Abstract
Context: Fitzroya cupressoides (Molina) I. M. Johnst. and Austrocedrus chilensis (D. Don) Pic.Serm. & Bizzarri are two Chilean Cupressaceae that are naturally resistant to biodegradation. Secondary metabolites from these species display a variety of biological activities.
Objective: To evaluate the antiproliferative activity of two lignans, a diterpene and a flavonol isolated from A. chilensis and F. cupressoides, to elucidate their cytological effects on P3X murine myeloma cells.
Materials and methods: The antiproliferative activity of yatein, isotaxiresinol, ferruginol, and isorhamnetin was evaluated in vitro using the MTT assay. The effect of yatein at the cellular level, due to its high antiproliferative activity was evaluated. P3X cells treated for 24 h with 12.5 and 25 µg/mL of yatein were also examined at the cytological level using immunofluorescence and scanning and transmission electron microscopy.
Results: Yatein, a lignan isolated from A. chilensis, potentially inhibited P3X murine myeloma cell proliferation, resulting in approximately 75% cell death in response to a 25 µg/mL treatment with the lignan. P3X cells lost membrane integrity at the nuclear and cytoplasmic levels, including organelles, in response to yatein treatment (12.5 µg/mL), and we observed changes in the cytoplasmic organization and distribution of microtubules. The other compounds tested had low activity.
Discussion and conclusions: Yatein is a lignan precursor of podophyllotoxin, a key agent in anticancer drugs. Due to its structural similarities to podophyllotoxin, yatein could have similar cytoplasmic target(s), such as the microtubular apparatus. These findings suggest that yatein may be of potential pharmacological interest and warrants further investigation in human cell lines.
Introduction
Austrocedrus chilensis (D.Don) Pic.Serm. & Bizzarri and Fitzroya cupressoides (Molina) I. M. Johnst are species belonging to the Cupressaceae, and are endemic to the southern portion of South America, naturally being found only in Chile and Argentina. The wood from these plants is renowned for its durability and resistance to biological degradation (Donoso, Citation1993; Rodríguez, Citation2003). There is evidence that the quality of the wood results from the presence of a particular class of secondary metabolite (Donoso et al., Citation2008).
Throughout history, secondary metabolites have provided a rich source of molecules that have biological properties, particularly as anticancer agents with different chemical structures and varied bioactivity (Liu et al., Citation2009). To date, some of the most important studied metabolites are podophyllotoxin and its hemisynthetic derivatives (etoposide, teniposide, and etoposide phosphate), which are particularly interesting due to their ability to inhibit tumor cell proliferation, making them useful in chemotherapy for the treatment of lung cancer (Ayres & Loik, Citation1990; Gordaliza et al., Citation2006; Yang et al., Citation2007).
Previous studies in our laboratory on the secondary metabolites present in extracts of Austrocedrus chilensis and Fitzroya cupressoides led to the isolation and chemical characterization of diterpenes and lignans, their major constituents (Bittner et al., Citation1997; Donoso et al., Citation2008; Flores et al., Citation2001). It is well established that lignans and diterpenes play a role in the defense mechanisms of plants and that they are biologically active, showing antibiotic, antifungal, antiviral, immune stabilizing, antiasthmatic, antioxidant, antineoplastic, gastroprotective, and cytotoxic properties (Adlercreutz, Citation1995; Ayres & Loik, Citation1990; Banskota et al., Citation2003; Charlton, Citation1998; Chen & Thompson, Citation2003; Chen et al., Citation2011; Donoso et al., Citation2008; Donoso-Fierro et al., Citation2009; Raffaelli et al., Citation2002; Theoduloz et al., Citation2011). Therefore, studies that examine their biological characteristics are very important, because they may be useful for pharmaceutical applications.
The present study evaluated the antiproliferative activity of two lignans, a diterpene and a flavonol isolated from A. chilensis and F. cupressoides, to elucidate their cytological effects on P3X murine myeloma cells in vitro using immunofluorescence techniques and scanning and transmission electron microscopy.
Materials and methods
Chemicals and reagents
All solvents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO); Dulbecco’s modification of Minimum Essential Medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin, and l-glutamine were purchased from Lonza Verviers SPRL (Verviers, Belgium). The anti-α-tubulin antibody was purchased from GE Healthcare (Buckingamshire, UK), and fluorescein isothiocyanate conjugated anti-mouse IgG secondary antibody and ProLong® Gold antifade reagent with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) were obtained from Molecular Probes (Eugene, OR).
Plant material
Austrocedrus chilensis (247 m.a.s.l. 41°55′S–71°52′W) and Fitzroya cupressoides (314 m.a.s.l. 41°57′S–71°52′W) were collected in February 2009, by Dr. Donoso-Fierro in the Patagonia North X Region, Province of Llanquihue, Cochamó, Llanada Grande, Chile. Dr. Roberto Rodríguez, a specialist from the Department of Botany at the Universidad de Concepción, identified the plant samples, which were then deposited in the Herbarium of the Universidad de Concepción (CONC.) under the following collection numbers: CONC 169166 (Austrocedrus chilensis) and CONC 169169 (Fitzroya cupressoides).
Extraction and identification
The extraction procedure followed that reported by Donoso et al. (Citation2008). Briefly, 800 g of heartwood samples from F. cupressoides and A. chilensis was manually separated and subsequently chipped and extracted three-times with n-hexane to remove fats. Then, the heartwood of F. cupressoides and A. chilensis was extracted with methanol and methanol/H2O (80:20), respectively. Heartwood extracts were suspended in distilled H2O, and then extracted three times with the same volumes of ethyl acetate. The extracts from each species were filtrates, and they were concentrated under a vacuum at 40 °C. Austrocedrus chilensis and F. cupressoides extracts (30 g) were subjected to column chromatography using silica gel 60. Extensive gradient elution was then employed using n-hexane, n-hexane-ethyl acetate, and ethyl acetate-methanol. The purity of each fraction was determined by thin layer chromatography using silica gel 60 F254 (TLC) and fractions with the same TLC profile pattern were pooled and further purified by rechromatography to yield pure compounds.
The structural identification of the yatein and isotaxiresinol (lignans), ferruginol (diterpene), and isorhamnetin (o-methylated flavonol), was performed using spectroscopic methods, including UV, GC-MS, 1H-NMR, and 13C-NMR and the structures were compared with previously reported data (Cao et al., Citation2009; Hendrawati et al., Citation2011; King et al., Citation1952; Ulubelen et al., Citation1994). Specifically, to analyze the pure compounds, 2–3 mg of each purified fraction was dissolved in 500 μL of deuterated chloroform (CDCl3) and transferred to an NMR tube. The samples were analyzed and recorded using a Varian 400 NMR spectrometer operating at 250 MHz for 1H and 13C nuclei. The chemical shift in δ (ppm) was assigned with a reference to the signal from the residual protons in the deuterated solvent and TMS was used as an internal standard. The mass spectra were determined using a 5972 series Hewlett Packard mass spectrometer (Agilent Technologies, Santa Clara, CA). GC/MS analyses (MS detection at 70 eV) were performed under the following conditions: column, HP-5, 30 m × 0.25 mm × 0.25 µm; temperature, 100 °C isothermal for 5 min, with 10° increments per minute up to 275 °C (held constant for 20 min); split injection, 100:1; injector temperature, 275 °C; detector temperature, 300 °C; and carrier gas, helium.
Murine myeloma cell culture
The murine myeloma P3X63-Ag8.653 cell line, which is derived from Balb/c mice, is a well-known cell model that is widely used by labs worldwide to produce monoclonal antibodies and for assays and biological studies. Due to their relative ease of handling and culturing, these cells currently serve as a cell model for preliminary work in our lab. This myeloma cell line was propagated and maintained in DMEM supplemented with 10% FBS, penicillin/streptomycin, and glutamine (2 mM) in a humidified incubator with an atmosphere of 5% CO2 and 90% humidity at 37 °C. For each treatment, 100 000 cells/well were seeded into a 24-well culture plate containing 1 mL of complete DMEM per well and cells were incubated for 20 h in the absence (control) or presence of the compounds.
Pure compounds were added to obtain final concentrations of 12.5 and 25 µg/mL. Data were calculated as a percentage of control, and all experiments were performed in replicates of 24.
Cell proliferation assay (MTT test)
The MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] system is commonly used to assess the activity of living cells through mitochondrial dehydrogenases (Mosmann, Citation1983). After 24 h of treatment with the compounds, 100 µL from each of the 24 wells was transferred to 96 well/plates. About 10 µL of MTT solution was added to each well, and the cells were incubated for 4 h at 37 °C. The blue MTT-formazan product was solubilized by the addition of 100 µL DMSO and the absorbance was measured at 595 nm using a NOVOstar microplate reader (BMG Labtechnologies, Offenburg, Germany). Antiproliferative activity was expressed as the percentage of viable cells relative to untreated cells (control). The results for each experimental condition represent the mean of 24 replicate wells.
Immunofluorescence microscopy
Among the compounds tested, yatein was selected for evaluation of its effect at the cellular level, due to its high antiproliferative activity. The microtubular component of the murine myeloma cell cytoskeleton was investigated as a possible target of yatein. Untreated P3X cells (control) and P3X cells treated for 24 h with 12.5 and 25 µg/mL yatein were seeded in 75 mL flasks (approximately 3 000 000 cells/flask in 30 mL of DMEM). Samples were collected by centrifugation at 1000 rpm, supernatants were discarded, and the pellets were re-suspended in 100 mM phosphate-buffered saline (PBS), pH 7.4.
Cell suspensions were placed on poly-l-lysine coated coverslips and incubated at room temperature for 60 min. Excess cell suspension was aspirated and the coverslips were rinsed briefly with PBS. Cell-coated coverslips were immersed in ice-cold methanol:acetone (1:1), incubated at −20 °C for 10 min and air-dried.
After two washes in PBS, each coverslip was placed on a drop of blocking buffer (1% bovine serum albumin in PBS) for 30 min at 37 °C to block non-specific binding sites, followed by two washes with PBS. To remove the blocking buffer, each coverslip was held on its edge using forceps, and the blocking buffer was drained onto a sheet of paper. Then, samples were incubated with a primary monoclonal antibody against α-tubulin (diluted 1:400 in blocking buffer) for 1 h at room temperature, using a 60 µL drop of antibody solution for each coverslip. After rinsing three-times in PBS, coverslips were dried and incubated with a fluorescein isothiocyanate conjugated anti-mouse IgG secondary antibody (diluted 1:100 in blocking buffer). To rule out non-specific staining, control samples were prepared without the primary antibody. After three washes in PBS, nuclei were stained by mounting coverslips on the slides using a drop of ProLong® Gold antifade reagent containing DAPI. The images were captured using a computer-assisted image analysis system that includes an Axiophot Microscope equipped with a color video camera (AxioCam MRC, Watertown, MA) and the AxioVision software package (Zeiss, Watertown, MA).
Scanning and transmission electron microscopy
P3X control cells and P3X cells treated for 24 h with 12.5 and 25 µg/mL yatein were processed for electron microscopy.
Approximately 3 000 000 P3X cells were seeded in 30 mL of DMEM in 75 mL flasks. Cells were collected in tubes and centrifuged. The supernatants were discarded, and the pellets were fixed with 4% paraformaldehyde and 5% glutaraldehyde (pH 7.2) in 0.1 M cacodylate buffer for 1 h at 4 °C (Karnovsky, Citation1965). After rinsing overnight in the same buffer, samples were post-fixed in 1% osmium tetroxide in cacodylate buffer for 1 h at 4 °C. After two washings in the same buffer, samples were dehydrated in a series of graded ethanol.
For scanning electron microscopy (SEM), cells were dried according to the critical point method, using CO2 in a Balzers Union CPD 020, sputter-coated with gold in a Balzers MED 010 unit, and observed with a JEOL JSM 5 200 electron microscope (JOEL Inc, Peabody, MA).
For transmission electron microscopy (TEM), samples were fixed and dehydrated as described above and embedded in an Epon mixture resin. Thin sections (50–70 nm) were cut with Reichert Ultracut and LKB Nova ultramicrotomes using a diamond knife, collected on copper grids, stained with uranyl acetate and lead citrate, and observed on a JEOL 1200 EX II electron microscope.
Statistical analysis
Significant differences between the control group and each experimental group were determined using one-way analysis of variance (ANOVA). Differences were considered significant at the 5% probability level.
Results
Antiproliferative activity
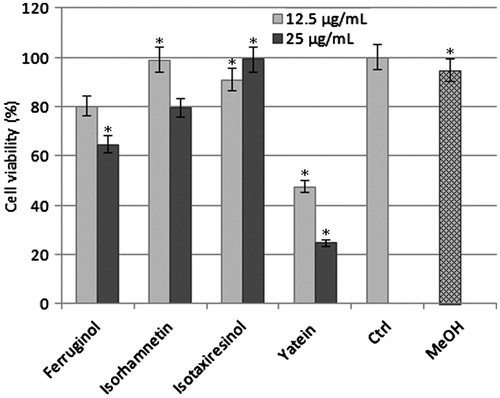
MTT assays were used to test the cytotoxic effects of yatein, isotaxiresinol, ferruginol, and isorhamnetin on P3X cells. Yatein exhibited potent cytotoxicity, inducing 75% cell death at 25 µg/mL after 24 h of treatment (). Furthermore, yatein was toxic toward P3X cells in a dose-dependent manner. In contrast, isotaxiresinol, isorhamnetin, and ferruginol did not significantly affect P3X cell proliferation (ANOVA: p < 0.001, ).
Figure 1. Antiproliferative activity of ferruginol, isorhamnetin, isotaxiresinol, and yatein toward murine myeloma cells. Cells were treated with 12.5 and 25 µg/mL of compounds for 24 h. Pure compounds were solubilized in methanol and added at final concentrations of 12.5 and 25 μg/mL in DMEM. MeOH, methanol treated cells; Ctrl, untreated cells; *Significantly different from control cells (p < 0.001, one-way ANOVA). Cell viability was determined using MTT assays and expressed as the mean ± SD of 24 replies.
Immunofluorescence
To evaluate the effect of yatein at the cytological level, we performed immunofluorescence staining using a monoclonal antibody against α-tubulin. In control cells (untreated cells, ), microtubule organizing centers (MTOCs, arrows) were visible as bright spots within the cytoplasm, whereas microtubules were widely distributed in the cytoplasm. DAPI staining demonstrated that the nucleus was localized in a defined zone in the cytoplasm (). P3X murine myeloma cells treated with low concentrations (12.5 µg/mL) of yatein were affected at the microtubular level, as demonstrated by the disappearance of MTOCs and changes in the organization and distribution of microtubules (). In addition, diffuse cytoplasmic fluorescence was observed, likely due to the presence of unpolymerized tubulin. DAPI staining revealed a reduction in the volume of the nucleus in yatein-treated cells () compared with control cells.
Figure 2. Immunofluorescence analyses of the cytoplasmic effects of yatein in murine myeloma cells (P3X). In control cells, (A) microtubules are widely distributed in the cytoplasm. Microtubule organizing centers (MTOCs) are clearly visible (arrows), and the nucleus is localized in a defined zone of cell cytoplasm (B). In P3X cells treated with 12.5 µg/mL yatein, (C) MTOCs disappeared, the organization and distribution of microtubules were altered (C), and DAPI staining (D) showed a reduction in the volume of the nucleus. Treatment with 25 µg/mL yatein led to the formation of large tubulin aggregates that were visible as brilliant cytoplasmic areas (E), and DAPI staining revealed a reduction in the volume of the nucleus (F). (n) nucleus; bars 10 µm.
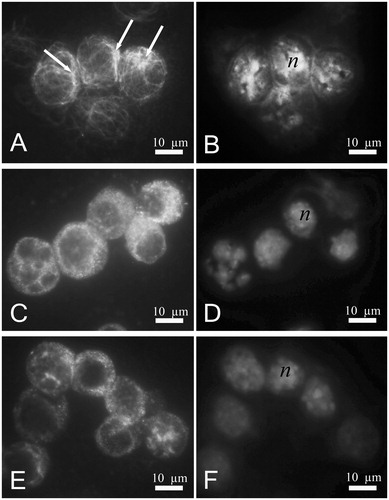
In P3X cells treated with higher concentrations of yatein (25 µg/mL), we observed brilliant cytoplasmic areas, indicating the formation of large tubulin aggregates (). DAPI staining revealed an apparent reduction in the volume of the nucleus compared with control cells ().
SEM and TEM
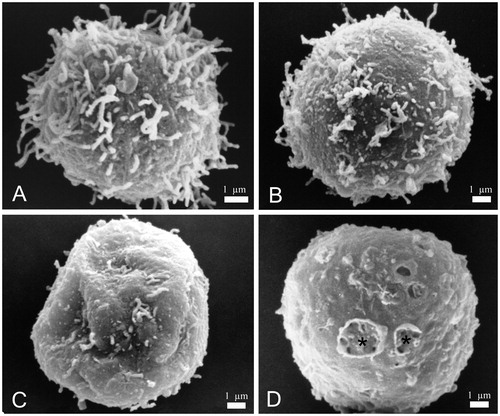
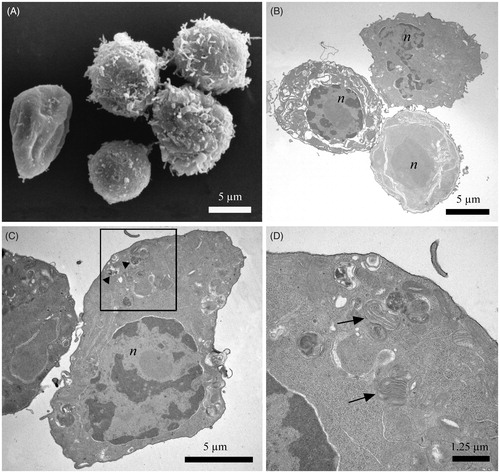
SEM imaging revealed that control cells (untreated cells) had a regular round shape with numerous filamentous extensions on the cell surface (). Cells treated with 12.5 µg/mL yatein increased in size and transitioned to an “egg-like” shape (). Additionally, we observed a consistent loss of filamentous extensions on the cell surface () and the formation of holes in the cell membrane (, stars).
Figure 3. Scanning electron microscopy (SEM) images of P3X control cells and cells treated with 12.5 µg/mL yatein. (A) Control cells showed a regular round shape with characteristic filamentous extensions on the surface. (B–D) Treated cells revealed alterations in their shape (B), loss of the filamentous structures (C), and holes on the surface (D, stars). Bars: 1 µm.
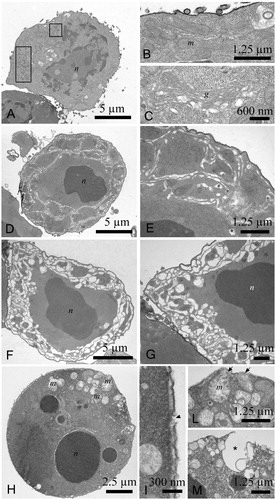
TEM imaging revealed that control murine myeloma cells were round, with a heterochromatic nucleus (). Mitochondria () and the Golgi apparatus () displayed a defined and ordered ultrastructure; viruses were also visible in the cytoplasm as they are residents of this cell line (, rectangles). In contrast, cells treated with 12.5 µg/mL yatein displayed a highly condensed nucleus () and a completely disorganized membrane system in the cytoplasm (); in some cells, the membrane system was highly compromised (). In addition, treated cells displayed nuclear fragmentation and mitochondria with damaged cristae (). We also observed cellular membrane interruptions (, arrowheads) and the extrusion of cytoplasmic materials from the cell (, arrows). Extruded materials located in proximity to cavities (, star) were also noted in SEM observations ().
Figure 4. Transmission electron microscopy (TEM) images of P3X control cells and cells treated with 12.5 µg/mL yatein. (A–C) Control cells were characterized by a round shape, a heterochromatic nucleus, and a mitochondria and Golgi apparatus with a normal ultrastructure; viruses were also visible in the cytoplasm, as they are resident to this cell line (rectangles). (D–M) TEM micrographs evidenced morphological alterations in cells treated with 12.5 µg/mL yatein, including condensation of the nucleus (D) and the complete disorganization of the membrane system in the cytoplasm (D and E). The membrane system was highly compromised in some cells (F and G). Nuclear fragmentation and mitochondria with damaged cristae were also observed (H). Cellular membrane rupture (I, arrowhead) and the extrusion of cytoplasmic materials from the cell were evidenced (L, arrows), and some cavities originated by the extrusion of cytoplasmic material were noted in proximity of the cell surface (M, star). (n) nucleus; (m) mitochondria; (g) Golgi apparatus. Bars: (A) 5 µm, (B) 1.25 µm, (C) 600 nm, (D) 5 µm, (E) 1.25 µm, (F) 5 µm, (G) 1.25 µm, (H) 2.5 µm, (I) 300 nm, (L) 1.25 µm, and (M) 1.25 µm.
As demonstrated by SEM investigations, treatment with 25 µg/mL did not substantially generate additional morphological alterations (data not shown). The membrane system was dramatically altered () by 12.5 µg/mL, as demonstrated by SEM and TEM. Cellular inclusions were subsequently extruded (, arrowheads), and multilamellar bodies were present in the cytoplasm (, arrows).
Figure 5. Electron microscopy images of control P3X cells and P3X cells treated with 25 µg/mL. (A) SEM micrographs showing normal and damaged cells after treatment. (B–D) TEM observation did not reveal new alterations with respect to the previous experiments. Cells displayed a high level of modification to the membrane system and cellular inclusions (B) (arrowheads). Multilamellar bodies (arrows) were also observed in the cytoplasm (C, rectangle). (n) nucleus; bars: (A) 5 µm, (B) 5 µm, (C) 5 µm, and (D) 1.25 µm.
Discussion
The wood extracts of Chilean Cupressaceae are composed of fatty acids, mono and sesquiterpenes, diterpenes, lignans, and phytosterols. The most active compounds are phenolic diterpenes and lignans, and among them matairesinol, podophyllotoxin, yatein, ferruginol, and 6,7-dehydroferruginol (Banskota et al., Citation2003; Donoso et al., Citation2008, Donoso-Fierro et al., Citation2009). Lignans represent one of the most important and interesting classes of biologically active compounds, because lignan molecules can induce cancer cell apoptosis (Ayella et al., Citation2010; Chavez et al., Citation2011; Huong et al., Citation2011; Singh et al., Citation2007; Xu et al., Citation2006, Citation2011). Podophyllotoxin inhibits microtubule assembly of the mitotic apparatus, acting as a competitive inhibitor of the binding site for colchicine to tubulin (Loike et al., Citation1978); this process occurs during mitosis, where the microtubules are rearranged to form the mitotic spindle, which is essential for cell division (Ayres & Loik, Citation1990; Islam & Iskander, Citation2004; Schmidt & Bastians, Citation2007).
Among the compounds tested here, only yatein was active against P3X murine myeloma cells, inhibiting proliferation and killing 75% of cells. The molecular structure of yatein, a lignan previously isolated from A. chilensis heartwood (Donoso et al., Citation2008), is characterized by a podophyllotoxin ring system, which includes a four member fused ring system containing a lactone ring and a pendent aromatic ring (). Studies on the structure–activity relationship (SAR) revealed that the antiproliferative activity of compounds with a podophyllotoxin ring system depends on the presence of rings A and E. Specifically, studies on the structural modification of podophyllotoxin analogues, such as VP-16 (4′-demethylepipodophyllotoxin-4-(4,6-o-ethy-lidene-β-d-glucopyranoside), revealed that removal of the A ring reduced their activity by one-third. The E ring is responsible for activity due to its ability to rotate freely (Long & Casazza, Citation1994).
In contrast, recent studies indicate that yatein is toxic toward DLD-1, CCRF-CEM, and HL-60 cell lines (Chen et al., Citation2011) and significantly suppresses herpes simplex virus type 1 replication in HeLa cells (Kuo et al., Citation2006). Here, we show that yatein affects P3X cells in a dose-dependent manner, and we investigated the possible cytoplasmic targets of yatein.
P3X cells that survived yatein treatment clearly displayed an altered microtubular apparatus, with the disappearance of filamentous structures and diffuse cytoplasmic fluorescence, which is an indicative of tubulin depolymerization. There is no evidence to establish whether yatein can bind microtubules and directly affect the tubulin depolymerization process. However, considering the similarity in the molecular structure between yatein and podophyllotoxin, our data suggest a direct (not unique) effect of yatein on microtubules.
SEM and TEM were used to investigate of the effects of yatein at the ultrastructural level. In response to yatein, we observed the loss of filamentous structures and a change in the size and shape of P3X cells. Inside the cell, we observed deep alterations to the membrane system, with the extrusion of cytoplasmic materials and condensation and fragmentation of the nucleus. Taken together, these results confirm the effect of yatein on different cellular targets and biochemical and physiological cell parameters. Further studies focused on the characterization of the molecular mechanisms underlying the effect of yatein on P3X cells are warranted.
In addition, similar research in other mammalian cell models is being carried out in our laboratory to elucidate the mechanism of action of this interesting lignan molecule.
Conclusions
We tested several purified compounds from Patagonia Cupressaceae plants and yatein consistently inhibited P3X murine myeloma cell proliferation. Other compounds from the same plants did not show similar biological properties. In cells that survived to yatein treatment, the microtubular apparatus was altered, as determined by immunofluorescence techniques, and SEM and TEM analyses displayed changes at the morphological and ultrastructural level. Yatein treatment altered cell shape and damaged the membrane system.
To elucidate its mechanism of cytotoxic action on P3X and other cell lines, the biological activity of yatein and its chemically modified analogs warrants further study.
Acknowledgements
We gratefully acknowledge Dr. Roberto Rodríguez for his assistance with the identification of plant samples and Zenón Rosas for his technical assistance.
Declaration for interest
This work was supported by the Università degli Studi della Tuscia, Bicentennial Program in Science, Technology Project PBCT ACT-38, Basal Financing Project PFB-27, FONDEF-IDEA Project CA12I10142, and the Universidad Católica del Norte and Research Council of the Universidad de Concepción.
References
- Adlercreutz H. (1995). Phytoestrogens: Epidemiology and a possible role in cancer protection. Environ Health Persp 103:103–12
- Ayella A, Lim S, Jiang Y, et al. (2010). Cytostatic inhibition of cancer cell growth by lignan secoisolariciresinol diglucoside. Nutr Res 30:762–9
- Ayres DC, Loike JD. (1990). Lignans: Chemical, Biological and Clinical Properties. Cambridge, United Kingdom: Cambridge University Press
- Banskota AH, Tezuka Y, Nguyen NT, et al. (2003). DPPH radical scavenging and nitric oxide inhibitory activities of the constituents from the wood of Taxus yunnanensis. Planta Med 69:500–5
- Bittner M, Silva M, Becerra J, et al. (1997). Metabolitos secundarios de gimnospermas chilenas familia Cupressaceae. Bol Soc Chil Quim 42:501–5
- Cao X, Wei Y, Itob Y. (2009). Preparative isolation of isorhamnetin from Stigma maydis using high-speed countercurrent chromatography. J Liq Chromatogr Relat Technol 32:273–80
- Charlton JL. (1998). Lignan compounds and 4,4′-dihydroxybiphenyl protect C2C12 cells against damage from oxidative stress. J Nat Prod 61:1447–51
- Chavez KJ, Feng XH, Fanders JA, et al. (2011). Spirocyclic lignans from Guaiacum (Zygophyllaceae) induce apoptosis in human breast cancer cell lines. J Nat Prod 74:1293–7
- Chen JJ, Thompson LU. (2003). Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res Treat 80:163–70
- Chen JJ, Hung HC, Sung PJ, et al. (2011). Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis. Phytochemistry 72:523–32
- Donoso C. (1993). Bosques templados de Chile y Argentina. Variación, estructura y dinámica. Ecología Forestal. Santiago, Chile: Editorial Universitaria
- Donoso CA, Becerra J, Bittner M, et al. (2008). Allelochemicals and natural durability in Chilean Cupressaceae heartwoods. Allelopathy J 21:119–32
- Donoso-Fierro C, Becerra J, Bustos E, Silva M. (2009). Chelating and antioxidant activity of lignans from Chilean woods (Cupressaceae). Holzforschung 63:559–63
- Flores C, Alarcón J, Becerra J, et al. (2001). Extractable compounds of native trees chemical and biological study I: Bark of Prumnopytis andina (Podocarpaceae) and Austrocedrus chilensis (Cupressaceae). Bol Soc Chil Quim 46:61–4
- Gordaliza M, Castro MA, García-Grávalos MD, et al. (2006). Antineoplastic and antiviral activities of podophyllotoxin related lignans. Archi Pharm 327:175–9
- Hendrawati O, Woerdenbag HJ, Michiels PJA, et al. (2011). Identification of lignans and related compounds in Anthriscus sylvestris by LC-ESI-MS/MS and LC-SPE-NMR. Phytochemistry 72:2172–9
- Huong DTM, Hang NTM, Pham VC, et al. (2011). Lignans and other constituents from the roots of the Vietnamese medicinal plant Pseuderanthemum palatiferum. Planta Med 77:951–4
- Islam MN, Iskander MN. (2004). Microtubulin binding sites as target for developing anticancer agents. Mini-Rev Med Chem 4:1077–104
- Karnovsky MJ. (1965). A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27:137A–8A
- King FE, Jurd L, King TJ. (1952). Isotaxiresinol (3′-demethylisolariciresinol), a new lignan extracted from the heartwood of the English yew, Taxus baccata. J Chem Soc 17–24
- Kuo YC, Kuo YH, Lin YL, Tsai WJ. (2006). Yatein from Chamaecyparis obtusa suppresses herpes simplex virus type 1 replication in HeLa cells by interruption the immediate-early gene expression. Antiviral Res 70:112–20
- Liu EH, Qi LW, Wu Q, et al. (2009). Anticancer agents derived from natural products. Mini-Rev Med Chem 9:1547–55
- Loike JD, Brewer CF, Sternlicht H, et al. (1978). Structure–activity study of the inhibition of microtubule assembly in vitro by podophyllotoxin and its congeners. Cancer Res 38:2688–93
- Long BH, Casazza AM. (1994). Structure–activity relationships of VP-16 analogues. Cancer Chemother Pharmacol 34:s26–31
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
- Raffaelli B, Hoikkala A, Leppälä E, Wähälä K. (2002). Enterolignans. J Chromatogr B 777:29–43
- Rodríguez R. (2003). Monografía del Ciprés de la Cordillera (Austrocedrus chilensis), especie con problemas de conservación en Chile. Santiago, Chile: ENDESA
- Schmidt M, Bastians H. (2007). Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Updates 10:162–81
- Singh SK, Shanmugavel M, Kampasi H, et al. (2007). Chemically standardized isolates from Cedrus deodara stem wood having anticancer activity. Planta Med 73:519–26
- Theoduloz C, Pertino MW, Rodríguez JA, Schmeda-Hirschmann G. (2011). Gastroprotective effect and cytotoxicity of carnosic acid derivatives. Planta Med 77:882–7
- Ulubelen A, Topçu G, Eriş C, et al. (1994). Terpenoids from Salvia sclarea. Phytochemistry 36:971–4
- Xu LJ, Huang F, Chen SB, et al. (2006). New lignans and cytotoxic constituents from Schisandra propinqua. Planta Med 72:169–74
- Xu XQ, Gao XH, Jin LH, et al. (2011). Antiproliferation and cell apoptosis inducing bioactivities of constituents from Dysosma versipellis in PC3 and Bcap-37 cell lines. Cell Div 6:1–14
- Yang L, Liu YQ, Tan H, et al. (2007). Design, synthesis, and biological evaluation of novel spin-labeled derivatives of podophyllotoxin as potential antineoplastic agents. Part XII. Nat Prod Res 21:998–1008