Abstract
Context: Artemisia iwayomogi Kitamura (Compositae) has been very widely used for the treatment of acute or chronic hepatitis, jaundice, and gastritis. In the course of our continuing efforts to identify and quantify peroxynitrite scavengers from Compositae plants, A. iwayomogi was used in this study.
Objective: The present study was aimed to identify and quantify the peroxynitrite scavengers of A. iwayomogi.
Materials and methods: Silica gel and ODS were used for column chromatography. The isolated compounds were quantified using an HPLC equipped with a Capcell Pak C18 column (5 μm, 250 mm × 4.6 mm i.d.), and the method was validated for the quality control. Peroxynitrite (ONOO−)-scavenging activities of the compounds and extracts were evaluated on the measurement of highly fluorescent rhodamine 123 converted from non-fluorescent dihydrorhodamine (DHR)-123 under the presence of peroxynitrite.
Results: Based on the spectroscopic evidences, a new compound, 2″-O-caffeoylrutin (2″-O-trans-caffeic acid ester of quercetin 3-O-α-l-rhamnopyranosyl(1 → 6)-β-d-glucopyranoside) was isolated and determined together with patuletin 3-O-glucoside, scopolin, scopoletin, rutin, 3,4-dicaffeoylquinic acid, and chlorogenic acid. All of them were potent peroxynitrite scavengers (IC50 ≤ 1.88 μg/mL).
Discussion and conclusion: The peroxynitrite scavengers were mainly distributed in the EtOAc fraction rather than the ether and BuOH fractions. The 70% MeOH extract exhibited a high peroxynitrite-scavenging activity. Through the validation, the present HPLC method was verified to be sufficiently sensitive, accurate, precise, and stable. Therefore, this method can be used for the quality control of A. iwayomogi.
Introduction
We have studied the isolation and the simultaneous HPLC quantification of mountainous vegetables mainly belonging to the Compositae family. In this study, we studied the isolation and the HPLC analysis of peroxynitrite scavengers from Artemisia iwayomogi Kitamura (Compositae), which has been very widely used for the treatment of acute or chronic hepatitis, jaundice, and gastritis (Kim, Citation1996). Of A. iwayomogi and A. capillaris, the former plant is used more often in Korea to treat hepatic diseases instead of the latter, although the latter is used in China for the same purpose (Wang et al., Citation2012).
Peroxynitrite (ONOO−) refers to a reactive nitrogen species (RNS) that is formed through the reaction between a superoxide anion radical () and a nitric oxide radical (√NO). The excess production of this anion species can cause lipid peroxidation, cytotoxicity, or rapid neurotoxicity, and consequently, hypercholesterolemia, atherosclerosis, obesity, diabetes mellitus, and Alzheimer’s disease (Drel et al., Citation2007; Korda et al., Citation2008; Patcher et al., Citation2005).
It has been pharmacologically reported mainly by Korean researchers that A. iwayomogi inhibits adipogenesis, osteoporosis, hepatic fibrosis, allergy, and inflammation (Choi et al., Citation2012; Ding et al., Citation2010; Han et al., Citation2012; Shin et al., Citation2006). In particular, scopoletin and scopolin inhibit osteoporosis by scavenging reactive oxygen species (Lee et al., Citation2013). As constituents of A. iwayomogi, the isolation of coumarins and aceophenones, in addition to potent peroxynitrite scavengers such as chlorogenic acid, genkwanin and scopoletin, was reported (Ding et al., Citation2010; Kim et al., Citation2004).
Although several compounds have been identified from A. iwayomogi, those compounds have not been so far quantified. Simultaneous quantification is important in the analysis of A. iwayomogi since several compounds contribute to its bioactivity. In the present study, the potent peroxynitrite-scavenging activities of 2″-O-caffeoylrutin, 3,4-dicaffeoylquinic acid (3,4-DQ), and patuletin 3-O-glucoside (fully, patuletin 3-O-β-d-glucopyranoside) in addition to scopoletin and scopolin were observed. In particular, 2″-O-caffeoylrutin has not been isolated from a natural source.
It would be very meaningful to establish the HPLC method for the simultaneous quantification of A. iwayomogi for quality control. The present experiment on the validation employed the calculation of the R2 value of the regression equation, measurement of LOD (limit-of-detection) and LOQ (limit-of-quantification), and tests on intra-day and inter-day variabilities and recovery rates. Using the established method, the quantitative evaluation of seven peroxynitrite scavengers was performed in several extracts and fractions.
Materials and methods
Plant material
The aerial part of Artemisia iwayomogi was collected in Wonju, Gangwon-do, Korea, on August in 2012. This plant was identified by Professor Sang-Cheol Lim (Department of Horticulture and Landscape Architecture, Sangji University). The voucher specimen (natchem#49) was deposited in the Laboratory of Natural Products Chemistry, Department of Pharmaceutical Engineering, Sangji University, Wonju, Korea. This plant was used for extraction after drying and crushing.
Instruments and reagents
Silica gel 60 (70–230 mesh, Fuji Silysia Chemical Ltd., Aichi, Japan) and ODS (12 nm, S-75 µm, YMC-Co., Ltd., Kyoto, Japan) were used for column chromatography as the stationary phase. Reversed and normal thin layer chromatographies (TLCs) were purchased from Merck Co. (Darmstadt, Germany). The Varian HPLC System (Palo Alto, CA) consisted of two Prostar 210 pumps and a Prostar 325 UV–Vis detector equipped with a Shiseido Capcell PAK C18 column (5 µm, 4.6 × 250 mm, Shiseido, Tokyo, Japan). A MetaTherm temperature controller (Varian Inc., Palo Alto, CA) was used to maintain the column temperature. The solvents H2O, CH3CN, and MeOH that were used as the mobile phase were HPLC grades purchased from J.T. Baker Co. (Phillipsburg, NJ). Data of HPLC chromatograms were processed using the Varian Star Workstation.
The melting point (mp) was measured using an Electrothermal digital melting point apparatus (Bibby Scientific Limited, Staffordshire, UK). The UV spectrum and IR spectrum were recorded on a UV-160A UV–Vis recording spectrophotometer (Shimadzu, Kyoto, Japan) and JASCO 4200 FT-IR spectrometer (JASCO, Japan), respectively. The 1H-, 13C-nuclear magnetic resonance (NMR) spectra, 2D-NMR spectra, and HMBC spectrum were taken on a Bruker AM-600 spectrometer (Bruker, Rheinstetten Germany). Tetramethylsilane (TMS) was used as an internal standard to measure the NMR spectra. The ESI-HR-MS was measured using Synapt G-2 (Waters, Milford, MA) with a mode of Q-TOF (quadrupole time-of-flight).
In the assay of peroxynitrite-scavenging activity, the reagents, diethylenetriaminepentaacetic acid (Sigma Chemical Co., St. Louis, MO), dihydrorhodamine (DHR) 123 (Molecular Probes, Eugene, OR), and peroxynitrite (Cayman Chemicals Co., Ann Arbor, MI), were used.
Extraction and fractionation
The plant material, dried and crushed A. iwayomogi (1000 g), was extracted three times with 6 L of MeOH for 6 h. The extracted solution was filtered and evaporated using a rotary evaporator under reduced pressure to give the MeOH extract (133.2 g). This extract was suspended in 1 L distilled water and then partitioned with diethyl ether (Et2O). The Et2O soluble portion was concentrated to dryness to give an Et2O fraction (31.8 g). In the same way, the residual aqueous layer was further partitioned with EtOAc and BuOH three times, successively, to give an EtOAc fraction (14.6 g) and a BuOH fraction (13.6 g), respectively.
Isolation
The BuOH fraction (10 g) was subjected to silica gel column chromatography (ø 55 mm × 38 cm, SiO2 331.2 g) with CHCl3:MeOH:H2O (75:25:10, lower layer) and each 50 mL was collected. After checking the TLC, the aliquots were concentrated to dryness and grouped into 10 fractions (Bu-1–Bu-10).
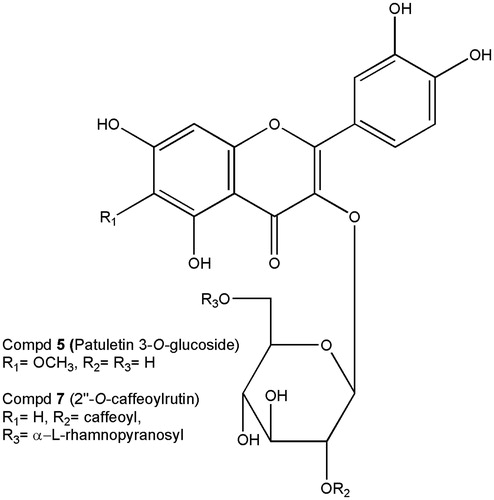
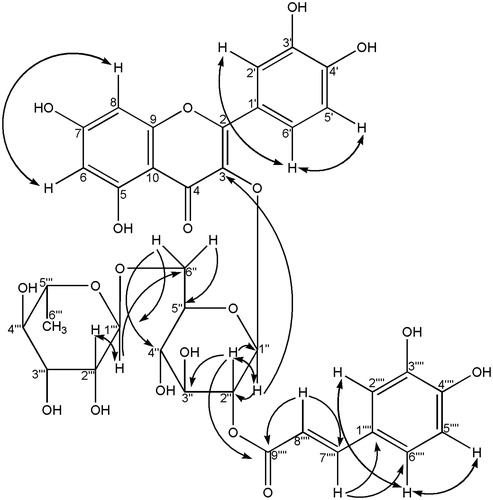
Recrystallization of Bu-3 yielded compound 1. For more purification, Bu-6 was further chromatographed on the ODS column (ø 27 mm × 37.5 cm) with MeOH–H2O (1:1) to yield compound 5. Bu-9 was subjected to ODS column chromatography (ø 27 mm × 37.5 cm) with MeOH (1:1) and each 20 mL was collected. Aliquots corresponding to the eluted volume, 100–160 mL, 180–240 mL, and 740–980 mL, were concentrated, and then crystallized to yield compounds 3, 4, and 7. By comparisons of the 1H- and 13C-NMR data with the literature data, compounds 1, 3, 4, 5, and 7 were identified as scopolin, scopoletin, rutin, and the uncommon compounds of patuletin 3-O-β-d-glucopyranoside and 2″-O-caffeoylrutin, respectively. The chemical structure of these two compounds is shown in . Since compound 7 has not been reported before, its 1H- and 13C-NMR data are shown in . The structure of compound 7 was determined to consist of 2″-O-trans-caffeic acid ester of quercetin 3-O-α-l-rhamnopyranosyl(1 → 6)-β-d-glucopyranoside named 2″-O-caffeoylrutin.
Table 1. 1H- and 13C-NMR assignments of compound 7.
To confirm the moieties of the new compound, compound 7 (20 mg) was refluxed with 5% H2SO4 (10 mL) for 2 h. The reaction mixture was partitioned with EtOAc three times and the organic phase was concentrated and purified by recrystallization from MeOH to afford the aglycones, and then was identified by direct comparison with the authentic specimens on a reversed TLC (MeOH–H2O, 3:2). Furthermore, the aqueous phase was adjusted to pH 7 with BaCO3 and filtered. The filtrate was concentrated to afford the glycones.
Scopoletin: white powder, mp 204–205 °C, 1H and 13C-NMR: literature, Jung et al. (Citation2011).
Scopolin: white powder, mp 216–218 °C, 1H and 13C-NMR: literature, Meng et al. (Citation2009).
Rutin: yellowish needles, mp 194–196 °C, 1H and 13C-NMR: literature, Meng et al. (Citation2009).
Patuletin 3-O-glucoside: yellowish powder, mp 253–254 °C, 1H and 13C-NMR: literature, Wei et al. (Citation2011).
Compound 7 (2″-O-caffeoylrutin)
Yellowish powder, mp 216–218 °C; ESI-HR-MS m/z: 773.1862 (Calcd for C36H36O19: 772.1851); UV λmax (MeOH) nm (log ɛ): 306.0 (4.245), 238.0 (4.218); IR (KBr) cm−1: 3418 (br., O–H), 2926 (aromatic C–H), 1703 (α,β-unsaturated ketone), 1655 (ester), 1606 (aromatic C=C), 1447 (CH2), 1362 (CH3), 1073 (glycosidic C–O); 1H and 13C-NMR (DMSO) ().
Preparation of standard and sample solutions
Standard stock solutions were prepared by dissolving each standard compound in MeOH and storing them at 4 °C in a refrigerator. The working standard solutions were prepared by serial dilutions prior to use. Regression equations were determined by calculation of the peak areas (y) versus the six concentrations (x, μg/mL). Each plant material (5 g) was added with 100 mL of MeOH, EtOH, 70% MeOH, 30% MeOH and H2O was extracted under ultrasonication at 45 °C for 5 h. The extracted solution was filtered through a filter paper (Advantec, Yamanashi, Japan) and adjusted to a volume of 100 mL. And this solution was filtered again through a disposable syringe filter (0.50 μm, Dismic-25JP Advantec, Yamanashi, Japan) before injection into an HPLC system.
HPLC method
The two solvents (A and B solvents) used for the gradient elution were 0.05%-trifluoroacetic acid (TFA) (as solvent A) and 0.05%-TFA in MeOH:CH3CN (60:40) (as solvent B). Gradient elution was programmed at a constant flow rate of 1.0 mL under the following program: (A)/(B) = 85/15 (0 min) → 35/65 (35 min, hold for 5 min) → 0/100 (42 min; hold for 4) → 85/15 (49 min; hold for 6 min). The wavelength of the UV detector was fixed at 254 nm and monitored for 40 min. The column temperature was maintained at 40 °C.
Validation of the HPLC method
The validation experiment for the HPLC method was performed in accordance to the ICH guidelines (International Conference on Harmonization) with respect to the linearity, sensitivity, precision, stability, and accuracy. Linearity was assessed by calculating the R2 values (correlation coefficient) of the regression equation. Sensitivity was evaluated by the calculation of LOD and LOQ values. The values of LOD and LOQ were determined by the signal-to-noise (S/N) ratio, where the S/N ratios were measured three times for LOD, and 10 times for LOQ.
Precision and stability were investigated by the intermediate evaluation method using the intra-day and inter-day variabilities. The tests on the intra-day variability were performed for 24 h and the tests on the inter-day variability were undertaken by injecting five times a day for four consecutive days. The relative standard deviation (RSD) was calculated from the retention times and peak areas, which were considered as measures of the precision and accuracy. To find the accuracy, a recovery test was performed by spiking the standard compounds in the sample solution. Recovery rates were determined by calculating the peak ratio of the spiked solution versus the non-spiked sample solution.
Peroxynitrite-scavenging assay
Peroxynitrite-scavenging activity was performed using a modification of a method that was first described by Kooy et al. (Citation1994). This is the method of monitoring strongly fluorescent rhodamine 123 produced from non-fluorescent DHR 123 under the presence of peroxynitrite. Rhodamine buffer (pH 7.4) is a solution composed of 50 mM sodium phosphate dibasic, 50 mM sodium phosphate monobasic, 90 mM sodium chloride, 5 mM potassium chloride, and 100 μM DTPA. The final concentration of DHR 123 was 5 μM. The activities of the plant extract and fractions were measured at the concentrations of 2, 10, and 50 μg/mL dissolved in dimethyl sulfoxide (DMSO), and the activities of the compounds were at 0.4, 2, and 10 μg/mL.
The final fluorescent intensity was measured with or without 0.3 N NaOH. The fluorescent intensity of oxidized DHR 123 was measured in the excitation and emission states at 480 nm and 530 nm, respectively, using a microplate fluorescence reader FL 500 (Bio-Tek Instruments Inc., Winooski, VT). The peroxynitrite-scavenging activity was determined from the final fluorescent intensity minus the background fluorescence. Data were expressed as the mean ± SEM. l-Penicillamine was used as a positive control.
Results
Identification of the constituents
Five compounds were isolated from the BuOH fraction. Four of the compounds were identified as scopolin, patuletin 3-O-glucoside, scopoletin, and rutin by comparisons of the spectroscopic data of 1H- and 13C-NMR with the literature data (Jung et al., Citation2011; Meng et al., Citation2009; Wei et al., Citation2011). In the IR spectrum of compound 7 (2″-O-caffeoylrutin), two carbonyls were observed, at 1655 cm−1 and 1703 cm−1; the former peak was attributed to an α,β-unsaturated ketone of a typical flavonol skeleton, and the latter peak was considered to be the other carbonyl that probably formed due to an α,β-unsaturated ester.
In the 1H-NMR spectrum, the J value (7.8 Hz) at δ 5.57 assignable to H-1″ indicated that the glycoside linkage of d-glucose had a β-configuration. A methyl group of l-rhamnose was observed at δC 18.2. And the glycoside linkage of l-rhamnose had an α-configuration, identified by a broad singlet shape of the anomeric proton (δH 4.39). The linkage position of l-rhamnose was determined to be the 6-position of d-glucose from the HMBC correlation between δH 4.39 and δC 67.6. Based on the 1H–1H COSY and HMBC spectra, the peaks of H-2″ and C-2″ of d-glucose were observed at δH 4.90 and δC 74.5, respectively.
As shown in and , the peak at δH 4.90 was correlated with the peak at the δC 166.2, indicating that caffeic acid is esterified to C–2″–OH in d-glucose. A closely related compound was also identified by Veitch et al (Citation2011). Although it had identical sugars, aglycone, and acylation position with compound 7, it was not a caffeoyl derivative, but a feruloyl derivative as indicated by the presence of a methoxyl group at δC 56.3.
As shown in , 1H- and 13C-NMR peaks similar to the peaks of rutin and caffeic acid were observed, respectively, but the peaks were not exactly the same as the peaks of the respective compounds, implying that this compound is the caffeic acid ester of rutin. This fact is again supported by [M + H]+ at m/z 773.1862 (Calcd. for C36H36O19: 772.1851). Thus, the structure of compound 7 was determined to consist of the 2″-O-trans-caffeic acid ester of quercetin 3-O-α-l-rhamnopyranosyl (1 → 6)-β-d-glucopyranoside (2″-O-caffeoylrutin). This elucidation was also supported by the hydrolysis result, which produced quercetin, caffeic acid, together with the sugars as observed on TLC by comparing with the authentic specimens.
In addition to those five isolate compounds, chlorogenic acid and 3,4-dicaffeoylquinic acid were also used for the experiment after their identification in the A. iwayomogi extract by co-TLC and co-HPLC. Therefore, seven compounds were used for the analysis and assay.
Optimization and validation of the HPLC method
Four factors affecting the quality of the chromatograms, mobile composition, gradient elution, column temperature, and UV wavelength, were considered to optimize the HPLC method. The solvents H2O, MeOH, and CH3CN were used since they are environment friendly and good for resolution.
The use of H2O as solvent A and MeOH–CH3CN (60:40) as solvent B led to a better peak shape and improved resolution; thus, the two solvents were chosen. About 0.05% TFA was added to both solvents because the addition of TFA produced better chromatograms, probably due to the deionization of the solutes. Gradient elution was employed, so a chromatogram should include peaks that range from high polar to less polar substances. The gradient elution was programmed as in the Materials and Methods section, including using a constant flow rate of 1.0 mL/min and a constant column temperature of 40 °C. Under these conditions, more constant retention times and improved peak separation were observed. Of the three detection wavelengths, 254 nm was more sensitive against a wide range of compounds than the other wavelengths; therefore, 254 nm was used as the detection wavelength.
These optimized HPLC conditions were validated in terms of the linearity, sensitivity, precision, stability, and accuracy. Linearity was established since the R2 values calculated from the data at six concentrations were more than 0.9997 (). The RSD values in the intra-day and inter-day variability tests were over 0.13–0.55% and over 0.48–1.92%, respectively, indicating that the method was sufficiently precise and stable (). The accuracy was also shown from the recovery rates, which were over 97.07–105.01%. Therefore, this method was used to simultaneously analyze seven compounds in the extracts or fractions. Furthermore, it could be used for the quality control of A. iwayomogi.
Table 2. Linearity of standard curves and detection/quantification limits for the standard compounds.
Table 3. Precision and recovery data of the analytes.
Peroxynitrite-scavenging effect of the extracts, fractions, and seven compounds
The peroxynitrite-scavenging effects and the IC50 values of the extracts and fractions are shown in . The effect of the 70% MeOH extract was more potent (IC50, 2.29 µg/mL) than the other three extracts, MeOH-, 30% MeOH, and H2O extracts. Therefore, the 70% MeOH extract from A. iwayomogi would be more suitable as a potent peroxynitrite scavenger than the other extracts tested. This extract could prevent atherosclerosis (Heeba et al., Citation2009) and hepatic (Han et al., Citation2012; Wang et al., Citation2012) or bowel (Singer et al., Citation1996) diseases that can be mediated through the excess production of peroxynitrite.
Table 4. Peroxynitrite-scavenging activity of extracts and fractions of A. iwayomogi.
The MeOH extract was prepared on a large scale, using the reflux method. The IC50 values of the two MeOH extracts prepared by the reflux method and the ultrasonication method were not very different. The IC50 of the MeOH extract by the reflux method was 5.65 μg/mL and that by ultrasonication was 6.21 µg/mL. Among the fractions partitioned from the MeOH extract, the activity of the EtOAc fraction was the highest (IC50, 2.21 μg/mL). These results suggest that the active compounds are mainly distributed in the EtOAc fraction. In addition, the activity of the EtOAc fraction was comparable with the 70% MeOH extract, suggesting that the latter is an extract of A. iwayomogi with potent peroxynitrite-scavenging activity.
Since Kim et al. (Citation2004) reported the peroxynitrite activities of scopolin and scopoletin of A. iwayomogi, we used other compounds, chlorogenic acid, rutin, patuletin 3-O-glucoside, 3,4-dicaffeoylquinic acid, and 2″-O-caffeoylrutin, for the study as well as the two compounds. The activities of the seven compounds ranged from 0.37 to 1.88 μg/mL () and were comparable with that of the positive control (l-penicillamine; IC50: 0.51 μg/mL). Therefore, those compounds were considered to have high activity.
Table 5. Peroxynitrite-scavenging activity of eight compounds identified in A. iwayomogi.
Content of the peroxynitrite scavenger in the extracts and fractions
The contents of peroxynitrite scavengers were generally higher in the 70% MeOH- and MeOH extracts than in the 30% MeOH and H2O extracts ( and ). In the 70% MeOH extract, which had the highest activities, the patuletin 3-O-glucoside (18.15 mg/g) content was the highest; the order of content was patuletin 3-O-glucoside (18.15 mg/g) > scopoletin (5.90 mg/g) > 3,4-dicaffeoylquinic acid (5.59 mg/g) > chlorogenic acid (4.20 mg/g) > rutin (3.33 mg/g) > scopolin (2.62 mg/g) > 2″-caffeoylrutin (1.59 mg/g). Although these phenolic substances were generally higher in the MeOH extract than in the 70% MeOH extract, chlorogenic acid with its high peroxynitrite-scavenging activity (IC50, 0.37 μg/mL) was higher in the 70% MeOH extract.
Figure 3. HPLC chromatograms of five extracts of A. iwayomogi produced by different extraction methods and the solvents. (1: scopolin, 2: chlorogenic acid, 3: scopoletin, 4: rutin, 5: patuletin 3-O-glucoside, 6: 3,4-dicaffeoylquinic acid, and 7: 2″-O-caffeoylrutin).
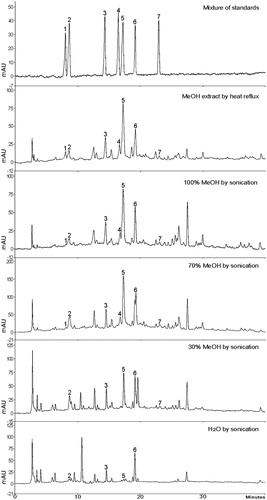
Table 6. Content of analytes in the lyophilized extracts and fractions of A. iwayomogi (mg/g).
Of the fractions, the peroxynitrite scavengers were higher in the EtOAc fraction than the BuOH fraction, while they were very low in the Et2O fraction. However, the content of scopolin was higher in the BuOH fraction (27.45 mg/g) but, it had lower LOQ than the other two fractions.
Discussion
Excess production of peroxynitrite can cause hypercholesterolemia, obesity, diabetes mellitus (Drel et al., Citation2007; Korda et al., Citation2008; Patcher et al., Citation2005), gastric ulcer (Beserra et al., Citation2011), and gastrointestinal disease (Singer et al., Citation1996). Recently, it was reported that ellagic acid, a potent peroxynitrite scavenger, can prevent gastrointestinal diseases such as gastric ulcer (Beserra et al., Citation2011) and Crohn’s disease (Rosillo et al., Citation2011). Furthermore, acetaminophen-induced- or galactosamine/lipopolysaccharide-induced hepatic injuries (Cover et al., Citation2005; Yokoyama et al., Citation2009), which are accepted as models in vivo animal experiments, are closely associated with the production of peroxynitrite. In addition, it was reported that quercetin metabolites are responsible for protection against the hepatotoxic action of peroxynitrite (Yokoyama et al., Citation2009).
The fact that scopoletin contained A. iwayomogi is active in osteoporosis (Lee et al., Citation2013) and hepatic diseases (Atmaca et al., Citation2011) was reported. However, it was revealed in the present study that the five compounds, chlorogenic acid, 3,4-dicaffeoylquinic acid, rutin, patuletin 3-O-glucoside, and 2″-O-caffeoylrutin, are also potent peroxynitrite scavengers, in addition to scopolin and scopoletin, which are already known to be peroxynitrite scavengers from A. iwayomogi. This is the first report to show that the two compounds, patuletin 3-O-glucoside and 2″-O-caffeoylrutin, have potent peroxynitrite-scavenging activities. The three compounds, rutin, patuletin 3-O-glucoside, and 2″-O-caffeoylrutin, have not been isolated before from A. iwayomogi, and in particular, the isolation of 2″-O-caffeoylrutin has not been reported before.
Although several researchers have shown interest in scopoletin or scopolin as the active constituents of A. iwayomogi, it is expected that many compounds will be revealed to be implicated in many hepatic diseases and other diseases. Therefore, we attempted to quantify phenolic compounds with peroxynitrite-scavenging activity using HPLC. Validation of the HPLC analytical method makes it possible to perform quality control of A. iwayomogi using a more reliable method.
The experimentation on the validation included the calculation of the R2 values on regression equations, and LOD and LOQ, and the tests on intra-day- and inter-day variabilities. Through the validation, the present HPLC method was verified to be sufficiently sensitive, accurate, precise, and stable. Therefore, this method can be used for the quality control of A. iwayomogi.
In particular, the 70% MeOH extract exhibited a high peroxynitrite-scavenging activity (IC50, 2.29 μg/mL) and the contents of the constituents with potent peroxynitrite-scavenging activity were determined. The peroxynitrite scavengers were mainly distributed in the EtOAc fraction rather than the other two fractions. Therefore, the 70% MeOH extract could be used for the better use of A. iwayomogi, a famous medicinal herb that is especially known for its hepatoprotective effects.
In conclusion, seven compounds (scopolin, scopoletin, chlorogenic acid, 3,4-dicaffeoylquinic acid, rutin, patuletin 3-O-glucoside, and 2″-O-caffeoylrutin) with peroxynitrite-scavenging activity were simultaneously quantified through the present study, and this HPLC method was validated in terms of the sensitivity, precision, accuracy, and stability for the quality control of A. iwayomogi. The structure of 2″-O-caffeoylrutin was determined using the spectroscopic method. In addition, the 70% MeOH extract could be used for the treatment of diseases that are caused by the excess production of peroxynitrite.
Declaration of interest
The authors report no conflicts of interest. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (# 20100021039).
References
- Atmaca M, Bilgin HM, Obay BD, et al. (2011). The hepatoprotective effect of coumarin and coumarin derivatives on carbon tetrachloride-induced hepatic injury by antioxidative activities in rats. J Physiol Biochem 67:569–76
- Beserra AME, Calegari PI, Souza Mdo C, et al. (2011). Gastroprotective and ulcer-healing mechanisms of ellagic acid in experimental rats. J Agric Food Chem 59:6957–65
- Choi YG, Yeo S, Kim SH, Lim S. (2012). Anti-inflammatory changes of gene expression by Artemisia iwayomogi in the LPS-stimulated human gingival fibroblast: Microarray analysis. Arch Pharm Res 35:549–63
- Cover C, Mansouri A, Knight TR, et al. (2005). Peroxynitrite-induced mitochondrial and endonulease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther 315:879–87
- Ding Y, Liang C, Yang SY, et al. (2010). Phenolic compounds from Artemisia iwayomogi and their effects on osteoblastic MC3T3-E1 cells. Biol Pharm Bull 33:1448–53
- Drel VR, Patcher P, Vareniuk I, et al. (2007). A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur J Pharmacol 569:48–58
- Han JM, Kim HG, Choi MK, et al. (2012). Aqueous extract of Artemisia iwayomogi Kitamura attenuates cholestatic liver fibrosis in a rat model of bile duct ligation. Food Chem Toxicol 50:3505–13
- Heeba G, Moselthy ME, Hassan M, et al. (2009). Anti-atherogenic effect of statins: Role of nitric oxide, peroxynitrite and haem oxygenase-1. Br J Pharmacol 156:1256–66
- Jung HA, Islam MN, Kwon YS, et al. (2011). Extraction and identification of three major aldose reductase inhibitors from Artemisia montana. Food Chem Toxicol 49:376–84
- Kim AR, Zou YN, Park TH, et al. (2004). Active compounds from Artemisia iwayomogi displaying ONOO− scavenging activity. Phytother Res 18:1–7
- Kim TJ. (1996). Plant Resources in Korea. Seoul: Publishing Center of Seoul National University, 262
- Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. (1994). Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med 16:149–56
- Korda M, Kubant R, Patton S, Malinski T. (2008). Leptin-induced endothelial dysfunction in obesity. Am J Physiol Heart Circ Physiol 295:1514–21
- Lee SH, Ding Y, Yan XT, et al. (2013). Scopoletin and scopolin isolated from Artemisia iwayomogi suppress differentiation of osteoclastic macrophage RAW 264.7 cells by scavenging reactive oxygen species. J Nat Prod 76:615–20
- Meng L, Liu R, Sun A, et al. (2009). Separation and purification of rutin and acaciin from the Chinese medicinal herb Herbacirsii by combination of macroporous absorption resin and high-speed counter-current chromatography. J Chromatogr Sci 47:329–32
- Patcher P, Obrosova IG, Mabley JG, Szabo C. (2005). Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem 12:267–75
- Rosillo MA, Sanchez-Hidalgo M, Cárdeno A, Lastra CA. (2011). Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem Pharmacol 82:737–45
- Shin TY, Park JS, Kim SH. (2006). Artemisia iwayomogi inhibits immediate-type allergic reaction and inflammatory cytokine secretion. Immunopharmacol Immunotoxicol 28:421–30
- Singer I, Kawka DW, Scott S, et al. (1996). Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 111:871–85
- Veitch NC, Regos I, Kite GC, Treutter D. (2011). Acylated flavonol glycosides from the forage legume, Onobrychis viciifolia (sainfoin). Phytochemistry 72:423–9
- Wang JH, Choi MK, Shin JW, et al. (2012). Antifibrotic effects of Artemisia capillaris and Artemisia iwayomogi in a carbon tetrachloride-induced chronic hepatic fibrosis animal model. J Ethnopharmacol 140:179–85
- Wei Y, Xie Q, Fisher D, Sutherland IA. (2011). Separation of patuletin-3-O-glucoside, astragalin, quercetin, kaempferol and isorhamnetin from Flaveria bidentis (L.) Kuntze by elution-pump-out high-performance counter-current chromatography. J Chromatogr A 36:6206–11
- Yokoyama A, Sakakibara H, Crozier A, et al. (2009). Quercetin metabolites and protection against peroxynitrite-induced oxidative hepatic injury in rats. Free Radic Res 43:913–21