Abstract
Context: Doxorubicin is a chemotherapy agent used in non-Hodgkin's lymphoma but side effects limit its use. Citral is a mixture of neral and geranial found in essential oils of lemon grass.
Objectives: We evaluated the activity of citral, doxorubicin, and combination on cytotoxicity, apoptosis, and anti-proliferative effects using human lymphoma Ramos cells.
Materials and methods: Cells were treated with doxorubicin alone or in combination with citral (10, 20, and 40 μM). Cytotoxic and apoptosis studies were done after 24 and 18 h incubations, respectively. Cytotoxic effects of citral on normal human peripheral blood mononuclear cells (PBMCs) were also investigated for its safety. Changes in the expression of BCL-2 family genes were analyzed by quantitative RT-PCR.
Results: Citral had cytotoxicity on cells with an IC50 value of 77.19 ± 4.95 µM. Citral at concentrations of 10, 20, and 40 µM additively increased the cytotoxic and apoptotic effects of doxorubicin, leading to decreased IC50 (µM) of the drug from 2.50 ± 0.01 to 2.16 ± 0.03, 1.90 ± 0.04, and 1.23 ± 0.04, respectively. Enhanced cytotoxicity was not observed in normal human PBMCs. Citral (40 µM) in combination with doxorubicin (1.5 µM) increased the expression of pro-apoptotic protein BAK but significantly decreased the expression of anti-apoptotic protein BCL-XL to 5.26-fold compared with doxorubicin-treated cells. It did not change the anti-proliferative activity of drug.
Discussion and conclusion: Citral potentiated cytotoxicity of doxorubicin by increasing apoptotic effects. We conclude that citral may have beneficial effects in patients with B cell lymphoma treated with chemotherapy.
Introduction
Non-Hodgkin's lymphoma (NHL) is one of the most common hematologic malignancies worldwide. More than 80% of all NHL cases are B-cell lymphoma (Zhong, Citation2006). The incidence is still rising with an estimated 385 741 new cases and 199 630 deaths from NHL in 2012 (Ferlay et al., Citation2012). Combination chemotherapy has been standard treatment for NHL patients, especially aggressive or advanced stage types of this disease (Hennessy et al., Citation2004). Doxorubicin is a chemotherapeutic drug widely used in many combined regimens for NHL. However, fatal cardiotoxicity has been a major limitation (Soni et al., Citation2011). Strategies to decrease doxorubicin toxicity by minimizing doxorubicin effects in chemotherapeutic doses and improving its efficacy are urgently needed to better treat cancer patients.
Over the past decade, medicinal plants have played an important role in cancer therapy research (Siripong et al., Citation2006). Many natural compounds such as curcumin, genistein, resveratrol, proanthocyanidin, emodin, silymarin, and flavopiridol have been reported to potentiate cytotoxicity of chemotherapeutic drugs in various cancer cell lines by enhancing chemotherapy-induced apoptosis (Garg et al., Citation2005; Sarkar & Li, Citation2006). Citral or 3,7-dimethyl-2,6-octadienal is a volatile oil found in essential oils from several herbs and edible plants such as lemongrass [Cymbopogon citratus (DC) Stapf. (Poaceae)], lemon balm [Melissa officinalis L. (Lamiaceae)], and Litsea cubeba (Lour.) Pers. (Lauraceae). It is the main component (70–85%) in lemon grass essential oil (Edris, Citation2007). Citral is the mixture of two isomeric acyclic monoterpene aldehydes, the trans-isomer geranial or citral A and the cis-isomer neral or citral B (Pihlasalo et al., Citation2007). Citral is used as a flavor additive and cooking ingredient where average daily intake of citral in humans is about 5 mg/kg (Ress et al., Citation2003). Several pharmacological activities of citral have been documented such as anti-inflammatory effects (Lee et al., Citation2008), antimicrobial activities against Gram-positive and Gram-negative bacteria, fungi, and some protozoa (Hayes & Markovic, Citation2002; Saddiq & Khayyat, Citation2010), and anticancer activities against some cancer cell lines including leukemic, breast, and lymphoma cells. Treatment with citral resulted in an activation of caspase-3, leading to apoptosis in human U937, HL60, and NB4 and mouse RL12 and BS-24-1 leukemic cell lines (Dudai et al., Citation2005). Moreover, it inhibited human breast cancer cell MCF-7 growth and induced cycle arrest in G2/M phase (Chaouki et al., Citation2009). Recently, Xia et al. (Citation2013) found that citral has apoptotic activity on acute promyelocytic leukemia cell lines (NB4 cells) and this effect was attributed to the activation of caspase-3 and down-regulation of nuclear factor kappa B (NF-κB) expression. Similarly, previous work from our laboratory have found that citral induced human lymphoma Ramos cell apoptosis by activating caspase activity in a concentration and time-dependent manner but has no effect on cell distribution in the cell cycle (unpublished observation). It would be a better strategy to combine natural anticancer compounds, such as citral, with potent existing cytotoxic therapeutic agents such as doxorubicin. To our knowledge, the effect of citral on anticancer activity of chemotherapeutic drugs has not been investigated. Therefore, this study was carried out to examine effects of citral on anticancer activity of doxorubicin in human lymphoma Ramos cell line.
Materials and methods
Cells
Ramos (Human Burkitt's lymphoma cell line), an aggressive type of B-cell lymphoma, was obtained from ATCC (Rockville, MD). Cells in the exponential growth phase with over 95% viability were used in the experiments. Human PBMCs were isolated from healthy male blood donors, age between 20 and 35 years old to use for safety screening of cytotoxic agents (Meng et al., Citation2013). This study was approved by the Human Research Ethics Committee from the Faculty of Medicine, Chulalongkorn University, Thailand, with COA no. 642/2012. Cells were maintained in RPMI 1640 medium supplemented with 10% (v/v) fetal bovine serum, penicillin (100 units/ml), and streptomycin (0.1 mg/ml) (Gibco BRL, Gaithersburg, MD) at 37 °C in a humidified atmosphere of 5% carbon dioxide.
Preparation of tested compounds
Freshly prepared citral (Sigma-Aldrich, St. Louis, MO) was diluted in ethanol to various final concentrations with constant 0.5% ethanol. Doxorubicin was freshly prepared from doxorubicin injection solution (Pfizer, New Yok, NY) by diluting in sterile double distilled water to the required concentrations. Ethanol of 0.5% was used as a negative control.
Isolation of human peripheral blood mononuclear cells
Collected whole blood from healthy volunteers in ethylenediaminetetraacetic acid (EDTA) was centrifuged at 2000 × g 25 °C for 10 min. The buffy coat was collected into 15 ml tubes and re-suspended with 5 ml incomplete RPMI medium. Diluted blood was slowly overlaid on Ficoll-hypaque solution (Sigma-Aldrich, St. Louis, MO) and centrifuged at 400 × g 25 °C for 30 min. Cells from interface were carefully collected, washed twice with 10 ml RPMI medium by centrifugation at 250 × g 25 °C for 10 min, re-suspended in complete RPMI medium, and incubated overnight at 37 °C with 5% carbon dioxide.
Resazurin cytotoxicity assay
Ramos cells at 1 × 106 cells/ml in 96-well plates were treated with various concentrations of citral (0–160 µM) or doxorubicin (0–3 µM) for 24 h at 37 °C. Ramos and PBMCs (1 × 106 cells/ml) were also treated with doxorubicin in combination with citral (10, 20, and 40 µM) for 24 h at 37 °C. Cytotoxicity of treated cells was determined by adding 5 µl of 1 mg/ml resazurin solution (Sigma-Aldrich, St. Louis, MO) into each well. Resazurin and its product resorufin were quantitatively determined by measuring their absorbances at 570 and 600 nm using a microplate reader (Labsystems Multiskan, New Hampshire, MS).
Combination index analysis
Half maximal inhibitory concentrations (IC50) of citral and doxorubicin, alone or in combination, on Ramos cells were calculated by Probit analysis using SPSS version 21.0 (SPSS Inc., Chicago, IL). These IC50 values were used to determine the type of citral–drug interaction using the combination index (CI) method of Chou and Talalay (Citation2010). This method has been widely used for assessing drug interaction at indicated effects by determining CI values from the following equation:
where C50 (drug) and C50 (citral) are concentrations of doxorubicin and citral used in combination to achieve 50% cytotoxicity. IC50 (drug) and IC50 (citral) are concentrations of each compound when used alone to produce the same effect. CI values are interpreted as follows; CI > 1.3 antagonism; CI 1.1–1.3 moderate antagonism; CI 0.9–1.1 additive effect; CI 0.8–0.9 slight synergism; CI 0.6–0.8 moderate synergism; CI 0.4–0.6 synergism; CI 0.2–0.4 strong synergism; CI < 0.1 very strong synergism (Chougule et al., Citation2011).
Induction of apoptosis
Ramos cells (1 × 106 cells/ml) were seeded in 24-well plates and incubated overnight at 37 °C. Cells were then treated with citral (10, 20, and 40 µM), doxorubicin (1.5, 2, and 2.5 µM), or doxorubicin combined with citral for 18 h. The treated cells were washed twice with cold phosphate buffer saline solution (PBS) and then stained with 1 µl of 200 µg/ml annexinV-FITC and 1 µl of 0.5 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) solution (Invitrogen, Carlsbad, CA) in annexin V-binding buffer (0.1 M HEPES/NaOH pH 7.4, 1.4 M NaCl, 25 mM CaCl2). Patterns of cell death were detected by fluorescence flow cytometer (BD Biosciences, San Jose, CA).
Anti-proliferative study
Ramos cells (1 × 106 cells/ml) were seeded in 24-well plates and incubated overnight at 37 °C. Cells were then treated with citral (10, 20, and 40 µM), doxorubicin (1.5 µM), or a combination of doxorubicin and citral for 3 h. Treated cells were collected and washed with the RPMI 1640 medium and incubated in freshly complete medium for 48 h. Cells were collected, re-suspended in 1 ml cold PBS, and counted using Scepter™ Handheld Automated Cell Counter with 40 µm Scepter Sensors (Millipore, Billerica, MA).
Expression of proteins in BCL-2 family by quantitative real-time PCR
After Ramos cell treatments with either citral (20 and 40 µM), or doxorubicin (1.5 µM) alone or in combination for 18 h, these cells were then subjected to total RNA isolation by TRIzol reagent (Invitrogen, Carlsbad, CA). RNA samples were reverse transcribed to cDNA using Improm II reverse transcription system according to the manufacturer's protocol (Promega, Madison, WI). cDNA samples were used for determining the expression of BCL-2 family proteins; BAX (Hs00180269_m1), BAK (Hs00832876_g1), BCL-2 (Hs00608023_m1) and BCL-XL (Hs00236329_m1) by quantitative reverse transcription polymerase chain reactions (qRT-PCR) using TaqMan® Gene Expression Assay (Applied Biosystems, Carlsbad, CA). GAPDH was used as endogenous control for normalization. Quantification of gene expression levels was performed using StepOnePlus™ Real-Time PCR System version 2.3 (Applied Biosystems, Carlsbad, CA) with comparative threshold cycle method (ΔΔCT) to provide calibrator-normalized relative quantification (RQ) that was indicated as either a fold-change or a fold-difference of gene expression levels between different samples.
Statistical analysis
All data obtained from this study were presented as mean with standard error of mean (mean ± SEM). Statistical evaluation was determined by one-way ANOVA followed by Tukey's post hoc test. SPSS program version 21.0 (SPSS Inc., Chicago, IL) was used to perform all statistical analysis. Any p value less than 0.05 was considered statistically significant.
Results
Effects of citral on cytotoxicity of doxorubicin
Both doxorubicin and citral had cytotoxic effects on Ramos cells after 24 h exposure with IC50 values of 77.19 ± 4.95 and 2.50 ± 0.01 µM, respectively (). Citral at 10, 20, and 40 µM was lower than its IC50 and were used in combination with 1–3 µM doxorubicin to treat Ramos cells for 24 h. Interestingly, the IC50 value of doxorubicin was reduced from 2.50 ± 0.01 to 2.16 ± 0.03, 1.90 ± 0.04, and 1.23 ± 0.04 µM when combined with 10, 20, and 40 µM of citral, respectively ( and ). This indicates that citral significantly increased the cytotoxicity of doxorubicin. CI values of the doxorubicin and citral combination were 0.99–1.02 as indicated in and suggest that citral additively enhances the cytotoxic effect of doxorubicin on Ramos cells. However, it would be worth mentioning that citral did not potentiate the cytotoxicity of doxorubicin in normal human PBMCs (). In addition, either citral or doxorubicin alone had very little effect on these normal cells. Together, these results suggest that citral is not toxic to normal cells.
Figure 1. Effect of citral on cytotoxic activity of doxorubicin against Ramos cells. The cells were treated with doxorubicin (0–3 µM) alone or in combination with citral at 10, 20, and 40 µM for 24 h. Cytotoxicity was determined by the resazurin reduction assay. The data represent means ± SEM of three independent experiments. *p < 0.05 compared with doxorubicin alone.
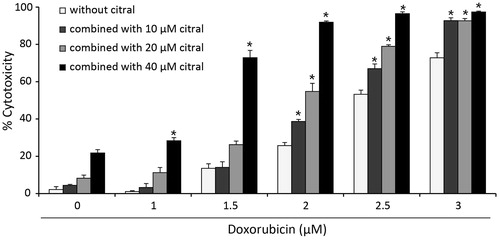
Figure 2. Effect of citral on cytotoxic activity of doxorubicin against human PBMCs. The cells were treated with doxorubicin (1.5, 2, and 2.5 µM) alone or combined with citral at 10, 20, and 40 µM for 24 h. Cytotoxicity was determined by the resazurin assay. The data are expressed as means ± SEM of five independent experiments. *p < 0.05 compared with the solvent control.
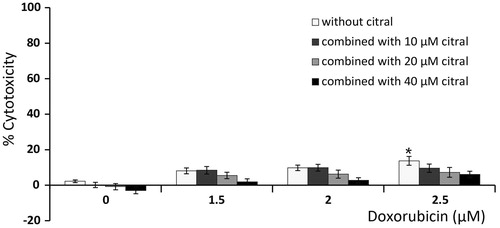
Table 1. IC50 and combination index (CI) values of doxorubicin combined with citral at 10, 20, and 40 µM on Ramos cells after 24 h incubation.
Effects of citral on apoptosis induction activity of doxorubicin
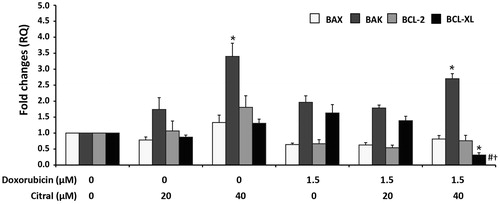
Apoptosis is an important mechanism of cell death involved in antitumor activities of many chemotherapeutic anticancer drugs. Apoptotic cells can be identified as annexin V-FITC+ cells after staining with annexin V-FITC/DAPI and detection with fluorescence flow cytometer. As shown in , single treatment of 40 µM citral significantly induced Ramos cell apoptosis. Combined with doxorubicin, citral 20 and 40 µM significantly enhanced apoptotic activity of doxorubicin when compared with doxorubicin- or citral-treated cells. Citral at 40 µM increased the percentage of apoptotic cells to 4.79- and 3.30-fold in citral-doxorubicin combination when compared to the effect of doxorubicin alone at 1.5 and 2 µM, respectively. Citral at 40 µM in combination with 2.5 µM doxorubicin increased total cell death but the cell death pattern was shifted from early apoptosis to late apoptosis (annexin V-FITC+/DAPI+ cells). Doxorubicin at 1.5 µM was further used to investigate the effect of citral on the expression of BCL-2 family proteins in Ramos cells. Modulation in pro-apoptotic and anti-apoptotic BCL-2 family protein expression is the main regulatory mechanism of caspase activation in the intrinsic or mitochondrial-dependent apoptosis pathway. Ramos cells were treated with either citral or doxorubicin alone or a combination of the two. mRNA expression of pro-apoptotic (BAX and BAK) and anti-apoptotic (BCL-2 and BCL-XL) proteins of treated cells was analyzed by qPCR. Results were presented as RQ numbers which indicate fold changes in expression levels of interested genes, normalized to endogenous gene control GAPDH, and 0.5% ethanol. As shown in , doxorubicin at 1.5 µM did not statistically change the expression of both pro-apoptotic and anti-apoptotic proteins when compared with the solvent control. Citral at 40 µM but not at 20 µM significantly increased the expression of pro-apoptotic protein BAK. The ratio of pro-apoptotic proteins (BAX plus BAK) to anti-apoptotic proteins (BCL-2 plus BCL-XL) favored apoptotic stage to that of survival stage of the treated cells. Citral at 40 µM in combination with 1.5 µM doxorubicin increased the expression of BAK but significantly decreased the expression of anti-apoptotic BCL-XL to 5.26-fold compared with doxorubicin-treated alone. The potentiating effect of citral on doxorubicin-induced apoptosis in Ramos cells may be due to a decrease in the anti-apoptotic gene BCL-XL.
Figure 3. Effect of citral on doxorubicin-induced Ramos cell death. The cells were treated with doxorubicin (1.5, 2, and 2.5 µM) alone or combined with citral at 10, 20, and 40 µM for 18 h. Treated cells were stained with annexin V-FITC/DAPI and analyzed by flow cytometry. Types of Ramos cell death were assessed as follows; annexin V-FITC+ cells were apoptotic cells, DAPI+ cells were necrotic cells, and annexin V-FITC+/DAPI+ cells were late apoptotic cells. The percentage of total cell death (apoptosis, late apoptosis, and necrosis) was plotted. The data represent means ± SEM of three independent experiments. *p < 0.05 compared with doxorubicin alone.
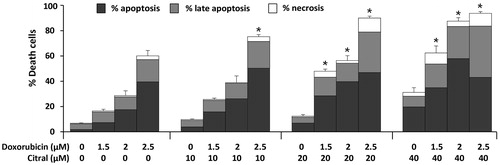
Figure 4. Effect of citral on the expression of BAX, BAK, BCL-XL, and BCL-2 in Ramos cells treated with doxorubicin (1.5 µM) alone or combined with citral at 20 and 40 µM for 18 h. The total RNA from the treated cells was reverse transcribed to cDNA for amplification of the mRNA expression levels of these genes by quantitative RT-PCR. GAPDH was used as the endogenous gene control for normalization. The expression levels of these genes from 0.5% ethanol-treated Ramos cells were regarded to have RQ = 1. The data are expressed as means ± SEM of four independent experiments. *p < 0.05 compared with solvent control, #p < 0.05 compared with doxorubicin alone, †p < 0.05 compared with 40 µM citral alone.
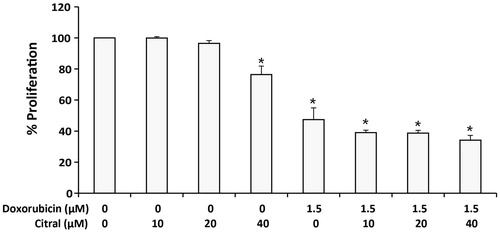
Effects of citral on anti-proliferative activity of doxorubicin
We also elucidated whether or not citral can potentiate the anti-proliferative activity of doxorubicin in Ramos cells. The cells were treated with doxorubicin in the presence and the absence of citral. The treated cells were washed, further incubated in fresh medium without the tested compounds for 48 h, and counted by Scepter™ Handheld Automated Cell Counter. As shown in , doxorubicin at 1.5 µM significantly inhibited Ramos cell proliferation. Treatment of citral alone at 40 µM significantly decreased cell proliferation. However, citral did not significantly affect the anti-proliferative activity of doxorubicin.
Figure 5. Effect of citral on the anti-proliferative effect of doxorubicin against Ramos cells. The cells were treated with doxorubicin (1.5 µM) alone or combined with citral at 10, 20, and 40 µM for 3 h. Treated cells were then washed and incubated in fresh medium without doxorubicin and citral for another 48 h. Numbers of the cells were counted by Scepter™ Handheld Automated Cell Counter. The results were expressed as a percentage of cells compared with 0.5% ethanol-treated Ramos cells (100% cell proliferation). The data are expressed as means ± SEM of three independent experiments. *p < 0.05 compared with solvent control.
Discussion
Treatment for NHL patients has been improved dramatically. Benefit of combining one targeted cancer drug such as rituximab, a monoclonal antibody against CD20, with a gold standard regimen, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) is an example of successful combination anticancer therapy. However, its use is restricted due to high costs of target-based antibodies. Recently, several natural-derived compounds have been explored and could serve as potentially effective anticancer compounds in combination therapy when coupled with standardized cancer drug treatment. The combination would not only reduce the dose of concurrent chemotherapy to achieve effective anticancer activity but may also improve upon the adverse events of potent therapeutic agents such as doxorubicin.
In the present study, we demonstrated that a volatile and edible oil, citral, may have this beneficial effect when used in combination with doxorubicin, an anticancer drug in CHOP regimen. Citral at the concentrations of 10, 20, and 40 µM additively increased the cytotoxicity of doxorubicin against Ramos cells ( and ). It had no cytotoxic effect on normal human PBMCs and did not potentiate doxorubicin cytotoxicity on these normal cells (). Taken together, these results indicate that citral is not harmful to normal cells but selectively potentiates the cytotoxicity of doxorubicin against cancer cells. To investigate the mechanism(s) underlying these beneficial effects, we determined citral effects on doxorubicin-mediated apoptosis. Citral potentiated doxorubicin-induced early apoptosis when it was combined with the drug at 1.5 and 2 μM. In contrast, potentiating effects of citral on total cell death, mainly in early and late apoptotic cells, were observed when it was combined with 2.5 µM doxorubicin (). It is possible that the higher concentration of doxorubicin, the early apoptotic cells in vitro, which were not removed by phagocytes as in vivo (Nagata et al., Citation2010), changed to late apoptotic cells quicker than when there was a lower concentration of citral in the drug.
We also confirmed the potentiating effect of citral on doxorubicin-induced apoptosis by evaluating the expression of proteins in BCL-2 family. Modulation of pro-apoptotic and anti-apoptotic BCL-2 family protein expression is the main regulatory mechanism of caspase activation in the intrinsic or mitochondrial-dependent apoptosis pathway (Chipuk & Green, Citation2008). The mRNA expression of pro-apoptotic BAK was dramatically increased in Ramos cells treated with either 40 μM citral alone or in combination with the anticancer drug. This effect correlated with apoptotic induction activity of citral that was mediated by caspase activation in many cancer cell lines (Chaouki et al., Citation2009; Dudai et al., Citation2005). It has been reported that the function of BAK, which is usually on the outer membrane of mitochondria (Willis et al., Citation2005) to initiate apoptosis, is faster than pro-apoptotic BAX which has to change its location from cytosol to mitochondria for oligomerization (Yao & Marassi, Citation2009). In contrast, we found that the expression of anti-apoptotic BCL-XL was significantly attenuated with the combination of doxorubicin and citral (40 μM) when comparing the effect of the doxorubicin or citral treatment alone on Ramos cells. Down-regulation of BCL-XL may diminish its anti-apoptotic character that inhibits BAX and BAK functions by directly forming heterodimer with these pro-apoptotic proteins (Martinou & Youle, Citation2011; Willis et al., Citation2005). From several studies, it has been suggested that BCL-XL is the main anti-apoptotic protein to protect B-cells from apoptosis. Its cellular levels could also predict the sensitivity of many B-lymphoma cells (Yeo et al., Citation2012) and hepatoblastoma HepG2 cells (Luo et al., Citation2000) to anticancer agents as well as better amplifying apoptotic stimulus than BCL-2 cellular levels. Moreover, inhibiting BCL-XL seems to be the target of potentiating agents in NHL B cell lines (Jazirehi et al., Citation2003; Li et al., Citation2008). Taken together, our results demonstrated that citral could potentiate anticancer activity of doxorubicin by up-regulating BAK and down-regulating BCL-XL mRNA expressions.
NF-κB, a family of transcription factors, plays a critical role in anti-apoptosis, cell proliferation, and tumorigenesis (Karin et al., Citation2002). The constitutive activation of NF-κB is found in B-cell lymphoma contributing to chemotherapy failure in patients (Fu et al., Citation2006). In fact, suppression of NF-κB activity has become an interesting approach to render cancer cells more susceptible to chemotherapy (Karin et al., Citation2002). A recent study in leukemic NB4 cells illustrated that an inhibition of NF-κB protein involved in apoptotic effects of citral on these cells (Xia et al., Citation2013). It may be possible that citral may exert potentiating activity by this manner but this hypothesis needs further investigation. It is reported that NF-κB directly regulates the anti-apoptotic BCL-XL genes but not BCL-2 (Chen et al., Citation2000). Taking this into consideration, it may be possible that NF-κB partly attributes to the potentiating effect of citral on doxorubicin-mediated apoptosis. Additional studies to determine the effect of citral on the NF-κB expression are warranted.
Chaouki et al. (Citation2009) have shown the citral inhibited human breast cancer cell MCF-7 growth and induced cycle arrest in G2/M phase. In this study, the effect of citral on anti-proliferative activity of doxorubicin was investigated by shortly treating Ramos cell for 3 h, washing the cells and further incubating in fresh medium without any tested agents for 48 h for cell to proliferate. The results demonstrated that citral (40 μM) reduced Ramos cell proliferation but it did not change the anti-proliferative effect of doxorubicin.
Conclusion
This study revealed the effects of citral on the anti-cancer activity of doxorubicin against human cancer B cells without any harm to normal blood cells. Citral potentiated doxorubicin-induced cytotoxicity and apoptosis in Ramos cells. The molecular effect of its potentiating effect is likely due to a decrease in the anti-apoptotic protein BCL-XL. Therefore, a combination of citral with doxorubicin may represent an alternative strategy to reduce the dose of doxorubicin. It also enhances the safety profile of such a potent anti-cancer agent in NHL patients.
Acknowledgements
We thank Miss Praewphan Ingrungruanglert for technical assistance with flow cytometry.
Declaration of interest
The authors have no conflicts of interest to declare. This study is supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University, Thailand, Grant number RA56/033.
References
- Chaouki W, Leger DY, Liagre B, et al. (2009). Citral inhibits cell proliferation and induces apoptosis and cell cycle arrest in MCF-7 cells. Fund Clin Pharmacol 23:549–56
- Chen C, Edelstein LC, Gélinas C. (2000). The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol 20:2687–95
- Chipuk JE, Green DR. (2008). How do Bcl-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18:157–64
- Chou T-C. (2010). Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res 70:440–6
- Chougule MB, Patel A, Sachdeva P, et al. (2011). Enhanced anticancer activity of gemcitabine in combination with noscapine via antiangiogenic and apoptotic pathway against non-small cell lung cancer. PLoS One 6:e27394
- Dudai N, Weinstein Y, Krup M, et al. (2005). Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med 71:484–8
- Edris AE. (2007). Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother Res 21:308–23
- Ferlay J, Soerjomataram I, Ervik M, et al. (2012). Cancer incidence and mortality worldwide [Online]. Available from: http://globocan.iarc.fr. [last accessed 28 Jan 2014]
- Fu L, Lin-Lee Y-C, Pham LV, et al. (2006). Constitutive NF-κB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood 107:4540–8
- Garg AK, Buchholz TA, Aggarwal BB. (2005). Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal 7:1630–47
- Hayes AJ, Markovic B. (2002). Toxicity of Australian essential oil Backhousia citriodora (Lemon myrtle). Part 1. Antimicrobial activity and in vitro cytotoxicity. Food Chem Toxicol 40:535–43
- Hennessy BT, Hanrahan EO, Daly PA. (2004). Non-Hodgkin lymphoma: An update. Lancet Oncol 5:341–53
- Jazirehi AR, Gan XH, Vos SD, et al. (2003). Rituximab (anti-CD20) selectively modifies Bcl-xl and apoptosis protease activating factor-1 (Apaf-1) expression and sensitizes human non-Hodgkin's lymphoma B cell lines to paclitaxel-induced apoptosis. Mol Cancer Ther 2:1183–93
- Karin M, Cao Y, Greten FR, Li Z-W. (2002). NF-κB in cancer: From innocent bystander to major culprit. Nat Rev Cancer 2:301–10
- Lee HJ, Jeong HS, Kim DJ, et al. (2008). Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264.7 Cells. Arch Pharm Res 31:342–9
- Li Z-M, Jiang W-Q, Zhu Z-Y, et al. (2008). Synergistic cytotoxicity of Bcl-xl inhibitor gossypol and chemotherapeutic agents in non-Hodgkin's lymphoma cells. Cancer Biol Ther 7:51–60
- Luo D, Cheng SC-S, Xie H, Xie Y. (2000). Effects of Bcl-2 and Bcl-xl protein levels on chemoresistance of hepatoblastoma HepG2 cell line. Biochem Cell Biol 78:119–26
- Martinou JC, Youle RJ. (2011). Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21:92–101
- Meng Q-x, Roubin RH, Hanrahan JR. (2013). Ethnopharmacological and bioactivity guided investigation of five TCM anticancer herbs. J Ethnopharmacol 148:229–38
- Nagata S, Hanayama R, Kawane K. (2010). Autoimmunity and the clearance of dead cells. Cell 140:619–30
- Pihlasalo J, Klika KD, Murzin DY, Nieminen V. (2007). Conformational equilibria of citral. J Mol Struct (Theochem) 814:33–41
- Ress NB, Hailey JR, Maronpot RR, et al. (2003). Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol Sci 71:198–206
- Saddiq AA, Khayyat SA. (2010). Chemical and antimicrobial studies of monoterpene: Citral. Pestic Biochem Phys 98:89–93
- Sarkar FH, Li Y. (2006). Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res 66:3347–50
- Siripong P, Yahuafai J, Shimizu K, et al. (2006). Antitumor activity of liposomal naphthoquinone esters isolated from Thai medicinal plant: Rhinacanthus nasutus Kurz. Biol Pharm Bull 29:2279–83
- Soni H, Pandya G, Patel P, et al. (2011). Beneficial effects of carbon monoxide-releasing molecule-2 (CORM-2) on acute doxorubicin cardiotoxicity in mice: Role of oxidative stress and apoptosis. Toxicol Appl Pharm 253:70–80
- Willis SN, Chen L, Dewson G, et al. (2005). Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xl, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19:1294–305
- Xia H, Liang W, Song Q, et al. (2013). The in vitro study of apoptosis in NB4 cell induced by citral. Cytotechnology 65:49–57
- Yao Y, Marassi FM. (2009). Bax and Bak caught in the act. Mol Cell 36:353–4
- Yeo AT, Porco JA, Gilmore TD. (2012). Bcl-xl, but not Bcl-2, can protect human B-lymphoma cell lines from parthenolide-induced apoptosis. Cancer Lett 318:53–60
- Zhong Y. (2006). Non-Hodgkin's lymphoma: What primary care professionals need to know?. J Nurse Pract 2:309–15
