Abstract
Context: P-selectin is a promising target for inflammatory-related diseases. Polysaccharides are the active ingredients of Sanguisorba officinalis L. (Rosaceae) responsible for its anti-inflammatory activities; however, the molecular mechanism is not clear yet.
Objective: This study evaluates the effects of polysaccharides (SOPs) from Sanguisorba officinalis on their antagonistic function against P-selectin-mediated leukocyte adhesion.
Materials and methods: The antagonistic function of SOPs was investigated by flow cytometry and static adhesion assay at the concentrations of 25 and 100 μg/ml. The dynamic interaction between HL-60 cells and CHO-P cell monolayer treated with SOPs (25 and 100 μg/ml) was analyzed in a parallel plate flow chamber, and quantitatively calculated by ImageJ software (NIH, Bethesda, MD). In vitro protein binding assay was carried out to evaluate the blocking effects of SOPs (25 and 100 μg/ml) on the interaction between P-selectin and PSGL-1.
Results: SOPs-treatment (100 μg/ml) significantly reduced the percentage of HL-60 cells binding to P-selectin (p < 0.01) determined by flow cytometry. In addition, SOPs (25 and 100 μg/ml) markedly blocked the adhesion between HL-60 cells and CHO-P cells under static condition, and the inhibitory rates reached 39.9% and 71.2%, respectively. Compared with the positive control group, SOPs-treatment (25 and 100 μg/ml) significantly reduced the percentage of HL-60 cells rolling on CHO-P cell monolayers by 43.5% and 75.2%, respectively. Protein binding assay showed the interaction between P-selectin and PSGL-1 was significantly blocked by SOPs.
Discussion and conclusion: SOPs possess a significant antagonistic function against P-selectin-mediated leukocyte adhesion, and SOPs could be considered as a promising candidate for amelioration of inflammation-related diseases.
Introduction
The recruitment of leukocytes from circulation to infected tissue, mediated by selectin family of adhesion molecule, is an important step of the innate immune response. P-selectin (CD62P) is stored in Weibel–Palade bodies of endothelial cells (Barthel et al., Citation2007). Once endothelial cell activated, the Weibel–Palade bodies rapidly fuse with the plasma membrane and present P-selectin on the cell surface, promoting the immediate attachment and rapid rolling of leukocytes over endothelial cells (Bernimoulin et al., Citation2003; Zarbock et al., Citation2011). Therefore, P-selectin has been considered as a promising therapeutic target for interfering inflammation-related pathological processes (Woollard & Chin-Dusting, Citation2010).
Sanguisorba officinalis L. (Rosaceae) is a traditional Chinese herbal plant with multiple pharmacological functions, such as anti-infection, anti-inflammatory, anti-cancer, anti-allergic, and neuroprotective activities (Cai et al., Citation2012; Lee et al., Citation2010; Nguyen et al., Citation2008; Yu et al., Citation2001). However, few reports explicitly explain the mechanisms that underlie the anti-inflammatory properties of S. officinalis. Among natural carbohydrate compounds, polysaccharides extracted from medicinal herbs might prove to be the promising candidate for anti-inflammatory agents with antagonistic function against P-selectin (Fei et al., Citation2008; Tong et al., Citation2011). In the present study, we isolated the water-soluble polysaccharides (SOPs) from S. officinalis and evaluated their antagonistic effects against P-selectin-mediated functions, and these results provide theoretical support for the polysaccharides as anti-inflammatory agents
Materials and methods
Plant material
The plant material of S. officinalis was purchased from a local pharmaceutical market in September 2011, and identified by Dr. Yu Hou, School of Life Science, Beihua University. The specimen was deposited in School of Life Science, Beihua University, with voucher specimen number P-2013-00784.
Antibodies and reagents
Recombinant human P-selectin/Fc chimera protein (rhP-Fc) and blocking mAb to P-selectin (9E1) were obtained from R&D Systems (Minneapolis, MN). A non-blocking mAb to P-selectin (AC1.2) was purchased from BD PharMingen (Franklin Lakes, NJ). The monoclonal antibodies for P-selectin (P8G6) and PSGL-1 (PL1) were obtained from Santa Cruz Biotechnology, Santa Cruz, CA. All other chemical reagents used were of analytical grade.
Cell culture
Human promyelocytic leukemia cells (HL-60) and CHO cells were obtained from the Cell Bank of Type Culture Collection of Chinese Academy of Science (Shanghai, China). CHO-P cells stably expressing human P-selectin were obtained by transfecting full-length human P-selectin vector into CHO cells. All cells were grown in IMDM (Gibco, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin in a humidified atmosphere containing 5% CO2 at 37 °C.
Isolation and purification of polysaccharides
After being cleaned and dried, the roots of S. officinalis were ground, and the powders were extracted with distilled water at 80 °C for 3 h. The whole extract was filtered, concentrated, and centrifuged, and then the supernatant was treated with three volumes of ethanol at 4 °C overnight. The crude polysaccharide precipitate was collected by centrifugation, and dried under reduced pressure. The sample was dissolved in distilled water, then frozen at −20 °C, thawed at room temperature and centrifuged to remove insoluble materials. The supernatant was deproteinated by a combination of the proteinase and Sevag method. And then, the supernatant was collected, dialyzed, and lyophilized to obtain water-soluble S. officinalis polysaccharides (SOPs). The yield of SOPs was 8.6% of dried material, and the carbohydrate content of SOPs was 97.7% determined by the phenol–sulfuric acid colorimetric method (Dubois et al., Citation1956). In addition, SOPs had a negative response to the Bradford assay (Bradford, Citation1976) and no absorption at 260 or 280 nm in UV spectrum was detected indicating the absence of protein and nucleic acid.
Flow cytometric assays
HL-60 cells were washed twice with PBS, and then incubated with rhP-Fc or human IgG at 4 °C for 30 min. After washing, the cells were incubated with FITC-labeled goat anti-human IgG and incubated at 4 °C for 30 min. Cells were washed twice and 10 000 cells were counted by flow cytometry. For inhibition experiment, rhP-Fc was pre-incubated with mAb 9E1 or AC 1.2, or SOPs at 37 °C for 30 min.
Static adhesion assay
CHO-P or CHO cells were seeded in 24-well plates overnight to form monolayers. For inhibition experiments, CHO-P monolayers were pre-incubated with mAb 9E1 or AC 1.2, or SOPs at 37 °C for 30 min. HL-60 cells were fluorescently-labeled with 5 µM calcein-AM (Molecular Probes, Leiden, The Netherlands) for 30 min at 37 °C. The fluorescently-labeled HL-60 cells were added to CHO-P or CHO monolayers at room temperature for 1 h. After gently washing, fluorescence was measured by a Molecule Devicer CytoFluor II microplate reader (Sunnyvale, CA) using excitation/emission wavelengths of 485/530 nm.
Laminar flow assays
The inhibitory effect of SOPs on the dynamic interaction between HL-60 cells and CHO-P cell monolayer was analyzed in a parallel plate flow chamber. CHO or CHO-P cells were seeded in 35 mm culture dishes, and incubated overnight to form cell monolayers. Blocking mAb (9E1), non-blocking mAb (AC1.2), and SOPs were added onto the cell monolayers, then incubated at 37 °C for 30 min. The culture dishes were assembled in a parallel-plate flow chamber and mounted onto an inverted microscope. After washing the flow chamber with PBS, HL-60 cells (2 × 106 cells/ml) were perfused through the chamber driven by a syringe pump. After the appearance of HL-60 cells, the fields were randomly selected and recorded via a CCD-camera connected to the inverted microscope. The percent of rolling HL-60 cells and relative rolling speed were calculated by NIH ImageJ software.
Protein binding assay between P-selectin and PSGL-1
Recombinant protein rhP-Fc was incubated with 20 μl of Protein G-Sepharose beads for 1 h at 4 °C, and then the beads were washed three times. HL-60 cells (1 × 107 per sample) were lysed in lysis buffer (50 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Nonidet P-40, 2.5 mM sodium pyrophosphate, 1 mM NaF, 1 mM Na3VO4, 1 mM β-glycerophosphate, 20 μg/ml aprotin/leupeptin, and 1 mM PMSF) on ice for 15 min. After centrifuged at 15 000 × g for 25 min, the supernatants of cell lysates were incubated with 20 μl Protein G-Sepharose beads coated with rhP-Fc for 2 h. The beads were collected by centrifugation and washed with ice-cold lysis buffer. After boiling in the Laemmli sample buffer, the binding proteins (PSGL-1) were detected by immunoblotting. Quantification was performed using NIH ImageJ software.
Statistical analysis
Data shown represent mean ± SEM. Statistical significance was determined by Student's t-test. Statistical analysis was performed using SPSS version 13.0 (SPSS Inc., Chicago, IL). p < 0.05 was considered statistically significant.
Results
SOPs inhibit the interaction between P-selectin and endogenous ligands under static conditions
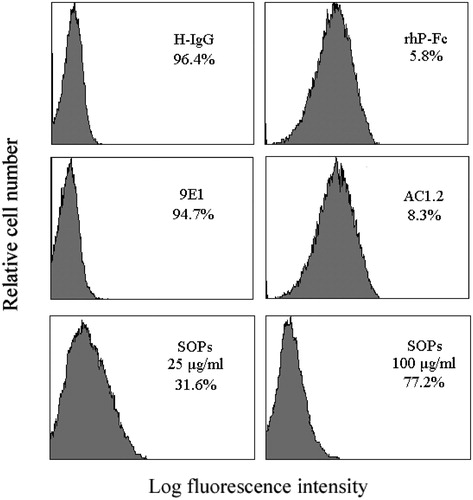
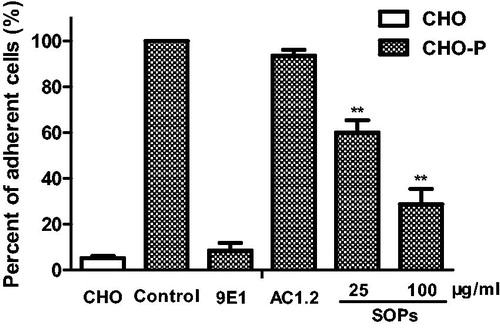
Flow cytometry was performed to evaluate the inhibitory effect on P-selectin binding to endogenous ligands using human promyelocytic leukemia HL-60 cells, which have a high expression of PSGL-1 (endogenous high-affinity P-selectin ligand). As shown in , the binding specificity was confirmed by using P-selectin antibodies, 9E1 (P-selectin functional blocking mAb) significantly reduced the binding of P-selectin to HL-60 cells, while AC1.2 (P-selectin non-blocking mAb) did not impact the binding. SOPs-treatment significantly reduced the percentage of HL-60 cells binding to P-selectin by 31.6% and 77.2% at the concentrations of 25 and 100 μg/ml, respectively. We further tested the inhibitory ability of SOPs in the adhesion of HL-60 cells onto CHO-P cells under static condition. As shown in , SOPs markedly blocked the adhesion between HL-60 cells and CHO-P cells, and the inhibitory rates reached 39.9% and 71.2%, respectively, at the concentration of 25 and 100 μg/ml.
The interaction between P-selectin and endogenous ligands was blocked by SOPs under flow conditions
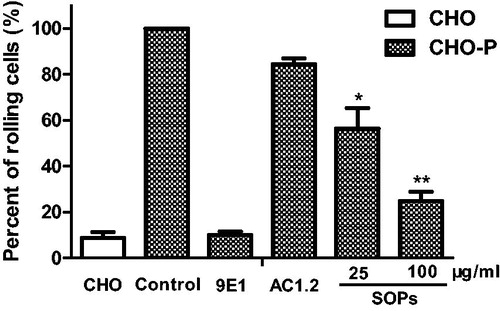
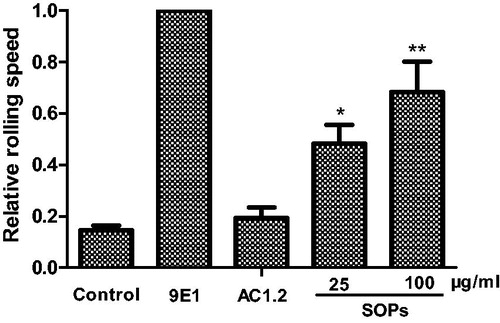
We investigated the inhibitory capacity of SOPs in blocking P-selectin mediated HL-60 cells adhesion to CHO-P cells under dynamic condition. As shown in , SOPs-treatment significantly decreased the percentage of HL-60 cells rolling on CHO-P cell monolayers, compared with the positive control group, the percent of rolling cells reduced by 43.5% and 75.2%, respectively, at the concentrations of 25 and 100 μg/ml, and the inhibitory effect at 100 μg/ml was similar to that treated with mAb 9E1 (p > 0.05). Furthermore, the relative rolling speed of HL-60 cells was calculated with NIH ImageJ software (). Since SOPs blocked the adhesion and rolling of HL-60 cells onto CHO-P cell monolayers, the relative rolling speed of HL-60 cells was significantly increased. These findings suggest that SOPs is effective P-selectin antagonist inhibiting P-selectin-mediated leukocyte adhesion and rolling under physiological conditions.
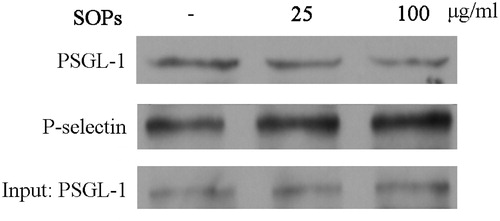
SOPs blocked the interaction between P-selectin and PSGL-1
Protein binding assay was carried out to evaluate the blocking effects of P-selectin and its physiological ligand PSGL-1. As shown in , rhP-Fc showed high affinity to PSGL-1. However, SOPs-treatment significantly eliminated the interaction between rhP-Fc and PSGL-1. These results suggest SOPs directly blocked the interaction between P-selectin and PSGL-1.
Discussion
The recruitment of leukocytes from peripheral blood into infected tissues is essential for the development of an appropriate inflammatory response to injury or infection (Kubes & Ward, Citation2006; Kelly et al., Citation2007). However, the excessive and improper recruitment of leukocytes often leads to serious tissue damage (Geng, Citation2001). Therefore, the recruitment of leukocytes into tissue compartments should be tightly controlled, which has been considered as an effective strategy in relieving inflammation-related diseases (Tong et al., Citation2013). P-selectin, an important cell adhesion molecule, is involved in leukocytes initial attachment and rolling on activated vascular endothelial cells, and plays a critical role in the inflammation response (Geng et al., Citation2004; Simon & Green, Citation2005). Therefore, the intervention in P-selectin-mediated adhesion is an attractive therapeutic strategy for inflammation-related diseases (Woollard & Chin-Dusting, Citation2010).
Polysaccharides are not only energy resources but also play crucial biological roles in many life processes. The structure and pharmaceutical mechanism of bioactive polysaccharides on various diseases have been extensively studied, and more natural polysaccharides with curative effects have been applied in clinical therapies. Previous reports indicate that polysaccharides are promising candidates with significant anti-inflammation effect, and the possible mechanism is related to their antagonizing function to P-selectin. Those polysaccharides, such as, PPS-2 [a polysaccharide fraction from Physalis alkekengi L. (Solanaceae)], p-PGBL [purified polysaccharides from Ginkgo biloba L. (Ginkgoaceae) leaves], and fucoidan (sulfated polysaccharide found mainly in various species of brown algae and brown seaweed), mimicked the selectin ligands and effectively blocked the binding of P-selectin to its native ligands on leukocyte, and then reduced the recruitment of leukocyte into inflammatory sites (Fei et al., Citation2008; Preobrazhenskaya et al., Citation1997; Tong et al., Citation2011).
In the present study, we evaluated the inhibitory capacity of SOPs in P-selectin-mediated leukocyte adhesion by flow cytometry, static adhesion assay, laminar flow assay, and protein binding assay. These results indicated that SOPs exhibited significant blocking capacity on the interaction between P-selectin and its endogenous ligands both under static and flow conditions. P-selectin glycoprotein ligand-1 (PSGL-1), a heavily glycosylated sialomucin specifically expressed on leukocyte, is a vital and high affinity ligand for P-selectin, and the interaction between P-selectin and PSGL-1 mediates the initial adhesion and rolling of leukocyte on the endothelium (Bernimoulin et al., Citation2003; Zarbock et al., Citation2011). A direct evidence, which SOPs blocked the interaction of P-selectin and PSGL-1, was observed by protein binding assay. Taken together, SOPs have been demonstrated their effects in inhibiting leukocyte adhesion and inflammation response. More importantly, SOPs could be considered as promising candidates for amelioration of inflammation-related disease with higher efficiency and lower side-effects.
Declaration of interest
This work was financially supported by the Innovative Team of Plant Chemical Engineering of Jilin Province (No. 20130521022JH), the Key Projects (Nos. 20140311056YY and 20140204039YY) and the Youth Foundation (No. 201201143) of Science and Technology Department of Jilin Province, Chunmiao Incubation Program for University Talents in Jilin Province (No. 2013-169).
References
- Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. (2007). Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Tar 11:1473–91
- Bernimoulin MP, Zeng XL, Abbal C, et al. (2003). Molecular basis of leukocyte rolling on PSGL-1. J Biol Chem 278:37–47
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248–54
- Cai ZB, Li W, Wang HT, et al. (2012). Anti-tumor and immunomodulating activities of a polysaccharide from the root of Sanguisorba officinalis L. Int J Biol Macromol 51:484–8
- Dubois M, Gilles KA, Hamilton JK, et al. (1956). Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–6
- Fei R, Fei Y, Zheng S, et al. (2008). Purified polysaccharide from Ginkgo biloba leaves inhibits P-selectin-mediated leukocyte adhesion and inflammation. Acta Pharmacol Sin 29:499–506
- Geng JG. (2001). Directional migration of leukocytes: Their pathological roles in inflammation and strategies for development of anti-inflammatory therapies. Cell Res 11:85–8
- Geng JG, Chen M, Chou KC. (2004). P-selectin cell adhesion molecule in inflammation, thrombosis, cancer growth and metastasis. Curr Med Chem 11:2153–60
- Kelly M, Hwang JM, Kubes P. (2007). Modulating leukocyte recruitment in inflammation. J Allergy Clin Immunol 120:3–10
- Kubes P, Ward PA. (2006). Leukocyte recruitment and the acute inflammatory response. Brain Pathol 10:127–35
- Lee NH, Lee MY, Lee JA, et al. (2010). Anti-asthmatic effect of Sanguisorba officinalis L. and potential role of heme oxygenase-1 in an ovalbumin-induced murine asthma model. Int J Mol Med 26:201–8
- Nguyen TT, Cho SO, Ban JY, et al. (2008). Neuroprotective effect of Sanguisorbae radix against oxidative stress-induced brain damage: In vitro and in vivo. Biol Pharm Bull 31:2028–35
- Preobrazhenskaya ME, Berman AE, Mikhailov VI, et al. (1997). Fucoidan inhibits leukocyte recruitment in a model peritoneal inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem Mol Biol Int 43:443–51
- Simon SI, Green CE. (2005). Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7:151–85
- Tong HB, Tian D, He ZM, et al. (2013). Polysaccharides from Bupleurum chinense impact the recruitment and migration of neutrophils by blocking fMLP chemoattractant receptor-mediated functions. Carbohydr Polym 92:1071–7
- Tong HB, Wang RF, Liu XM, et al. (2011). Structural characterization and in vitro inhibitory activities in P-selectin-mediated leukocyte adhesion of polysaccharide fractions isolated from the roots of Physalis alkekengi. Int J Biol Macromol 49:210–17
- Woollard KJ, Chin DJ. (2010). P-selectin antagonism in inflammatory disease. Curr Pharm Design 16:4113–18
- Yu T, Lee YJ, Yang HM, et al. (2001). Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE2 production is mediated by suppression of NF-κB and AP-1 activation signaling cascade. J Ethnopharmacol 134:11–17
- Zarbock A, Ley K, McEver RP, Hidalgo A. (2011). Leukocyte ligands for endothelial selectins: Specialized glycoconjugates that mediate rolling and signaling under flow. Blood 118:6743–51