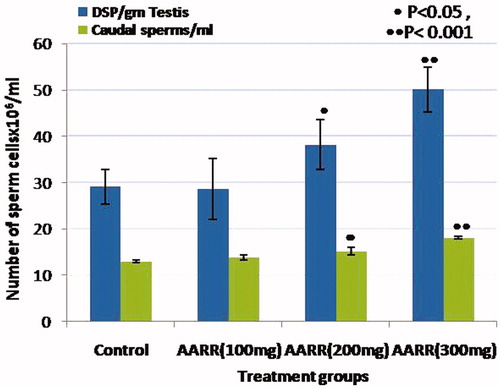
Table 2. Effect of Asapragus adscendens Roxb. roots extract on testicular germ cell population dynamics, tubular diameter and nuclear diameter of Leydig cells in rats.
Effect of AARR extract on sexual behavior
In the copulatory behavior test, a significant increase in ML was observed in treated rats at the 100 mg/kg dose of AARR extract. Other sexual behavioral parameters such as MF, IF, IL, EL, PEI, SPW, and hit rate were observed to be comparable with that of controls. However, treatment with 200 and 300 mg/kg doses of AARR extract produced a significant increase in MF, IF, and EL when compared with controls. The ML and IL were significantly decreased at both doses, and PEI decreased significantly only in 300 mg/kg dose of extract. The standard drug, Sildenafil citrate (5 mg/kg) treatment, produced a pronounced increase in MF, IF, and EL, but caused significant decrease in ML, IL, and PEI as compared with controls. The hit rate and seminal plug weights were similar in treated and control rats ().
Table 3. Effects of 50% ethanol extract of Asparagus adscendens Roxb. roots on the sexual behavioral performance of male Sprague–Dawley rats.
Effect of AARR extract on potency
The test of potency included the penile reflexes such quick flip (QF), long flip (LF), total penile reflexes (TPR), and penile erections (PE). Treatment of AAR extract at 100 mg/kg did not cause any effect on penile indices, but caused a significant increase in QF, LF, TPR, and PE in treated rats at 300 mg/kg extract. At the dose level of 200 mg/kg, there was an increase in QF and PE. The standard drug, Sildenafil citrate, caused significantly higher penile indices compared with control as well as AARR extract-treated groups ().
Table 4. Effects of Aspragus adscendens Roxb. roots extract on penile reflexes (test for potency) in male rats.
Toxicity study
No significant changes in gross behavior of AARR-treated as compared with control rats were observed during 1–2 h of the toxicity study.
Discussion
AARR extract has been widely traditionally acclaimed for its aphrodisiac action that increases libido, potency, sperm counts, and sexual pleasure in man. To investigate scientific rational behind the folk claim of this plant extract, the present study was conducted experimentally in a rat model to emphasize pharmacological basis and dose-dependent effects on the anabolic activity, reproductive organ function, and sexual behavioral performance. Oral administration of ethanol extract of AARR for 30 d produced a significant anabolic activity characterized by the weight gain in the body and reproductive organs (testis, epididymis, prostate, and seminal vesicles) at 200 and 300 mg/kg doses as compared with control rats. In addition, there was an increase in testicular tubular diameters at both doses. In concurrence, previous studies have also been demonstrated an increase in anabolic activity that improves the body and reproductive organ weights which is a biological indicator of steroidogenesis (El-Tantawy et al., Citation2007; Esfandiari & Dehghani, Citation2010; Gauthaman et al., Citation2003; Gauthaman & Adaikan, Citation2008). However, lower dose, i.e., 100 mg/kg of extract, exhibited no significant changes in body and reproductive organ weights. The quantitative analysis of testicular spermatogenesis showed an increase in the number of androgen-dependent spermatogenic cells, e.g., spermatids (round and elongated). The mature elongated spermatid releases from Sertoli cells into the seminiferous tubular lumen by spermiation process prior to their passage to epididymis have been used as a ‘marker’ for DSP rate in testis (Amann et al., Citation1976). Further, there was an increase observed in ESCs in treated (200 and 300 mg/kg) rats, which is a useful biochemical “marker” for testicular activity (Srivastav et al., Citation2010; Wang et al., Citation1999). The increased testicular function coincided with elevated weights of androgen-dependent accessory sex organs, sperm production/semen characteristics has shown to interfere with the normal testicular activity by enhancing the libido and sexual behavioral performance indicating testosterone-like effects in rats (Ganguly et al., Citation1992; Ganu et al., Citation2010; Thakur et al., Citation2009c). Interestingly, our results also showed an increase in the testicular activity as well as in androgen-dependent accessory sex organ weights (namely epididymis, prostate, and seminal vesicles) in AARR-treated rats at 200 and 300 mg/kg doses. Recent studies have also investigated an increase in the spermatogenic as well as aphrodisiac activity with other plant species like Curculia orchioides Gaertn. (Hypoxidaceae) and Tribulus terresteris L. (Zygophyllaceae) (Chauhan & Dixit, Citation2008; Esfandiari & Dehghani, Citation2010). Since AARR extract is rich in steroidogenic compounds like saponins and flavonoids, it may stimulate an increase in steroidal biosynthesis, testosterone secretion, gonadotrophic activity, and availability of precursors in the form of steroidal components such as gonads for the improvement of reproductive and copulatory performance (Chauhan et al., Citation2010; Haren et al., Citation2002; Jadhav & Bhutani, Citation2006; Thakur & Dixit, Citation2007; Thakur et al., Citation2009a–c). In contrast, the steroidogenic Leydig cells did not show any significant change in their nuclear diameters in treated rats, but represented insignificant increase at its higher dose (300 mg/kg) as compared with controls. The testicular Sertoli cell number as well as the germ cells, namely A-spermatogonia, non-pachytene, and pachytene spermatocytes, did not show any significant change in treated as compared with controls, which may indicate that there is no change in gonadotrophic and/or testicular testosterone hormone levels at the doses used in the present study (Gauthaman & Adaikan, Citation2008; Yakubu et al., Citation2008). But it has been well documented that biosynthesis and secretion of testosterone hormone are responsible for androgenic activity as well as for the development of male accessory sex organs, e.g., epididymis, prostate gland, seminal vesicles, and vas deferens (Gray et al., Citation1979). The molecular mechanism of action involves testosterone conversion into dihydrotestosterone (DHT), which binds with cytoplasm receptor proteins, translocate into the nucleus, and stimulate DNA and RNA transcription process that activate RNA polymerase and enhance the production of proteins in cells, resulting in increased body mass and weights of secondary sexual organs (Gilna, Citation2004; Thakur et al., Citation2009c). Previous reports have shown that the AARR extract is rich in steroidal saponin, protodioscin that increases levels of LH, DHT, DHEA, and testosterone, and enhances libido, spermatogenesis, and pro-erectile effects (Gauthaman & Adaikan, Citation2008; Padashetty & Mishra, Citation2007). Thus, the increased testicular and accessory sex organ weights (namely epididymis, prostate, and seminal vesicles) in AARR extract-treated rats may attribute to an increase in androgen levels that trigger libido-enhancing effect that needs to be explored.
In the copulatory behavior test, many aspects of male sexual behavior including an erection and mounting provide direct and prominent measures for determining the efficacy of aphrodisiacs (Linnankoski & Leinonen, Citation2010; Linnankoski et al., Citation1995). In the present investigation, a dose-dependent increase (300 > 200 > 100 mg/kg) in the MF and IF was observed in AARR extract-treated rats. These effects of extract have been demonstrated as the useful indices of vigor, libido, and potency (Chaturapanich et al., Citation2012; Tajuddin et al., Citation2005; Yakubu & Akanji, Citation2011). An increase in MF indicates increased sexual libido, and increased IF indicates increased potency parameters such as penile reflexes, erections, and penile orientation towards ejaculatory reflexes (Agmo, Citation1997; Suresh et al., Citation2009; Tajuddin et al., Citation2004; Yakubu & Akanji, Citation2011). There was a significant increase in ML at 100 mg/kg dose level of AARR extract which is an indicator of sexual motivation and arousability. But decreased ML and IL in treated rats at higher doses (200 and 300 mg/kg body weight) may indicate repeated stimulation in MF and IF. The PEI is a nitrous oxide (NO)-based mechanism and considered to be an index of potency, libido, and the rate of recovery from exhaustion after first series of mating (Suresh et al., Citation2009; Tajuddin et al., Citation2003). Existing reports have shown that the PEI of more than 5400 s causes sexual exhaustion, resulting a decline in the intensity of sexual behavior in subsequent mating (Agmo, Citation1997). The present study also showed a decline in PEI at 300 mg/kg extract, which may indicate enhanced sexual behavior marked by increased EL and prolongation in the duration of coitus in the AARR-treated rats. Also, it has been demonstrated that the display of pelvic thrusting during intromission and ejaculation may lead to close contact between the male copulatory organ and the vaginal orifice, resulting in higher lordosis in female rats (Agmo, Citation1997; Yakubu et al., Citation2008). The sexual behavior, desire, libido, and penile erections are androgen dependent and act through central and peripheral mechanisms (Mills et al., Citation1996; Thakur & Dixit, Citation2007; Yakubu et al., Citation2005, Citation2008). It may, therefore, be logical to attribute these sexual appetitive behavioral changes in male rats to the extract contents such as alkaloids, saponins, or flavonoids due to their engorgement, androgen enhancing, and antioxidant properties (Gauthaman & Adaikan, Citation2005; Jadhav & Bhutani, Citation2006; Sharma et al., Citation2012; Yakubu & Akanji, Citation2011).
It has been shown that penile erection depends on a well-coordinated system of vascular, endocrine, and neural network supplying to the male sexual organ. The plant extracts may cause sexual stimulation via simulation of parasympathetic nerves, which further activate the local factors thereby increasing vascular blood flow in the cavernous artery that induces a rapid increase in intracavernous pressure during penile erection (Anderson & Wagner, Citation1995; Chen et al., Citation1992; de Andrade et al., Citation2007; Lucio et al., Citation2001; Mizusawa et al., Citation2002; Naylor, Citation1998; Park et al., Citation2006; Tajuddin et al., Citation2003; Zhang et al., Citation2002). The relaxation of the cavernous smooth muscle is mediated by NO is a physiologic signal essential for penile erection that involves both neuronal NO synthase (nNOS) and endothelial NO synthase (eNOS) isoforms (Burnett, Citation2004), ACh (Furchgott & Zawadzki, Citation1980), guanylate cyclise and cyclic guanosine monophosphate (cGMP) (Chang et al., Citation2004), protein kinase G, (Francis et al., Citation2010) as well as phosphatidylinositol 3-kinase/Akt-induced eNOS phosphorylation mechanisms under the blood flow stimuli in the penis to sustain physiological penile erection (Dimmeler et al., Citation1999). There are several reports on plant-based mechanisms of penile erection and saponins acting as a nitric oxide donor may induce the relaxation of smooth muscle corpus cavernosum through the l-arginine/nitric oxide pathway (Kim et al., Citation1998). While alkaloids, which show induction of vasodilation of the blood vessels resulting in penile erection, may be signaling through the VEGF/eNOS signaling cascade (Agmo, Citation1997; Liu et al., Citation2010). The enhanced IF by the AARR extract may be associated with the flavonoids and/or saponins content of the plant that needs further exploration. However, increased ejaculation latency was supported by the presence of the vaginal plug in female rats.
Conclusion
The present study demonstrates the anabolic effects by AARR extract at 200 and 300 mg/kg doses indicating body and reproductive organ (testis, epididymis, ventral prostate, and seminal vesicle) weight gain. The testicular/epididymal function was evident by an increase in the testicular tubular diameter, spermatogenic germ cells (spermatids) population, DSP, and ESC in rats. Further, the extract also produced a significant increase in sexual behavioral aspects such as MF, IF, EL, and penile erections, and exhibited decreased ML, IL, and PEI in a dose-dependent manner in rat. Thus, the results of the study provide scientific rationale for traditional use of AARR extract as an aphrodisiac as well as for male sexual disorders.
Acknowledgements
The authors are thankful to Ms. T. Firdaus, Mr. Pradeep Singh and N. P. Yadav for technical assistance.
Declaration of interest
The authors report no declaration of interest. The authors are also grateful to the Ministry of Health and Family Welfare, Government of India, for financial support.
References
- Agmo A. (1997). Male rat sexual behavior. Brain Res Protoc 1:203–9
- Amann RP, Johnson L, Thomson DL, Pickett BW. (1976). Daily spermatozoal production, epididymal spermatozoal reserves and transit time of spermatozoa through the epididymis of the rhesus monkey. Biol Reprod 15:586–92
- Anderson KE, Wagner G. (1995). Physiology of penile erection. Physiol Rev 75:191–236
- Ang HH, Ikeda S, Gan EK. (2001). Evaluation of the potency activity of aphrodisiac in Eurycoma longifolia Jack. Phytother Res 15:435–6
- Asolkar LV, Kakkar KK, Chakre OJ. (1992). Glossary of Indian Medicinal Plants with Active Principles. New Delhi, India: CSIR Publication
- Bansode FW, Dwivedi AK, Maikhuri JP, Chowdhury SR. (1998). Quantitative analysis of spermatogenesis in rats made azoospermic with compound CDRI 84/35. Contraception 58:175–82
- Bhatt VP, Negi GCS. (2005). Ethnomedicinal plant resources of Jaunsari tribe of Garhwal Himalaya, Uttaranchal. Ind J Traditional Knowledge 5:331–5
- Burnett AL. (2004). Novel nitric oxide signaling mechanisms regulate the erectile response. Int J Impot Res 16:S15–19
- Chang S, Hypolite JA, Velez M, et al. (2004). Downregulation of cGMP-dependent protein kinase-1 activity in the corpus cavernosum smooth muscle of diabetic rabbits. Am J Physiol Regul Integr Comp Physiol 287:R950–60
- Chaturapanich G, Chaiyakul S, Verawatnapakul V, et al. (2012). Enhancement of aphrodisiac activity in male rats by ethanol extract of Kaempferia parviflora and exercise training. Andrologia 44:323–8
- Chauhan NS, Dixit VK. (2008). Spermatogenic activity of rhizomes of Curculigo orchioides Gaertn in male rats. Int J Appl Res Nat Prod 1:26–31
- Chauhan NS, Saraf DK, Dixit VK. (2010). Effect of vajikaran rasayana herbs on pituitary–gonadal axis. Eur J Integr Med 2:89–91
- Chen KK, Chan JY, Chang LS, et al. (1992). Intracavernous pressure as an experimental index in a rat model for the evaluation of penile erection. J Urol 147:1124–8
- Chin Rutgers CC. (2003). Enhancing the Value of Asparagus. USA: University New Jersey, JSHS. ASM
- de Andrade E, de Mesquita AA, Claro Jde A, et al. (2007). Study of the efficacy of Korean Red Ginseng in the treatment of ED. Asian J Androl 9:241–4
- Dimmeler S, Fleming I, Fisslthaler B, et al. (1999). Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399:601–5
- Dinan L, Savchenko T, Whiting P. (2001). Phytoecdysteroids in the genus Asparagus (Asparagaceae). Phytochemistry 56:569–76
- El-Tantawy WH, Temraz A, El-Gindi OD. (2007). Free serum testosterone level in male rats treated with Tribulus Alatus extracts. Int Br J Urol 33:554–9
- Esfandiari A, Dehghani R. (2010). Histomorphometrical study of seminiferous tubule in rats after used Tribulus terresteris. J Cell Anim Biol 4:68–72
- Francis SH, Busch JL, Corbin JD. (2010). cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62:525–63
- Furchgott RF, Zawadzki JV. (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–6
- Ganguly A, Misro MM, Chaudhury J, et al. (1992). Differential response of testis and serum gonadotrophins to testosterone in rats treated with a gonadotrophin releasing hormone antagonist or estradiol-17β. Indian J Exp Biol 30:567–73
- Ganu G, Nagore DH, Rangari M, et al. (2010). Pharmacological evaluation of ayurvedic plants for aphrodisiac activity in experimental animals. J Complement Integr Med 7. [Online]. Available from: http://dx.doi.org/10.2202/1553-3840.1418 [last accessed 10 Sept 2010]
- Gaur RD. (1999). Flora of the District Garhwal, North West Himalaya. Srinagar, Garhwal, India: Trans Media
- Gauthaman K, Adaikan PG. (2005). Effect of Tribulus terrestris on nicotinamide adenine dinucleotide phosphate-diaphorase activity and androgen receptors in rat brain. J Ethnopharmacol 96:127–32
- Gauthaman K, Adaikan PG. (2008). The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction-an evaluation using primates, rabbits and rats. Phytomedicine 15:44–54
- Gauthaman K, Ganesan AP, Prasad RN. (2003). Sexual effects of productive (Tribulus terrestris) extract (protodioscin): An evaluation using a rat model. J Altern Complement Med 9:257–65
- Gilna S. (2004). Testosterone and erectile dysfunction. J Med Health Gonads 1:407–12
- Gray JM, Nunez AA, Siegel LL, Wade GN. (1979). Effects of testosterone on body weight and adipose tissue: Role of aromatization. Physiol Behav 23:465–9
- Haren MT, Morley JE, Chapman IMO, et al. (2002). Defining relative androgen deficiency in aging men: How should testosterone be measured and what are the relationships between androgen levels and physical, sexual and emotional health. Climacteric 5:15–25
- Huang X, Kong L. (2006). Steroidal saponins from roots of Asparagus officinalis. Steroids 71:171–6
- Jadhav AN, Bhutani KK. (2006). Steroidal saponins from the roots of Asparagus adscendens Roxb and Asparagus racemosus Willd. Indian J Chem 45B:1515–24
- Johnson L, Petty CS, Neaves WB. (1980). A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod 22:1233–43
- Kanwar AS, Bhutani KK. (2010). Effects of Chlorophytum arundinaceum, Asparagus adscendens and Asparagus racemosus on pro-inflammatory cytokine and corticosterone levels produced by stress. Phytother Res 24:1562–6
- Kapoor LD. (2001). Handbook of Ayurvedic Medicinal Plants. New York, Washington D.C.: CRC Press, LCC
- Khan I, Nisar M, Khan N, et al. (2010). Structural insights to investigate conypododiol as a dual cholinesterase inhibitor from Asparagus adscendens. Fitoterapia 81:1020–5
- Kim HJ, Woo DS, Lee J, Kim JJ. (1998). The relaxation effects of ginseng saponins in rabbit corporal smooth muscle: Is it a nitric oxide donor? Br J Urol 82:744–8
- Kumar S, Sharma A. (2005). Anti-anxiety activity studies on homoeopathic formulations of Turnera aphrodisiaca Ward. ECAM 2:117–9
- Linnankoski I, Leinonen LM. (2010). Compatibility of male and female sexual behaviour in Macaca arctoides. Z Tierpsychol 70:115–22
- Linnankoski I, Grönroos M, Carlson S, Pertovaara A. (1995). Effect of cocaine on sexual behavior in male stump tail macaques (Macaca arctoides). Pharmacol Biochem Behav 52:211–16
- Liu G, Sun X, Dai Y, et al. (2010). Chronic administration of sildenafil modified the impaired VEGF system and improved the erectile function in rats with diabetic erectile dysfunction. J Sexual Med 7:3868–78
- Lucio RA, Flores-Rojas G, Aguilar F, et al. (2001). Effects of genitofemoral nerve transection on copulatory behavior and fertility in male rats. Physiol Behav 73:487–92
- Mathews JN, Flatt PR, Abdel-Wahab YH. (2006). Asparagus adscendens (Shweta musali) stimulates insulin secretion, insulin action and inhibits starch digestion. Br J Nutr 95:576–81
- Mehta SR, Subramanian RB. (2005). Direct in vitro propagation of Asparagus adscendens Roxb. Plant Tissue Cult 15:25–32
- Mercier O, Perraud J, Stadler J. (1987). A method for the routine observation of sexual behavior in rats. Lab Anim 21:125–30
- Mills TM, Reilly CM, Lewis RW. (1996). Androgens and penile erection: A review. J Androl 17:633–8
- Mizusawa H, Ishizuka O, Nishizawa O. (2002). Animal models for studying penile hemodynamics. Asian J Androl 4:225–8
- Naylor AM. (1998). Endogenous neurotransmitters mediating penile erection. Br J Urol 81:424–31
- Negi JS, Singh P, Joshi GP, et al. (2010). Chemical constituents of Asparagus. Pharmacogn Rev 4:215–20
- Padashetty SA, Mishra SH. (2007). Effect of terpenoidal fraction of Echinops echinatus roots on reproductive parameters of male rats. J Nat Med 61:452–7
- Panda H. (2000). Herbs Cultivation and Medicinal Uses. New Delhi, India: National Institute of Industrial Res., NPCS
- Park SW, Lee CH, Shin DH, et al. (2006). Effect of SA1, a herbal formulation, on sexual behavior and penile erection. Biol Pharm Bull 29:1383–6
- Ramachandran S, Sridhar Y, Sam SK, et al. (2004). Aphrodisiac activity of Butea frondosa Koen. ex Roxb. extract in male rats. Phytomedicine 11:165–8
- Ratnasooriya WD, Dharmasiri MG. (2000). Effects of Terminalia catappa seeds on sexual behaviour and fertility of male rats. Asian J Androl 2:213–19
- Sharma V, Thakur M, Dixit VK. (2012). A comparative study of ethanolic extracts of Pendulum murex Linn. fruits and sildenafil citrate on sexual behaviors and serum testosterone level in male rats during and after treatment. J Ethnopharmacol 143:201–6
- Singh R, Rai B. (2000). Antifungal potential of some higher plants against Fusarium udum causing wilt disease of Cajanus cajan. Microbios 102:165–73
- Singh R, Khan NU, Singhal KC. (1997). Potential antifilarial activity of roots of Asparagus adscendens Roxb, against Setaria cervi in vitro. Indian J Exp Biol 35:168–72
- Srivastav A, Chandra A, Singh M, et al. (2010). Inhibition of hyaluronidase activity of human and rat spermatozoa in vitro and antispermatogenic activity in rats in vivo by Terminalia chebula, a flavonoid rich plant. Reprod Toxicol 29:214–24
- Suresh S, Prithiviraj E, Prakash S. (2009). Dose- and time-dependent effects of ethanolic extract of Mucuna pruriens Linn. seed on sexual behaviour of normal male rats. J Ethnopharmacol 122:497–501
- Tajuddin, Ahmad S, Latif A, Qasmi IA. (2003). Aphrodisiac activity of 50% ethanolic extracts of Myristica fragrans Houtt. (nutmeg) and Syzygium aromaticum (L) Merr. and Perry. (clove) in male mice: A comparative study. BMC Complement Altern Med 3:6
- Tajuddin, Ahmad S. Latif A, et al. (2005). An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement Altern Med 5:16
- Tajuddin, Ahmad S, Latif A, Qasmi IA. (2004). Effect of 50% ethanolic extract of Syzygium aromaticum (L) Merr. & Perry. (Clove) on sexual behaviour of normal male rats. BMC Complement Altern Med 4:17–24
- Tandon M, Shukla YN. (1995). Phytoconstituents of Asparagus adscendens, Chlorophytum arundinaceum and Curculigo orchioides: A review. J Med Aromatic Plant Sci 17:42–50
- Thakur M, Dixit VK. (2007). Effect of some vajikaran herbs on pendiculation activities and in vitro sperm count in male rats. Sex Disabil 25:203–7
- Thakur M, Bhargava S, Dixit VK. (2009a). Effect of Asparagus racemosus on sexual dysfunction in hyperglycemic male rats. Pharm Biol 47:390–5
- Thakur M, Bhargava S, Praznik W, et al. (2009b). Effect of Chlorophytum Borivilianum Santapau and Fernandes on sexual dysfunction in hyperglycemic male rats. Chin J Integr Med 6:448–53
- Thakur M, Chauhan NS, Bhargawa S, Dixit VK. (2009c). A comparative study on aphrodisiac activity of some Ayurvedic herbs in male albino rats. Arch Sex Behav 38:1009–15
- Trivedi HK, Upadhyay KK. (1993). AIDS – A serious problem of modern world. Sachitra Ayurved 45:821–4
- Wang ZP, Gu ZP, Cao L, et al. (1999). Effect of tripchlorolide on the epididymides and testes of rats. Asian J Androl 1:121–5
- Yakubu MT, Akanji MA. (2011). Effect of aqueous extract of Massularia acuminata stem on sexual behaviour of male Wistar rats, Evid Based Complement Alternat Med [Online]. Available from: http://dx.doi.org/10.1155/2011/738103 [last accessed 22 Aug 2013]
- Yakubu MT, Akanji MA, Oladiji AT. (2005). Aphrodisiac potentials of the aqueous extract of Fadogia agrestis (Schweinf. Ex Hierr) on male albino rats. Asian J Androl 7:399–404
- Yakubu MT, Akanji MA, Oladiji AT, Adesokan AA. (2008). Androgenic potentials of aqueous extract of Massularia acuminata (G. Don) Bullock ex Hoyl. stem in male Wistar rats. J Ethnopharmacol 118:508–13
- Zhang XO, Hu LU, Yin JO, et al. (2002). Rat model of erectile dysfunction caused by cavernous nerve ablation. Chin Med J 115:1179–82
