Abstract
Context: Keloid is an excessive dermal scar occurring in response to skin injuries. Several therapeutic strategies have been proposed to ease the aggressiveness of keloid scarring. Even though the principle mechanism underlying the disease propagation still remains unidentified, several signaling pathways were highly focused as plausible pathways involving keloid formation, including transforming growth factor-beta 1 (TGF-β1), mitogen-activated protein kinase (MAPK), insulin-like growth factor-I (IGF-I), and integrin pathways. Natural compounds containing multiple bioeffective properties such as quercetin, asiaticoside, Astragalus membranaceus Bunge. (Leguminosae), and Salvia miltiorrhiza Bunge. (Lamiaceae) extracts, curcuminoids, oxymatrine, madecassoside, and Aneilema keisak Hassk. (Commelinaceae) are claimed as candidates for therapeutic treatment against keloid disorder.
Objective: This review investigates current mechanisms regarding keloid formation and provides scientific evidence supporting the therapeutic potential of natural compounds.
Methods: This review obtained and analyzed a number of literature data items from various databases including Pubmed, ScienceDirect, and Elton B. Stephens Company (EBSCO).
Result: Several phytochemical compounds are able to suppress keloid scar development through manipulating various components in the complex signaling cascades.
Conclusion: The present review may be helpful to future studies that further examine the molecular mechanism of keloid etiology as well as investigate the anti-keloid property in natural compounds.
Introduction
Keloids are raised and aggressive scar tissue. They grow and extend beyond the position of the original lesion and tend to persist after excision. In 1806, Jean Louis Alibert established the name “Keloid” to describe crab-claw-like extension of scar tissue into the neighboring skin (Butler et al., Citation2008). Keloid scarring occurs uniquely to humans in response to dermal damage (Bran et al., Citation2009; Lim et al., Citation2001) and is generally observed in African-American, Latin-American, and other pigmented skin ethnicities (Butler et al., Citation2008). The molecular characteristics of keloid include invasion and hyperproliferation of dermal fibroblasts along with an increased biosynthesis and accumulation of extracellular matrix (ECM). Collagen bundles produced in keloid are thicker, denser, and less structured, compared with regular collagen, resulting in node-like appearance in the deep dermal skin (Bran et al., Citation2009; Gauglitz & Korting, Citation2011). Keloid scarring can be seen as an end result of an unregulated wound healing process while the other end presents as a clean single-lined wound (Seifert & Mrowietz, Citation2009).
In this review, we focus on alternative keloid therapy provided by naturally derived compounds, which target the mechanism of keloid formation. Synthetic chemical treatment seems to be more commonly used in reducing keloid scarring; however, these treatments are usually used for general hyperscarring therapy and do not prevent the keloid formation recurrence. Therefore, the wide variety of pharmaceutically active compounds from natural sources may contain active ingredients with an anti-keloid activity and can be used to treat keloid disease.
Keloid pathogenesis
The pathobiology of keloid formation is not completely understood. Some hypotheses have been proposed for the etiology of keloid disorder. Keloid scarring has a varied prevalence among the populations worldwide. The disease frequently occurs in Blacks, Hispanics, and Orientals, and is less commonly found in Caucasians, suggesting a genetic factor in keloid etiology (Bran et al., Citation2009; Gauglitz & Korting, Citation2011). However, there are no reports on any linkage genes in the incidence of keloid disease. It is possible that keloid may have an inheritance mode with incomplete penetrance which environmental factors such as wound tension or hormonal stress are required to trigger the disease onset in genetically susceptible persons (Halim et al., Citation2012).
Autoimmune response
Moreover, some studies have found a correlation between an immune reaction against sebum exposure and keloid scar formation (Huang et al., Citation2013; Seifert & Mrowietz, Citation2009). A dermal injury causes leakage of pilosebaceous units from sebaceous glands into the systemic system. In some individuals who are sensitive to the sebum, this exposure may trigger a cell-mediated immune response resulting in over inflammation and the deregulated wound healing process (Al-Attar et al., Citation2006). This hypothesis explains the invasiveness of keloid as more sebaceous glands being disrupted causing the scar propagation.
Site of keloid
It appears that keloid scarring often occurs on certain areas of the body such as the sternum, deltoid region of the upper arm, and upper back which are susceptible to excessive stretch and tension (Butler et al., Citation2008). This can lead to a hypothesis that mechanical tension applied to the scar during the wound healing process may also contribute to the formation of keloid (Edriss & Smrcka, Citation2011). It was reported that mechanical tension can stimulate fibroblast proliferation and collagen production but disturb the arrangement of collagen structure (Huang et al., Citation2013). Even though the etiology of keloid disease is still debatable, there are several therapies to reduce the scarring size or to soothe the severity of the disease.
Deregulation of wound healing process
Keloid scarring can be seen as an end result of an unregulated wound healing process while the other end presents as a clean single-lined wound (Seifert & Mrowietz, Citation2009; Yang et al., Citation2003). Many studies described keloid scarring as a consequence of an inability to stop the wound healing process in genetically susceptible individuals (Bran et al., Citation2009; Gauglitz et al., Citation2011). At the cellular level, wound healing events can be divided into three overlapping phases: inflammatory, proliferation, and maturation. Disruptive regulations in each phase have been reported as a candidate for an origin of abnormal scar formation (Bran et al., Citation2009). Inflammatory phase happens shortly after wounding in response to a dermal disruption. Immune cells are induced to accumulate at the wound site. Neutrophils release proinflammatory cytokines and are responsible for killing bacteria to prevent infection. Activated macrophages migrate to the wound site to clean up cell debris and secrete growth factors as well as cytokines, which further stimulate the proliferative phase. A prolonged and intense inflammatory process leads to unbalanced cellular regulation in the proliferative phase, which was claimed to result in keloid scarring (Bran et al., Citation2009). Growth factors and cytokines released from inflammatory phase trigger migration and proliferation of dermal cells including keratinocytes and fibroblasts. Keloid fibroblast was found to be more responsive to some growth factors compared with normal fibroblast, for example, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and insulin-like growth factor-1 (IGF-1) (Shih et al., Citation2010). During proliferative phase, fibroblasts proliferate and produce ECM to substitute the damaged tissue. As wound healing progresses, a maturation phase must occur to inhibit the skin tissue production. Apoptosis or a programmed cell death is an important cellular event for the final scarring process in order to return to the normal skin homeostasis. An abnormal regulation of apoptosis pathway has been claimed to be a leading cause for keloid scar formation (Brown & Bayat, Citation2009). Due to the resistance to apoptosis behavior, keloid fibroblasts lack the ability to stop proliferation and collagen production resulting in an imbalance of collagen deposition and degradation (Chodon et al., 2000; Diao et al., Citation2011). Wound healing process is a complex, highly regulated cellular mechanism with multiple signaling pathways involved. A failure in regulating these pathways could result in keloid scar formation. Thus, further understanding in each phase of wound healing process is crucial to manage keloid disease.
Heterogeneity in keloid fibroblast population
The cellular components of keloid scarring were found to be heterogenic. The center of the keloid is densely packed with unstructured collagen, avascular, and acellular. In contrast, the boundary of keloid scarring contains numerous invasive and proliferative fibroblasts and is highly vascularized. The cell type differences and variations in underlying mechanisms may lead to inconsistency with the results regarding keloid in vitro studies (Babu et al., Citation1989; Tucci-Viegas et al., Citation2010). The biological differences between fibroblast derived from different regions in the keloid scar are evidenced in a study done by Lu et al. (Citation2007). It was reported from the cell-cycle analysis that fibroblast cells from the middle region of the scar were in an arrest or the G0–G1 phase while cells derived from the peripheral region were shown to be in a proliferative or G2–S phase (Lu et al., Citation2007). The difference in phases in the cell cycle reflects different cellular behavior and could consequently lead to diverse or bias in vitro results.
Molecular signalings
Transforming growth factor-beta 1 (TGF-β1) pathway
The fundamental molecular mechanism of keloid formation still remains unclear; however, the TGF-β1 pathway is strongly believed to be a major player in keloid pathogenesis (Bran et al., Citation2009; Seifert & Mrowietz, Citation2009; Pakyari et al., Citation2013; Shih & Bayat, Citation2010). The TGF-β1 signaling pathway is well known as an essential fibrotic cytokine for promoting ECM production and tissue fibrosis. In 2001, Chin and his team demonstrated an increase in the expression of TGF-β receptors type 1 and type 2 (TGF-βR1 and TGF-βR2) in keloid fibroblast compared with normal fibroblast. In addition, there are many reports confirming the overexpression of TGF-β1 in keloid fibroblast compared with normal fibroblast (Babu et al., Citation1989; Gauglitz & Korting, Citation2011; Hsu et al., Citation2010). Nevertheless, there is no conclusion whether increases in TGF-β1 is the cause or the effect of keloid disease. Some studies also claimed that the sensitivity of the TGF-β pathway leads to the abnormality in keloid formation (Wakefield & Roberts, Citation2002). An overexpressed TGF-β signaling cascade can be seen as a principle mechanism underlining keloid scar formation.
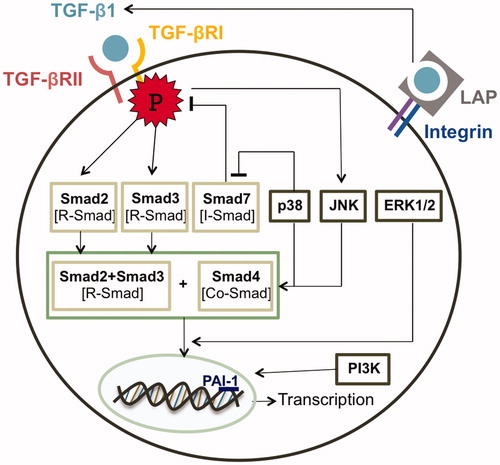
The TGF-β1 ligand facilitates the intracellular signal through two transmembrane serine/threonine kinase receptors, TGF-β receptors type 1 and type 2 (TGF-βR1 and TGF-βR2) (). The binding of the ligand to TGF-βR2 induces the recruitment and phosphorylation of TGF-βR1, which leads to downstream signaling by Smad proteins that are functionally categorized into three groups: R-Smads, Co-Smad, and I-Smads (Miyazono et al., Citation2000). R-Smads or receptor regulated Smads including Smad2 and Smad3 are phosphorylated directly by TGF-βR1. Then R-Smads form a heterogeneous complex with Co-Smad (Smad4) and translocate into the nucleus. In the nucleus, the Smads complex (Smad2/3/4) associates with a wide array of specific DNA-binding proteins to regulate transcription responses. In contrast, the third class of Smad, the inhibitory Smad (I-Smad) containing Smad7, counteracts the effects of the R-Smads and thus antagonizes TGF-β signaling (Itoh & Dijke, Citation2007; Shi & Massagué, Citation2003).
Mitogen-activated protein kinase pathway
An additional level of complexity of the TGF-β signaling pathway is the ability to crosstalk with other signaling cascades (). The mitogen activated protein kinase (MAPK) pathway was claimed to become activated through TGF-β transduction (Javelaud & Mauviel, Citation2005). Several reports have shown that TGF-β induced the activation of extracellular signal-regulated kinases (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 kinase in different cell types (He et al., Citation2010). Yu and colleagues (Citation2002) illustrated that despite a mutation on TGF-βR1 resulting in the inability to phosphorylate R-Smad, the receptor can still activate p38 kinase upon TGF-β ligand binding. This evidence shows that TGF-β operates through MAPK signaling independently from Smad proteins. Furthermore, the MAPK pathway is in turn able to moderate the TGF-β signaling through direct phosphorylation of Smad. ERK activation was proven to interrupt the Smad nuclear translocation by phosphorylating Smad2 and Smad3 (Miyazono et al., Citation2000). An in vitro keloid cellular mechanism study showed that JNK and p38 inhibitors significantly disturbed the Smad2/3/4 complex formation and thus decreased the invasiveness of keloid fibroblasts (He et al., Citation2010). Moreover, p38 pathway interfered with an I-Smad expression and was able to reverse the TGF-β induced suppression of Smad7 in keloid fibroblasts (He et al., Citation2010). Accumulative data have been leading to an assumption that the crosstalk between Smad-dependent pathway and Smad-independent mitogenic pathway responding to TGF-β stimulation may have a role in driving normal fibroblast cellular mechanism towards aggressive, tumor-like characteristics of keloid fibroblast.
Insulin-like growth factor-I (IGF-I) pathway
Besides a strengthened Smad downstream cascade, IGF-I accounted for an invasive growth of fibroblast as well as an excessive production of ECM proteins induced by TGF-β. IGF-1 is one of the growth factors found to be significantly increased in keloid tissue when comparing with normal fibroblast (Shih et al., Citation2010). It was reported that IGF-1 only had a minor effect on fibroblast proliferation; however, the invasiveness of keloid fibroblast was significantly enhanced by IGF-1 stimulation (Daian et al., Citation2003) which was shown to be done through the PI3K pathway (Song et al., Citation2012). In addition, a report done by Daian and his team (Citation2003) examined the accumulation of ECM components in fibroblasts responding to TGF-β and IGF-1 co-stimulation by using signaling activity that targets plasminogen activator inhibitor (PAI-1) a promoter region as a marker (Daian et al., Citation2003). PAI-1 is a protease inhibitor functioning to promote the buildup of connective tissue (Tuan et al., Citation1996). TGF-β treatment intensified the activity by 10-fold, whereas a 25-fold increase was observed in the co-stimulation with IGF-1. Nevertheless, IGF-1 treatment alone failed to cause any changes in the activity (Daian et al., Citation2003). The results indicated the synergistic effect of TGF-β and IGF-1 suggesting a complex crosstalk mechanism underlying the two pathways.
Integrin pathway
An integrin alteration behavior in keloid fibroblast has been observed in recent studies. Integrin is a transmembrane receptor of fibroblast cell binding to the surrounding ECM. Expressions of α2β1 and α1β1integrin were found to be up-regulated in keloid fibroblast population (Suarez et al., Citation2013; Szulgit et al., Citation2002). The consequence of integrin alteration was not discussed in the previous studies. Nevertheless, in 2012, Leask claimed that integrin β1 could act as a mechanotransduction receptor controlling a release of activated TGF-β (Leask, 2013). TGF-β is synthesized and released in a latent form forming a large complex with latency-associated peptide (LAP) which is linked to the ECM by TGF-β-binding protein-1 (LTBP-1) (). An increase in mechanical force during the scar formation sending high tension across fibroblast and exerted on integrin causes LAP to unfold and release an active TGF-β (Buscemi et al., Citation2011). The deletion of β1 integrin failed to activate the latent TGF-β resulting in a reduced in collagen production (Liu et al., Citation2010). Therefore, an alteration in integrin expression may influence the TGF-β signal transduction and consequently disturb the collagen production in keloid fibroblast.
Keloid therapy
The aggressive characteristics of keloid cause both physical and mental drawbacks to patients. The presence of keloid scar has a negative impact on patients' appearance. The invasive and enlarging ability of keloid tissue may also become symptomatic by causing deformity or limiting joint mobility (Butler et al., Citation2008; Seifert & Mrowietz, Citation2009). Consequently, there are many available therapeutic strategies to reduce the appearance and discomfort of the scar. Surgical incision remains the most common method for eliminating keloid and hypertrophic scars. Unlike hypertrophic scar, keloid excisional surgery alone is not often successful. The recurrence rate was reported between 45 and 100% (Edriss & Smrcka, Citation2011). Compression therapy has been another standard strategy to handle keloid expansion since the 1970s (Slemp & Kirschner, Citation2006). The fundamental mechanism behind compression theory is not totally comprehended. A decrease in scar tissue metabolism and fibroblast activity may occur in response to hypoxia or thermal difference in the compression area (Slemp & Kirschner, Citation2006). An effective pressure application requires a special garment designed specifically for each individual wound site, which is one of the main limitations of the therapy. Moreover, silicone treatment has proved to be favored by most of the patients (Edriss & Smrcka, Citation2011). There is no evidence of chemical reaction between the silicone and the underlying scar. Some reports claimed that the occlusive therapy causes hydration of local keratinocytes resulting in an altered growth factor secretion which affects the regulation of fibroblast (Durani & Bayat, Citation2008). Radiation therapy has been claimed as a more effective alternative to surgical excision. It was reported with a lower relapsing rate compared to surgery (Durani & Bayat, Citation2008; Slemp & Kirschner, Citation2006). Currently, it is believed that radiation can induce fibroblast apoptosis causing the number of fibroblast cells in keloid to reduce to normal level (Durani & Bayat, Citation2008). However, a long-term exposure to radiation can theoretically increase the malignancy rate and thus lead to development of cancer. Another novel non-invasive method for treating scarring is ultrasound. It was claimed that ultrasound could help speed up the wound healing process, relieve pain, and itching during scarring and smooth out the mature scar tissue (Bessonart et al., Citation2005). The ultrasound is used as a general therapy for reducing the scar appearance and does not help with the recurrence of keloid scar. Modern treatment of keloid scarring including laser therapy continues to improve; however, recent clinical data still shows no promising results (Chike-Obi et al., Citation2009). Mitomycin C, an antitumor medicine, also shows an anti-proliferative property exerted by forming a cross-linkage to DNA strands inhibiting cell proliferation. Topical application of mitomycin C prevents fibrotic formation after surgery. Still, the clinical usage of mitomycin C showed very poor results in preventing keloid scar recurrence (Sanders et al., Citation2005). Other invasive therapeutic approaches are intralesional corticosteroid and 5-fluorouracil (5-FU). Injecting corticosteroid has been shown as one of the base-established approaches to prevent recurrence rate up to 80% with reducing pruritus and pain (Wang et al., Citation2009). Intralesional 5-FU have been reported to show improvement; however, the recurrence of keloid scarring remains apparent (Gupta & Kalra, Citation2002; Kontochristopoulos et al., Citation2005). Severe side effects including dermal atrophy, hypopigmentation, telangiectasias, necrosis, ulceration together with the pain from injection, still strongly limit the success of the therapy.
Natural-based therapies
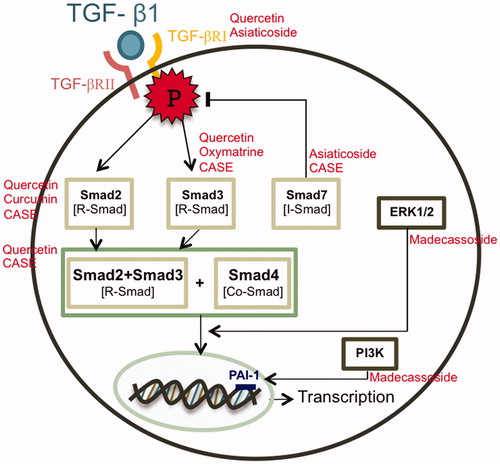
In recent years, there have been more studies exploring the anti-keloid properties of phytochemical compounds and herbal extracts because these naturally derived compounds cover a wide spectrum of activities in medicine, pharmacy, and general biology fields. Natural product is generally a secondary metabolite that can be produced by nature without any human interventions. As one of the main drawbacks of the synthetic chemical therapy is the severity of the adverse effects, natural compounds have been gaining considerable interest as other alternative therapeutic strategies. Besides, phytochemical compounds or compounds specifically derived from plants usually have several biological activities by influencing multiple components in the signaling cascades. Therefore, herbal compound therapy has a promising effect in targeting molecular signal pathways in keloid formation and is able to suppress the scar propagation. Compounds with anti-oxidant, anti-proliferative, and anti-inflammation properties such as quercetin, asiaticoside, Astragalus membranaceus Bunge. (Leguminosae), and Salvia miltiorrhiza Bunge. (Lamiaceae) extracts, curcuminoids and oxymatrine are likely to be proposed for anti-keloid treatment. A reduction in TGF-β production from keloid fibroblast was suggested to suppress collagen type 1 accumulation, reduce fibroblast proliferation, and induce apoptotic cell death. Resveratrol, a natural phenol compound, was claim as anti-fibrotic agent through the potential to reduce the TGF-β production specifically in keloid fibroblast (Ikeda et al., Citation2013). Since the suppression of the TGF-β/Smad signaling pathway has been a general strategy to treat keloids, quercetin, a flavonoid glycone compound identified as an active ingredient of Ginkgo biloba Linn. (Ginkgoaceae) (Phan et al., Citation2004), was shown to significantly inhibit the expression of both TGF-β receptors at various concentrations and prevent Smad2/3/4 nuclear translocation which are important for collagen type 1 gene expression (Phan et al., Citation2004). There were multiple clinical reports claiming that onion extract, which is one of the richest sources of quercetin, helped in the treatment of keloid and hypertrophic scarring (Hosnuter et al., Citation2007; Kakar et al., Citation2006; Koc et al., Citation2008). Another compound targeting TGF-receptors is asiaticosia, a saponin component from the leaves of Centella asiatica Linn. (Apiaceae). The compound was shown to reduce the amount of TGF-β receptor expression in mRNA level and increase the expression of Smad7 in a dose-dependent manner which resulted in deregulating the production of ECM proteins (Tang et al., Citation2011). The extracts from A. membranaceus Bunge. (Leguminosae) and Salvia miltiorrhiza Bunge. (Lamiaceae), named compound Astragalus and Salvia miltiorrhiza extract (CASE) that contained astragalosides, astragalus polysaccharides and salvianolic acids, showed an upregulated effect on Smad7 expression together with reducing the phosphorylation of Smad2/3 which posses a negative effect in the TGF-β pathway. Therefore, the treatment was able to inhibit cell proliferation and invasion as well as to reduce collagen production in keloid fibroblast (He et al., 2012). Furthermore, curcuminoids and oxymatrine were claimed as potential candidates for keloid therapy for the reason that they were shown to block the phosphorylation of Smad2 and Smad3, respectively (Fan et al., Citation2012; Hsu et al., Citation2010). In Gopinath et al., (Citation2004) incorporated curcumin in a collagen matrix film to improve the wound healing process in rats. This study showed a promising result for topical application of curcumin to enhance wound reduction compared to normal collagen film. The ability to down-regulate the level of Smad2 was also observed in Aneilema keisak extract which was claimed as a novel natural TGF-β signaling blocker (Kim et al., Citation2013). As most of the natural compounds mainly suppress the TGF-β pathway by targeting Smad transducing proteins, Madecassoside was proven to reduce keloid formation through the p38 kinase and the PI3K signaling pathway and substantially inhibit the migration of keloid fibroblast (Song et al., Citation2012). The molecular mechanisms of natural agents against keloids are summarized in . Since most of the natural compounds with a promising keloid therapy effect only exert a minimal effect on normal fibroblast (He et al., 2012; Hsu et al., Citation2010; Tang et al., Citation2011), they can be claimed as a potential keloid treatment alternative with no severe adverse effect. As a result, more studies should focus on the anti-keloid properties in naturally derived compounds.
Prospective
It was evidenced from an extensive number of studies on keloid pathobiology that keloid disease has been of major concern for centuries. Nonetheless, the principal mechanism of keloid formation has yet to be identified. As keloid scarring occurs selectively in humans, only human clinical and in vitro studies are available for studying keloid therapy (Theoret et al., Citation2013). This can be a crucial burden in understanding the underlying etiology of keloid disease. With the heterogeneity of keloid fibroblasts behavior in the population, the results from in vitro studies were often found to be inconsistent. Thus, a standard model to collect keloid fibroblasts from the scar should be used for unbiased cell culture studies. In addition, the binding of Smad complex to the target DNA sequence occurs in a relatively low specificity (Shih & Bayat, Citation2010) allowing the activation of the Smad pathway to result in various responses depending on different cell types. In contrast, the low DNA binding affinity can contribute to complexity in determining the downstream effect from the Smad-responding DNA element. Furthermore, there are many cytokines and growth factors that are reported to be increased in keloid scarring condition compared with normal tissue, for example, interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), platelet-derived growth factor (PDGF), and connective tissue growth factor (CTGF) (Shih & Bayat, Citation2010; Smith et al., Citation2007). However, it was demonstrated that the signaling cascades involving keloids propagation tend to interrupt each other in multidirectional aspects. This contributes to the difficulties in determining whether the alteration of protein expression is the cause or effect of the disease. Therefore, in examining the keloid formation mechanism, multiple pathways should be viewed as a complex regulation not as a linear directional pathway.
Conclusion
Although most reports consistently confirmed that the TGF-β pathway is the main signaling transduction for fibrotic keloid disease, there are other studies supporting keloid treatment with no involvement of the signaling pathway. Many available keloid therapies nowadays focus on treating the keloid at the lesion tissue, instead of targeting the formation mechanism. Thus, the results are often ineffective. Phytochemical compounds were demonstrated to have multiple targets in various signaling cascades, therefore, should be considered as a capable therapeutic option for keloid disease.
Declaration of interest
The authors report no conflict of interest.
This work was supported by The 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship.
Acknowledgements
The authors would like to thank Mr. Keith Kitson for proofreading.
References
- Al-Attar A, Mess S, Thomassen JM, et al. (2006). Keloid pathogenesis and treatment. Plast Reconstr Surg 117:286–300
- Babu M, Diegelmann R, Oliver N. (1989). Fibronectin is overproduced by keloid fibroblasts during abnormal wound healing. Mol Cell Biol 9:1642–50
- Bessonart MN, Macedo N, Carmona C. (2005). High resolution B-scan ultrasound of hypertrophic scars. Skin Res Technol 11:185–8
- Bran GM, Goessler UR, Hormann K, et al. (2009). Keloids: Current concepts of pathogenesis (Review). Int J Mol Med 24:283–93
- Brown JJ, Bayat A. (2009). Genetic susceptibility to raised dermal scarring. Br J Dermatol 161:8–18
- Buscemi L, Ramonet D, Klingberg F, et al. (2011). The single-molecule mechanics of the latent TGF-β1 complex. Curr Biol 21:2046–54
- Butler PD, Longaker MT, Yang GP. (2008). Current progress in keloid research and treatment. J Am Coll Surg 206:731–41
- Chike-Obi CJ, Cole PD, Brissett AE. (2009). Keloids: Pathogenesis, clinical features, and management. Semin Plast Surg 23:178–84
- Chodon T, Sugihara T, Igawa HH, et al. (2000). Keloid-derived fibroblasts are refractory to Fas-mediated apoptosis and neutralization of autocrine transforming growth factor-β1 can abrogate this resistance. Am J Pathol 157:1661–9
- Daian T, Ohtsuru A, Rogounovitch T, et al. (2003). Insulin-like growth factor-I enhances transforming growth factor-β-induced extracellular matrix protein production through the P38/activating transcription factor-2 signaling pathway in keloid fibroblasts. J Invest Dermatol 120:956–62
- Diao JS, Xia WS, Yi CG, et al. (2011). Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch Dermatol Res 303:573–80
- Durani P, Bayat A. (2008). Levels of evidence for the treatment of keloid disease. J Plast Reconstr Aesthet Surg 61:4–17
- Edriss AS, Smrcka V. (2011). Therapy of keloid and hypertrophic scars: A review. Eur J Plast Surg 34:425–36
- Fan DL, Zhao WJ, Wang YX, et al. (2012). Oxymatrine inhibits collagen synthesis in keloid fibroblasts via inhibition of transforming growth factorβ1/Smad signaling pathway. Int J Dermatol 51:463–72
- Gauglitz GG, Korting HC, Pavicic T, et al. (2011). Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol Med 17:113–25
- Gopinath D, Ahmed MR, Gomathi K, et al. (2004). Dermal wound healing processes with curcumin incorporated collagen films. Biomaterials 25:1911–17
- Gupta S, Kalra A. (2002). Efficacy and safety of intralesional 5-fluorouracil in the treatment of keloids. Dermatology 204:130–2
- Halim AK, Emami A, Salahshourifar I, Kannan TP. (2012). Keloid scarring: Understanding the genetics basis, advances, and prospects. Arch Plast Surg 39:184–9
- He S, Liu X, Yang Y, et al. (2010). Mechanisms of transforming growth factor beta(1)/Smad signalling mediated by mitogen-activated protein kinase pathways in keloid fibroblasts. Br J Dermatol 162:538–46
- He S, Yang Y, Liu X, et al. (2012). Compound Astragalus and Salvia miltiorrhiza extract inhibits cell proliferation, invasion and collagen synthesis in keloid fibroblasts by mediating transforming growth factor-β(1)/Smad pathway. Br J Dermatol 166:564–74
- Hosnuter M, Payasli C, Isikdemir A, Tekerekoglu B. (2007). The effects of onion extract on hypertrophic and keloid scars. J Wound Care 16:251–4
- Hsu YC, Chen MJ, Yu YM, et al. (2010). Suppression of TGF-β1/SMAD pathway and extracellular matrix production in primary keloid fibroblasts by curcuminoids: Its potential therapeutic use in the chemoprevention of keloid. Arch Dermatol Res 302:717–24
- Huang C, Murphy GF, Akaishi S, Ogawa R. (2013). Keloids and hypertrophic scars: Update and future directions. Plast Reconstr Surg 1:25–31
- Ikeda K, Torigoe T, Matsumoto Y, et al. (2013). Resveratrol inhibits fibrogenesis and induces apoptosis in keloid fibroblasts. Wound Repair Regen 21:616–23
- Itoh S, ten Dijke P. (2007). Negative regulation of TGF-β receptor/Smad signal transduction. Curr Opin Cell Biol 19:176–84
- Javelaud D, Mauviel A. (2005). Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad signaling downstream of TGF-β: Implications for carcinogenesis. Oncogene 24:5742–50
- Kakar AK, Shahzad M, Haroon TS. (2006). Keloids: Clinical features and management. J Pak Assoc Dermatol 16:162–72
- Kim WS, Lee LS, Bae GY, et al. (2013). Extract of Aneilema keisak inhibits transforming growth factor-β-dependent signalling by inducing Smad2 downregulation in keloid fibroblast. Exp Dermatol 1:69–71
- Koc E, Arca E, Surucu B, Kurumlu Z. (2008). An open, randomized, controlled, comparative study of the combined effect of intralesional triamcinolone acetonide and onion extract gel and intralesional triamcinolone acetonide alone in the treatment of hypertrophic scars and keloid. Dermatol Surg 34:1507–14
- Kontochristopoulos G, Stefanaki C, Panagiotopoulos A, et al. (2005). Intralesional 5-fluorouracil in the treatment of keloids: An open clinical and histopathologic study. J Am Acad Dermatol 52:474–9
- Leask A. (2013). Integrin β1: A mechanosignaling sensor essential for connective tissue deposition by fibroblasts. Adv Wound Care 4:160–6
- Lim IJ, Phan TT, Song C, et al. (2001). Investigation of the influence of keloid-derived keratinocytes on fibroblast growth and proliferation in vitro. Plast Reconstr Surg 107:797–808
- Liu S, Xu SW, Blumbach K, et al. (2010). Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci 123:3674–82
- Lu F, Gao J, Ogawa R, et al. (2007). Biological differences between fibroblasts derived from peripheral and central areas of keloid tissues. Plast Reconstr Surg 120:625–30
- Miyazono K, ten Dijke P, Heldin CH. (2000). TGF-β signaling by Smad proteins. Adv Immunol 75:115–57
- Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. (2013). Critical role of transforming growth factor beta in different phases of wound healing. Adv Wound Care 2:215–24
- Phan TT, Lim IJ, Chan SY, et al. (2004). Suppression of transforming growth factor beta/Smad signaling in keloid-derived fibroblasts by quercetin: Implications for the treatment of excessive scars. J Trauma 57:1032–7
- Sanders KW, Gage-White L, Stucker FJ. (2005). Topical mitomycin C in the prevention of keloid scar recurrence. Arch Facial Plast Surg 7:172–5
- Seifert O, Mrowietz U. (2009). Keloid scarring: Bench and bedside. Arch Dermatol Res 301:259–72
- Shi Y, Massagué J. (2003). Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700
- Shih B, Bayat A. (2010). Genetics of keloid scarring. Arch Dermatol Res 302:319–39
- Shih B, Garside E, McGrouther DA, Bayat A. (2010). Molecular dissection of abnormal wound healing processes resulting in keloid disease. Wound Repair Regen 18:139–53
- Slemp AE, Kirschner RE. (2006). Keloids and scars: A review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr 18:396–402
- Smith JC, Boone BE, Opalenik SR, et al. (2007). Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways. J Invest Dermatol 128:1298–310
- Song J, Xu H, Lu Q, et al. (2012). Madecassoside suppresses migration of fibroblasts from keloids: Involvement of p38 kinase and PI3K signaling pathways. Burns 38:677–84
- Suarez E, Syed F, Alonso-Rasgado T, et al. (2013). Up-regulation of tension-related proteins in keloids: Knockdown of Hsp27, α2β1-integrin, and PAI-2 shows convincing reduction of extracellular matrix production. Plast Reconstr Surg 131:158–73
- Szulgit G, Rudolph R, Wandel A, et al. (2002). Alterations in fibroblast alpha1beta1 integrin collagen receptor expression in keloids and hypertrophic scars. J Invest Dermatol 118:409–15
- Tang B, Zhu B, Liang Y, et al. (2011). Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch Dermatol Res 303:563–72
- Theoret CL, Olutoye OO, Parnell LK, Hicks J. (2013). Equine exuberant granulation tissue and human keloids: A comparative histopathologic study. Vet Surg 42:783–9
- Tuan TL, Zhu JY, Sun B, et al. (1996). Elevated levels of plasminogen activator inhibitor-1 may account for the altered fibrinolysis by keloid fibroblasts. J Invest Dermatol 106:1007–11
- Tucci-Viegas VM, Hochman B, Franca JP, Ferreira LM. (2010). Keloid explant culture: A model for keloid fibroblasts isolation and cultivation based on the biological differences of its specific regions. Int Wound J 7:339–48
- Wakefield LM, Roberts AB. (2002). TGF-β signaling: Positive and negative effects on tumorigenesis. Curr Opin Genet Dev 12:22–9
- Wang XQ, Liu YK, Qing C, Lu SL. (2009). A review of the effectiveness of antimitotic drug injections for hypertrophic scars and keloids. Ann Plast Surg 63:688–92
- Yang GP, Lim IJ, Phan TT, et al. (2003). From scarless fetal wounds to keloids: Molecular studies in wound healing. Wound Repair Regen 11:411–18
- Yu L, Hebert MC, Zhang YE. (2002). TGF-β receptor-activated p38 MAP kinase mediated Smad-independent TGF-β responses. EMBO J 21:3749–59