Abstract
Context: In Africa, Garcinia kola Heckel (Guttiferae) seed is commonly recommended in folklore medicine for the treatment of diabetes and its associated complications.
Objective: The present study evaluated this traditional claim by mechanistic investigation into the effect of G. kola seed administration on renal, hepatic, and testicular oxidative damage in streptozotocin (STZ)-induced diabetic rats.
Materials and methods: Diabetes mellitus was induced in adult male Wistar rats by an intraperitoneal injection of STZ (50 mg/kg). The diabetic rats were thereafter treated orally once per day with G. kola seed (250 mg/kg) and monitored for 14 d. Clinical observations, plasma biochemistry, hormonal profile, oxidative stress indices, sperm characteristics, and histopathological examination of the kidney, liver, and testes were evaluated to monitor treatment-related effects of G. kola seed in STZ-induced diabetic rats.
Results and discussion: Garcinia kola seed administration significantly ameliorated hyperglycemia mediated damage by decreasing the blood glucose level (72.8% and 84.6% on the 7th and 14th post-treatment days, respectively), enhancement of the antioxidant system, inhibition of lipid peroxidation, and improving the architecture of the kidney, liver, and testes in STZ-induced diabetic rats. In addition, G. kola seed intervention restored the kidney and liver function biomarkers, the sperm characteristics as well as the plasma levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, triiodothyronine (T3), and thyroxine (T4) to normal in STZ-induced diabetic rats.
Conclusion: The findings from this investigation provide persuasive scientific support for the traditional use of G. kola seed in the treatment of diabetes and its associated complications.
Introduction
Diabetes mellitus is a non-communicable metabolic disorder characterized by persistent hyperglycemia due to the defects in the secretion or action of endogenous insulin. The complications in diabetic patients could be lethal or non-lethal. Diabetes mellitus is one of the five leading causes of death worldwide. Diabetic nephropathy is a microvascular complication of the diabetic kidney. It is the most common cause of end-stage renal disease in the world, and accounts for a significant increase in morbidity and mortality in patients with diabetes (Ghule et al., Citation2012; Shang et al., Citation2013). The male reproductive function is one of the mammalian systems that is undoubtedly impaired by diabetes (Jelodar et al., Citation2010). Infertility is one of the non-lethal complications in diabetic men. Recently, 51% of subfertility among patients with diabetes mellitus was reported (La Vignera et al., Citation2009). Several pathological and biochemical alterations due to diabetes could lead to deficits in male fertility.
Furthermore, hyperglycemia is responsible for liver dysfunction which is a major secondary complication in diabetic patients (Harrison, Citation2006; Rashid et al., Citation2013). There is increasing evidence from both experimental and clinical studies indicating that oxidative stress plays an important role in the development and progression of diabetes and its complications (Baynes & Thorpe, Citation1999). Indeed, hyperglycemia causes overproduction of mitochondrial superoxides that ultimately leads to oxidative stress in a variety of tissues (Yu et al., Citation2006). The modulation of reactive oxygen species (ROS) concentration often generated in hyperglycemic condition by dietary treatment may contribute to the prevention of diabetic complications (Saravanan & Ponmurugan, Citation2012).
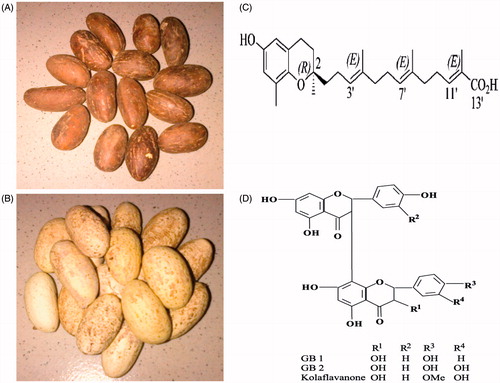
The seed of G. kola Heckel (Guttiferae) () is eaten raw as a stimulant by people because of its bitter astringent taste which is followed by a slight sweetness. Garcinia kola seed is recommended traditionally for the treatment of diabetes (Adaramoye & Adeyemi, Citation2006; Adesuyi et al., Citation2012; Iwu et al., Citation1990). It is also a potent remedy for liver disorders, hepatitis as well as an aphrodisiac and fertility enhancing substance (Iwu, Citation1993; Okoko, Citation2009; Ralebona et al., Citation2012). Traditionally, it is believed that regular consumption of Garcinia kola seed lowers blood glucose levels and improves the complications of diabetes mellitus. Garcinia kola seed contains abundance of natural compounds such as garcinoic acid and kolaviron, which have been isolated and characterized (). Garcinia kola seed is well reported to have several pharmacological effects including anti-hyperglycemic, anti-inflammatory, antioxidant, and antigenotoxic activities (Adedara & Farombi, 2012; Farombi et al., Citation2013; Iwu Citation1993; Mazzini et al., Citation2009; Omage et al., Citation2011; Terashima et al., Citation2002). The hypoglycemic and hypolipidemic effects of G. kola in streptozotocin (STZ)-induced diabetic rats have been reported (Adaramoye & Adeyemi, Citation2006; Duze et al., Citation2012). However, there is paucity of information on its mechanism of action as an ethnomedicinal seed in the treatment of diabetes and its complications.
Figure 1. The unpeeled (A) and peeled (B) ethnomedicinal seeds of Garcinia kola. Chemical structures of garcinoic acid (C) and kolaviron (D).
STZ-induced diabetes mellitus is a well-established experimental model and known to produce pathologic features that resemble diabetes in humans. The acclaimed health beneficial effects of G. kola seed against liver and reproductive disorders in traditional medicine, and its proven ability to suppress oxidative stress in different experimental models of organ toxicity led us to the present mechanistic investigation into its effect on hyperglycemia mediated renal, hepatic, and testicular oxidative damage in STZ-induced diabetic rats.
Materials and methods
Chemicals
STZ, thiobarbituric acid (TBA), 1-chloro-2,4-dinitrobenzene (CDNB), epinephrine, glutathione (GSH), 5′,5′-dithiobis-2-nitrobenzoic acid (DTNB), and hydrogen peroxide were purchased from Sigma Chemical Co. (St Louis, MO). All other reagents were of analytical grade and were obtained from the British Drug Houses (Poole, Dorset, UK).
Authentication and preparation of Garcinia kola stock solution
Garcinia kola seeds were procured from a local vendor in Bodija Market, Ibadan, Nigeria in April 2013. The seeds were authenticated as G. kola seeds at the Department of Botany, University of Ibadan, Ibadan, where a voucher specimen already exists in the herbarium. Subsequently, the fresh G. kola seeds were sliced, air dried, powdered, and stored in a sterile plastic container until needed. Stock solution of G. kola (100 mg/mL) was prepared fresh every other day with corn oil during this study.
Animal model and induction of experimental diabetes in rats
Sexually mature male Wistar rats (6–8 weeks old, 150 ± 6 g) obtained from the Department of Biochemistry, University of Ibadan, Ibadan, Nigeria, were used for this study. The rats were housed in plastic cages placed in a well-ventilated rat house, provided rat chow and water ad libitum and subjected to natural photoperiod of 12 h light/dark. All the animals received humane care according to the criteria outlined in the ‘Guide for the Care and Use of Laboratory Animals’ prepared by the National Academy of Science (NAS) and published by the National Institute of Health. The experiment was performed in accordance with the US NAS guidelines and approval of institutional animal ethics committee. Diabetes was induced with single intraperitoneal administration of STZ (50 mg/kg) in a freshly prepared citrate buffer (0.1 M, pH 4.5) to the rats after an overnight fast. The rats were provided food and water ad libitum after STZ administration. Following 72 h of STZ injection, blood samples were collected through the tail vein and blood glucose levels were determined with an Accu-check glucometer (Roche Diagnostics GmbH, Mannheim, Germany). The rats with fasting blood glucose levels above 250 mg/dL were considered as diabetic and selected for the study.
Experimental protocol
The following four groups of 10 rats each were maintained throughout the investigation period.
Group I: control rats received corn oil orally at 2 mL/kg for 14 d.
Group II: diabetic rats received corn oil orally at 2 mL/kg for 14 d.
Group III: diabetic rats treated with G. kola seed orally at 250 mg/kg for 14 d.
Group IV: control rats treated with G. kola seed orally at 250 mg/kg alone for 14 d.
Fasting blood glucose level and body weight were monitored throughout the treatment period. The dose of Garcinia kola seed was selected from the earlier data published from our laboratory (Farombi et al., Citation2013). Twenty-four hours following the last treatment, the overnight-fasted rats were sacrificed by cervical dislocation, and blood was collected from retro-orbital venous plexus. Plasma samples were separated from blood cells by centrifugation at 3000g for 10 min. Subsequently, plasma samples were stored frozen at −20 °C until the determination of hormones concentrations using Robonic S 2000 ELISA strip reader (Southampton, UK) and biochemical estimations. The kidney, liver, and testes were quickly removed and weighed. Samples from testes were fixed in Bouin’s solution whereas kidney and liver samples were fixed in formalin. The biopses were subsequently paraffin embedded, sectioned, and stained routinely with hematoxylin and eosin for microscopic analysis. The slides were examined with light microscope by pathologists who were blinded to control and treatment groups.
Plasma biochemistry
Plasma activities of aspartate and alanine aminotransferases (AST and ALT) were estimated according to the method of Reitmann and Frankel (Citation1957). Alkaline phosphatase (ALP) was determined according to the recommendation of German Society of Clinical Chemistry (Rec GSCC, Citation1972). The levels of serum bilirubin, urea, and creatinine were determined according to the methods of Jendrassik and Grof (Citation1983), Fawcett and Scott (Citation1960), and Henry (Citation1974), respectively, with kits obtained from Randox Laboratory Limited (London, UK).
Estimation of renal, hepatic, and testicular antioxidant status
The kidney, liver, and testes of the rats were homogenized in 50 mM Tris–HCl buffer (pH 7.4) containing 1.15% potassium chloride, and the homogenate was centrifuged at 10 000 g for 15 min at 4 °C. The supernatant was thereafter collected for the determination of protein concentration by the method of Lowry et al. (Citation1951). Superoxide dismutase (SOD) activity was determined according to the method described by Misra and Fridovich (Citation1972) whereas catalase (CAT) activity was estimated using hydrogen peroxide as a substrate according to the method of Clairborne (Citation1995). Hydrogen peroxide (H2O2) generation was assessed by the method of Wolff (Citation1994). Reduced glutathione (GSH) was estimated at 412 nm according to the method described by Jollow et al. (Citation1974). Lipid peroxidation was quantified as malondialdehyde (MDA) according to the method described by Farombi et al. (Citation2000) and expressed as micromoles of MDA per milligram protein.
Assay of plasma hormones
In accordance with the manufacturer’s instructions, the commercial enzyme immunoassay kits specific for rats were used to assay the plasma concentrations of testosterone (EIA-5179, DRG Diagnostics, Marburg, Germany), FSH (RPN 2560, GE Healthcare, Amersham, UK), and LH (RPN 2562, GE Healthcare, Amersham, UK). The sensitivity of the testosterone assay was 0.07 ng/mL and with negligible cross-reactivity with other androgen derivatives like androstenedione, 5α-dihydrotestosterone, and methyl testosterone. The intra-assay coefficients of variation for the testosterone assay were 4.1%. The sensitivity of LH was 0.06 ng at 80% whereas the FSH sensitivity was 0.05 ng at 98%. The intra-assay coefficients of variation were 3.1% for LH and 3.7% for FSH. The total plasma triiodothyronine and thyroxine concentrations were assayed using the commercial enzyme immunoassay kits (DiaSorin, Sauggia, Italy) according to the manufacturer’s instructions. Intra-assays coefficients of variation for total thyroxine were 2.8–3.6% whereas for total triiodothyronine, 3.2–5.8%. Sensitivity of the assays was 62 pg/mL for total thyroxine and 246 pg/mL for total triiodothyronine. All the samples were assayed on the same day to avoid the inter-assay variation. The total plasma triiodothyronine and thyroxine concentrations were expressed as ng/dL and ug/dL, respectively.
Determination of sperm parameters
The progressive motility of the sperm from the rats was determined according to the method of Zemjanis (Citation1970). Briefly, epididymal sperm was obtained by cutting the cauda epididymis with surgical blades and released onto a sterile clean glass slide. The sperm was then diluted with 2.9% sodium citrate dehydrate solution and thoroughly mixed to assess the sperm progressive motility with the aid of a microscope within 2–4 min of their isolation and data were expressed as percentages. Epididymal sperm number (ESN) was obtained by mincing the caudal epididymis in distilled water and filtering through a nylon mesh. The sperm were counted by a hemocytometer using the improved Neubauer (Deep 1/10 m; LABART, Munich, Germany) chamber according to Pant and Srivastava (Citation2003). A portion of the sperm suspension placed on a glass slide was smeared out with another slide and stained with Wells and Awa’s stain (0.2 g eosin along with 0.6 g fast green were dissolved in distilled water and ethanol in a 2:1 ratio) for morphologic examination. The sperm viability was determined using a stain containing 1% eosin and 5% nigrosine in 3% sodium citrate dehydrate solution according to Wells and Awa (Citation1970). A total of 400 sperm from each rat were used for morphologic examination.
Determination of daily sperm production (DSP) and testicular sperm number (TSN)
DSP was determined using the frozen left testes from the rats according to Joyce et al. (Citation1993). Briefly, after the testes were removed and weighed, they were homogenized for 3 min in 25 mL physiologic saline containing 0.05% (v/v) Triton X-100. Sample aliquots of the 5.5 µL were then placed on the hemocytometer and counted twice at 100 × magnification under a microscope to determine the average number of spermatids per sample. Elongated spermatid nuclei with spermatid characteristic of steps 17–19 of spermatogenesis were counted (Amann et al., Citation1976; Hess, Citation1990). These values were used to obtain the total number of spermatids per testis, and this number was then divided by the testes weight to yield spermatids per gram of testes. Developing spermatids spend 6.1 d in rat. Therefore, the values for the number of spermatids per testis were divided by 6.1 to obtain DSP.
Statistical analysis
Statistical analysis were carried out using one-way analysis of variance (ANOVA) to compare the experimental groups followed by Bonferroni’s test to identify significantly different groups (SPSS for Windows, version 17, SPSS Inc., Chicago, IL). p < 0.05 was considered statistical significance.
Results
Time-course evaluation of body weight and blood glucose level
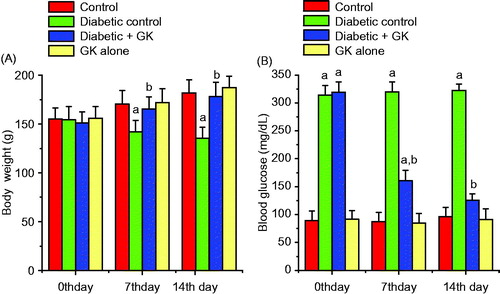
depicts the body weights of the all treatment groups at time zero and on the 7th and 14th days of the study. The body weight of STZ-treated diabetic rats decreased significantly when compared with the control rats. Oral administration of G. kola seed to the diabetic rats preserved the body weight close to that of the control rats. The blood glucose level of the control and experimental rats at time zero and on the 7th and 14th days of the study is presented in . The STZ-treated diabetic rats showed hyperglycemia as evidenced by a significant (p < 0.05) increase in the blood glucose level when compared with the control rats. The increase in the blood glucose level of STZ-induced diabetic rats was 245% above the control rats. However, oral administration of G. kola seed to the diabetic rats resulted in 72.8% and 84.6% blood glucose lowering activity on the 7th and 14th post-treatment days respectively, which were significant when compared with the diabetic control rats. The body weight and the blood glucose level of the rats treated with Garcinia kola seed alone remain unaffected during this study.
Figure 2. Effect of Garcinia kola seed on body weight (A) and blood glucose levels (B) in control and STZ-induced diabetic rats. Each bar represents mean ± SD of 10 rats. (a) Values differ significantly from control (p < 0.05). (b) Values differ significantly from the diabetic control group (p < 0.05).
The relative organ weights
The data on the relative organ weights, presented in , showed that there was a significant decrease in the relative weights of the testes and epididymis whereas those of the liver and kidney were significantly (p < 0.05) increased in the STZ-induced diabetic rats when compared with the control rats. However, the relative weights of these organs were restored to near control following treatment of G. kola seed. The relative weights of the seminal vesicle and prostate gland were not affected in the treatment groups.
Table 1. Effect of Garcinia kola seed on relative organ weights (g/100 g bw) in STZ-induced diabetic rats.
Liver and kidney function parameters
The functionality of the liver was confirmed by assessing the plasma levels of alanine ALT, aspartate AST, and ALP levels whereas renal functionality was confirmed by estimating the plasma urea and creatinine. The markers of hepatic and renal damage are depicted in . A significant (p < 0.05) increase in the ALT, AST, and ALP levels were observed in the plasma of STZ-induced diabetic rats when compared with the control rats. Oral treatment of STZ-induced diabetic rats with G. kola seed significantly restored the levels of these biomarkers to near normalcy. Administration of G. kola seed alone did not affect the liver and kidney function parameters during this study.
Table 2. Effect of Garcinia kola seed on liver and kidney function parameters in STZ-induced diabetic rats.
Plasma hormone concentrations
The influence of G. kola seed administration on plasma concentrations of LH, FSH, testosterone, T3, and T4 in STZ-induced diabetic rats is presented in . Circulatory concentrations of LH, FSH, testosterone, T3, and T4 in STZ-induced diabetic rats were significantly decreased when compared with those of control rats. However, oral treatment with G. kola seed significantly (p < 0.05) reversed the suppression of these hormones by elevating their levels to near control in spite of STZ-induced diabetic state.
Table 3. Effect of Garcinia kola seed on hormonal profile in STZ-induced diabetic rats.
Lipid peroxidation and antioxidant systems
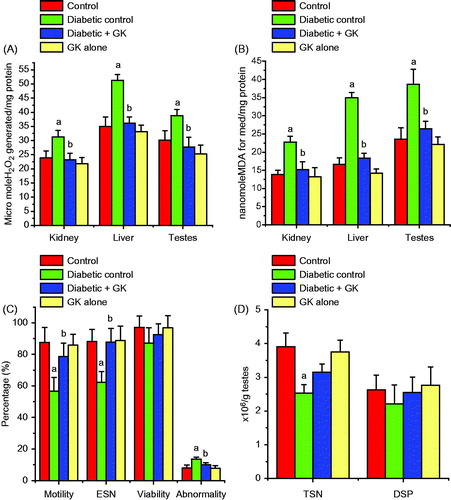
The activities of SOD and CAT and the level of GSH were significantly (p < 0.05) diminished in the kidney, liver, and testes of STZ-treated diabetic rats. Oral administration of G. kola seed ameliorated the decrease in the GSH level () and the activities of these enzymes () and maintained their normalcy in STZ-treated diabetic rats. The levels of H2O2 and MDA, a biomarker of lipid peroxidation, were significant increased in the kidney, liver, and testes of the STZ-treated diabetic rats. Oral administration of G. kola seed significantly ameliorated the diabetes-mediated adverse effects by decreasing the levels of H2O2 and MDA in the investigated organs when compared with the STZ-induced diabetic rats (). The renal, hepatic, and testicular antioxidant status in the rats administered with G. kola seed alone was comparable with the control rats.
Figure 3. Effects of Garcinia kola seed on the GSH level and activities of SOD and CAT in control and STZ-induced diabetic rats. Each bar represents mean ± SD of 10 rats. (a) Values differ significantly from control (p < 0.05). (b) Values differ significantly from the diabetic control group (p < 0.05).
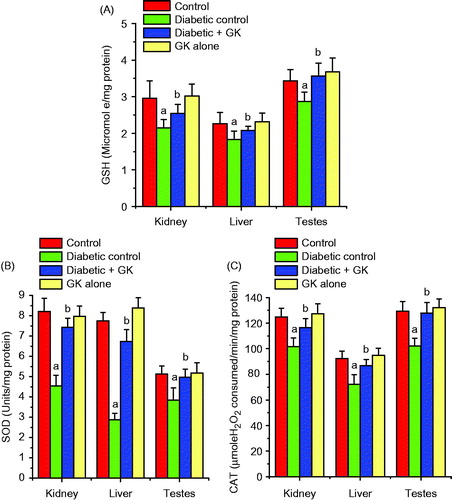
Figure 4. Effects of Garcinia kola seed on H2O2 (A) and MDA (B) levels and spermiogram (C and D) in control and STZ-induced diabetic rats. Each bar represents mean ± SD of ten rats. (a) Values differ significantly from control (p < 0.05). (b) Values differ significantly from the diabetic control group (p < 0.05).
Sperm characteristics
depicts the spermiogram of the experimental rats. The percentage of sperm progressive motility, ESN, and TSN decreased significantly in the STZ-induced diabetic rats (p < 0.05). The percentage of abnormal sperm with morphological defects increased significantly in the STZ-induced diabetic rats when compared with the control rats. The major abnormalities in the STZ-induced diabetic group include tailless heads, curved mid-pieces, and bent mid-pieces. However, except for TSN, the sperm progressive motility and ESN were restored to near control levels following treatment with Garcinia kola seed when compared with the STZ-induced diabetic rats. The decrease in the sperm viability and DSP in STZ induced diabetic rats were not significant when compared with the control rats.
Histopathological observations
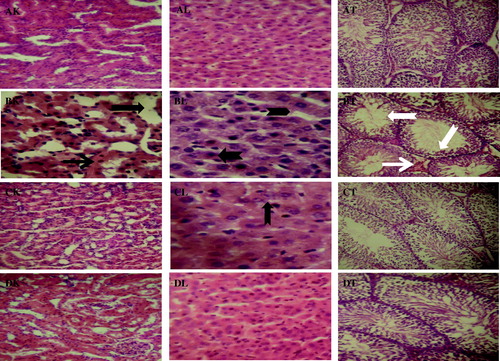
Histopathologic alterations observed with the light microscope in the kidney, liver, and testes sections from the experimental rats are depicted in . The kidney, liver, and testes of control rats (–AT) appeared structurally and functionally normal. Moreover, treatment-related histopathology was observed in the biopsies of kidney, liver, and testes from STZ-induced diabetic rats. The results revealed tubular necrosis (black arrow), interstitial, and periglomerular cellular infiltration by mononuclear cells (line arrow) in the kidney, hepatic tissue showed periportal fatty infiltration (notched arrow), and hydropic degeneration of hepatocytes (chevron) while severe interstitial congestion, edema (white arrow), and decreased germinal epithelium (white notched arrow) of the testes were seen in the STZ-induced diabetic rats (–BT). Conversely, the renal, hepatic, and testicular tissues of STZ-induced diabetic rats administered with G. kola seed appeared structurally and functionally normal but with a very mild renal interstial congestion and a few periportal cellular infiltration in the liver (–CT). The histology of the investigated tissues in the rats administered with G. kola seed alone was comparable with the control rats (–DT).
Figure 5. Histopathology guide of kidney, liver, and testes of control and STZ-induced diabetic rats treated with Garcinia kola seed. The upper panel (AK, AL, and AT) shows representative photomicrographs of kidney, liver, and testes from control rats, respectively. STZ-induced diabetic control rats showed severe histopathologic alterations in kidney, liver, and testes of rats (BK, BL, and BT). Tubular necrosis (black arrow), interstitial, and periglomerular cellular infiltration by mononuclear cells (line arrow), periportal fatty infiltration (notched arrow), hydropic degeneration of hepatocytes (chevron), interstitial congestion and edema (white arrow), and decreased germinal epithelium (white notched arrow). The investigated organs from STZ-induced diabetic rats administered with Garcinia kola seed appeared structurally and functionally normal but with a very mild renal interstial congestion and a few periportal cellular infiltration in the liver (CK, CL, and CT). Normal rats administered with Garcinia kola seed alone showed regular architecture (DK, DL, and DT). Original magnification: 160×.
Discussion
The present study was designed to scientifically scrutinize the ethno-medicinal potential of G. kola seed in the treatment of diabetes by mechanistic investigation of its effect on hyperglycemia mediated renal, hepatic, and testicular oxidative damage in STZ-induced diabetic rats. In the present study, STZ-induced diabetic rats showed significant decrease in the body weight with concomitant marked elevation in the plasma glucose level. The decrease in the body weight in these rats is attributable to excessive breakdown of tissue proteins. The increase in relative liver and kidney weights observed in the present study indicates hypertrophy of the organs. This apparent increase in the relative organ weights of STZ-treated rats is in agreement with the previous observations (Shang et al., Citation2013; Zafar & Naqvi, Citation2010). However, the significant increase in the body weight and the restoration of the blood glucose level to near normal following treatment with the G. kola seed indicates an improvement in the metabolic condition and prevention of tissue damage due to STZ-mediated hyperglycemic state.
It is well known that ROS production is accelerated under chronic hyperglycemia leading to oxidative damage in a variety of tissues. Oxidation of protein sulfhydryl groups by ROS at the local site of generation is associated with morphological alterations of membranes, enzyme inactivation, and cellular dysfunction (Halliwell, Citation2007). The mutually supportive cooperation between SOD, which accelerates the dismutation of endogenous cytotoxic superoxide radicals to H2O2, and CAT, which converts the harmful peroxide radicals into water and oxygen, offers the first line of protection to the cells (Adedara & Farombi, Citation2010; Johnson & Giulivi, Citation2005). Our data showed significant decrease in the renal, hepatic, and testicular SOD and CAT activities in the STZ-treated diabetic rats. The diminution in the SOD and CAT activities observed in the present study may indicate enzyme inhibition resulting from structural changes and loss of enzyme activity due to hyperglycemic condition in the STZ-induced diabetic rats (Cemek et al., Citation2008; Halliwell, Citation2007). However, the recovery of the defense system via increase in the activities of SOD and CAT was observed following administration of G. kola seed to the STZ-induced diabetic rats. Glutathione, a tripeptide containing cysteine with a reactive thiol group, is a potent reductive non-enzymatic antioxidant, which maintains the intracellular redox status against free radicals and peroxides generated by oxidative stress (Adedara & Farombi, Citation2013). It provides secondary line of defense against intracellular deleterious effects of ROS (Pompella et al., Citation2003). The diminution in the GSH level suggests overutilization in the detoxification process in order to cope with oxidative stress in the diabetic rats. However, administration of Garcinia kola seed restored the GSH level in the kidney, liver and testes of STZ-induced diabetic rats, thus suggesting its antioxidative action in the investigated organs.
MDA and 4-hydroxynonenal are the major oxidation products of peroxidized polyunsaturated fatty acids which play significant roles in tissue damage, and thus elevated MDA level is considered an important biomarker of oxidative damage (Demir et al., Citation2011). In the present study, the increased lipid peroxidation, evidenced by elevated MDA level, was accompanied by a concomitant elevation in the H2O2 level in kidney, liver, and testes of STZ-induced diabetic rats. The harmful effects of H2O2 are attributable to their direct oxidizing properties and the indirect activity in which they serve as a source for more deleterious species like hydroxyl radicals and hypochlorous acid (Kohen & Nyska, Citation2002). The decrease in cytotoxic H2O2 and MDA levels observed following administration of G. kola seed indicates the reduction of lipid peroxidation which could be a consequence of enhancement of the antioxidant defense systems in the kidney, liver, and testes of the STZ-induced diabetic rats. The histopathological observations of the kidney, liver, and testes confirmed that the decrease in the antioxidant status with elevated levels of MDA resulted in the oxidative damage in the STZ-induced diabetic rats. The present study revealed tubular necrosis, interstitial, and periglomerular cellular infiltration by mononuclear cells in the kidney, hepatic periportal fatty infiltration with hydropic degeneration of hepatocytes while severe interstitial congestion, edema, and decreased germinal epithelium were seen in the testes in the STZ-induced diabetic rats. However, administration of G. kola seed to the STZ-induced diabetic rats maintained architectures of the investigated organs similar to that of normal control rats.
The aminotransferases (ALT and AST) are localized in peripotal hepatocytes whereas ALP resides in cells lining biliary duct of the liver. These enzymes are known biomarkers of hepatic damage because their serum or plasma activities presumably increase as a result of loss of hepatocytes structural integrity and leakage (Kaplan, Citation1993). Elevated levels of ALT, AST, and ALP in circulation indicate a hepatic injury in the STZ-induced diabetic rats. Damaged hepatocytes result in hyperbilirubinemia due to inability of the liver to conjugate and secrete bilirubin into bile. Elevated levels of plasma creatinine and urea indicate renal dysfunction in STZ-induced diabetic rats. High plasma urea may indicate decreased reabsorption at the renal epithelium whereas high plasma creatinine revealed impairment in the kidneys, particularly for glomerular filtration rate in the STZ-induced diabetic rats. The restoration of all these liver and renal function biomarkers following G. kola seed administration confirmed it as a chemoprotective agent against kidney and liver damage in STZ-induced diabetic rats.
Alterations in the thyroid hormone and insulin levels are the two most frequent endocrine abnormalities which have been observed to exert significant effects on diabetes (Saravanan & Ponmurugan, Citation2012). Thyroid hormone is well known to regulate glucose metabolism. The post-challenge hyperglycemia on the thyroid gland could result in thyrotoxicosis (Loeb, Citation1996). The kidney and thyroid functions are interrelated through several mechanisms (Iglesias & Díez, Citation2009). The kidney regulates the metabolism and elimination of thyroid hormones whereas the kidney is an important target organ for thyroid hormones actions (den Hollander et al., Citation2005). Renal function has been reported to be influenced by the thyroid status in both animal models and human studies (Gopinath et al., Citation2013). In the present investigation, thyroid function was assessed by measuring the plasma levels of the thyroid hormones in the experimental animals. There were significant decreases in T3 and T4 levels as well as in the T3/T4 ratio in the STZ-induced diabetic rats. The observed hypothyroidism in STZ-induced diabetic rats could result in decreased renal blood flow, glomerular filtration rate, tubular function, electrolytes homeostasis and altered kidney structure (Montenegro et al., Citation1996). However, the restoration of T3 and T4 levels to near control following treatment with G. kola seed indicates an improvement in the condition of the STZ-induced diabetic rats.
The present study showed that hyperglycemia mediated male reproductive dysfunction by decreasing the plasma FSH, LH, and testosterone levels in the STZ-induced diabetic rats. Our result is in agreement with earlier reports (Schoeller et al., Citation2012). Increased oxidative stress may result in the malfunctioning of hypothalamus leading to the reduction in gonadotropins production by the pituitary (Muthuvel et al., Citation2006). Testosterone is an important hormone that regulates spermatogenesis and the male phenotype (Nanjappa et al., Citation2012) and also the weight of the testes and the accessory sex organs. The significant decrease in the relative testes weight, sperm progressive motility, ESN, and TSN with elevated abnormal sperm numbers in STZ-induced diabetic rats is attributable to inadequate hormone levels and oxidative stress. The restoration of the hormone homeostasis, relative organ weights, and spermiogram to normal following oral administration of G. kola seed could be attributed to its free radical quenching capacity in the STZ-induced diabetic rats.
Conclusion
Taken together, the findings from the present investigation demonstrated that administration of G. kola seed significantly ameliorated hyperglycemia-mediated renal, hepatic, and testicular oxidative damage in STZ-induced diabetic rats via multiple mechanisms including inhibition of lipid peroxidation, enhancement of the antioxidant system, and preservation of hypothalamus–pituitary–gonadal and thyroid axes. This study provides persuasive scientific support for the traditional use of G. kola seed in the treatment of diabetes and its associated complications.
Declaration of interest
The authors declare that there are no conflicts of interest. This work was supported by the Multidisciplinary Research Grants under the Staff Training and Research Capacity Building Programme of the John D. and Catherine T. MacArthur Foundation Grant (USA) endowment from the University of Ibadan, Nigeria, awarded to Professor E. O. Farombi.
References
- Adaramoye OA, Adeyemi EO. (2006). Hypoglycaemic and hypolipidaemic effects of fractions from kolaviron, a biflavonoid complex from Garcinia kola in streptozotocin-induced diabetes mellitus rats. J Pharm Pharmacol 58:121–8
- Adedara IA, Farombi EO. (2010). Induction of oxidative damage in the testes and spermatozoa and hematotoxicity in rats exposed to multiple doses of ethylene glycol monoethyl ether. Hum Exp Toxicol 29:801–12
- Adedara IA, Farombi EO. (2012). Chemoprotection of ethylene glycol monoethyl ether-induced reproductive toxicity in male rats by kolaviron, isolated biflavonoid from Garcinia kola seed. Hum Exp Toxicol 31:506–17
- Adedara IA, Farombi EO. (2013). Influence of kolaviron and vitamin E on ethylene glycol monoethyl ether-induced haematotoxicity and renal apoptosis in rats. Cell Biochem Function 32:31–8
- Adesuyi AO, Elumm IK, Adaramola FB, Nwokocha AGM. (2012). Nutritional and phytochemical screening of Garcinia kola. Adv J Food Sci Tech 4:9–14
- Amann RP, Johnson L, Thompson DL, Pickett BW. (1976). Daily spermatozoal production, epididymal spermatozoal reserves and transit time of spermatozoa through the epididymis of the rhesus monkey. Biol Reprod 15:586–92
- Baynes JW, Thorpe SR. (1999). Role of oxidative stress in diabetic complications: A new perspective on an old paradigm. Diabetes 48:1–9
- Clairborne A. (1995). Catalase activity. In: Greewald AR, ed. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press, 237–42
- Cemek M, Kaga S, Simsek N, et al. (2008). Antihyperglycemic and antioxidative potential of Matricaria chamomilla L. in streptozotocin-induced diabetic rats. J Nat Med 62:284–93
- Demir E, Kaya B, Soriano C, et al. (2011). Genotoxic analysis of four lipid-peroxidation products in the mouse lymphoma assay. Mutat Res 726:98–103
- den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. (2005). Correlation between severity of thyroid dysfunction and renal function. Clinic Endocrinol 62:423–7
- Duze BN, Sewani-Rusike CR, Nkeh-Chungag BN. (2012). Effects of an ethanolic extract of Garcinia kola on glucose and lipid levels in streptozotocin induced diabetic rats. Afr J Biotech 11:8309–15
- Farombi EO, Adedara IA, Oyenihi AB, et al. (2013). Hepatic, testicular and spermatozoa antioxidant status in rats chronically treated with Garcinia kola seed. J Ethnopharmacol 146:536–42
- Farombi EO, Tahnteng JG, Agboola AO, et al. (2000). Chemoprevention of 2-acetylaminofluorene-induced hepatotoxicity and lipid peroxidation in rats by kolaviron-a Garcinia kola seed extract. Food Chem Toxicol 38:535–41
- Fawcett JK, Scott JE. (1960). Rapid and precise method for the determination of urea. J Clinic Pathol 13:156–8
- Ghule AE, Jadhav SS, Bodhankar SL. (2012). Trigonelline ameliorates diabetic hypertensive nephropathy by suppression of oxidative stress in kidney and reduction in renal cell apoptosis and fibrosis in streptozotocin induced neonatal diabetic (nSTZ) rats. Int Immunopharmacol 14:740–8
- Gopinath B, Harris DC, Wall JR, et al. (2013). Relationship between thyroid dysfunction and chronic kidney disease in community-dwelling older adults. Maturitas 75:159–64
- Halliwell B. (2007). Biochemistry of oxidative stress. Biochem Soc Transact 35:1147–50
- Harrison SA. (2006). Liver disease in patients with diabetes mellitus. J Clinic Gastroenterol 40:68–76
- Henry RJ. (1974). Clinical Chemistry, Principles and Techniques, 2nd ed. Hagerstown (MD): Harper and Row, 525
- Hess RA. (1990). Quantitative and qualitative characteristics of the stages and transitions in the cycle of the rat seminiferous epithelium: Light microscopic observations of perfusion-fixed and plastic-embedded testes. Biol Reprod 43:525–42
- Iglesias P, Díez JJ. (2009). Thyroid dysfunction and kidney disease. Euro J Endocrinol 160:503–15
- Iwu MM. (1993). Handbook of African Medicinal Plants. CRC Press: London, 183–4
- Iwu MM, Igboko OA, Okunji CO, Tempesta MS. (1990). Antidiabetic and aldose reductase activities of Biflavanones of Garcinia kola. J Pharm Pharmacol 42:290–2
- Jelodar G, Khaksar Z, Pourahmadi M. (2010). Endocrine profile and testicular histomorphometry in neonatal rats of diabetic mothers. Veterinarski Arhiv 80:421–30
- Jendrassik LP, Grof P. (1983). Vereinfachte photometrische methoden zur bestimmung des blutbilirubins. Biochem Zeitschr Band 297:81–9
- Johnson F, Giulivi C. (2005). Superoxide dismutases and their impact upon human health. Mol Aspects Med 26:340–52
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. (1974). Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–69
- Joyce KL, Porcelli J, Cooke PS. (1993). Neonatal goitrogen treatment increases adult testes size and sperm production in the mouse. J Androl 14:448–55
- Kaplan MM. (1993). Laboratory tests. In: Schiff L, Schiff ER, eds. Diseases of the Liver, 7th ed. Philadelphia: JB Lippincott, 108–44
- Kohen R, Nyska A. (2002). Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30:620–50
- La Vignera S, Calogero AE, Condorelli R, et al. (2009). Andrological characterization of the patient with diabetes mellitus. Minerva Endocrinol 34:1–9
- Loeb JN. (1996). Metabolic changes in thyrotoxicosis. In: Braverman LE, Utiger RD, Ingbar SH, Werner SC, eds. Werner and Ingbar's the Thyroid: A Fundamental and Clinical Text, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 687–93
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with Folin phenol reagent. J Biol Chem 193:265–75
- Mazzini F, Betti M, Netscher T, et al. (2009). Configuration of the vitamin E analogue garcinoic acid extracted from Garcinia kola seeds. Chirality 21:519–24
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–5
- Montenegro J, Gonzalez O, Saracho R, et al. (1996). Changes in renal function in primary hypothyroidism. Am J Kidney Dis 27:195–8
- Muthuvel R, Venkataraman P, Krishnamoorthy G, et al. (2006). Antioxidant effect of ascorbic acid on PCB (Aroclor 1254) induced oxidative stress in hypothalamus of albino rats. Clin Chim Acta 365:297–303
- Nanjappa MK, Simon L, Akingbemi BT. (2012). The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod 86:1–12
- Okoko T. (2009). In vitro antioxidant and free radical scavenging activities of Garcinia kola seeds. Food Chem Toxicol 47:2620–3
- Omage K, Erifeta OG, Uhunmwangho SE, et al. (2011). Evaluation of hypoglycemic and antioxidative properties of aqueous extract of Garcinia kola seeds in Wistar rats. Curr Res J Biol Sci 3:326–9
- Pant N, Srivastava SP. (2003). Testicular and spermatotoxic effects of quinalphos in rats. J Appl Toxicol 23:271–4
- Pompella A, Visvikis A, Paolicchi A, et al. (2003). The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol 66:1499–503
- Ralebona N, Sewani-Rusike CR, Nkeh-Chungag, BN. (2012). Effects of ethanolic extract of Garcinia kola on sexual behaviour and sperm parameters in male Wistar rats. Afr J Pharm Pharmacol 6:1077–82
- Rashid K, Das J, Sil PC. (2013). Taurine ameliorate alloxan induced oxidative stress and intrinsic apoptotic pathway in the hepatic tissue of diabetic rats. Food Chem Toxicol 51:317–29
- Recommendation of German Society of Clinical Chemistry (Rec. GSCC). (1972). Optimised standard colorimetric methods. J Clinic Chem Clinic Biochem 10:182–3
- Reitmann S, Frankel S. (1957). Colorimetric method for the determination of serum transaminase activity. Am J Clinic Pathol 28:56–68
- Saravanan G, Ponmurugan P. (2012). Antidiabetic effect of S-allylcysteine: Effect on thyroid hormone and circulatory antioxidant system in experimental diabetic rats. J Diabetes Complicat 26:280–5
- Schoeller EL, Albanna G, Frolova AI, Moley KH. (2012). Insulin rescues impaired spermatogenesis via the hypothalamic–pituitary–gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 61:1869–78
- Shang G, Gao P, Zhao Z, et al. (2013). 3,5-Diiodo-l-thyronine ameliorates diabetic nephropathy in streptozotocin-induced diabetic rats. Biochim Biophys Acta 1832:674–84
- Terashima K, Takawa Y, Niwa M. (2002). Powerful antioxidative agents based on garcinoic acid from Garcinia kola. Bioorg Med Chem 10:1619–25
- Wells ME, Awa OA. (1970). New technique for assessing acrosomal characteristics of spermatozoa. J Dairy Sci 53:226–7
- Wolff SP. (1994). Ferrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 233:182–9
- Yu T, Robotham JL, Yoon Y. (2006). Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA 103:2653–8
- Zafar M, Naqvi SN. (2010). Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. Int J Morphol 28:135–42
- Zemjanis R. (1970). Collection and evaluation of semen. In: Zemjanis R, ed. Diagnostic and Therapeutic Technique in Animal Reproduction, 2nd ed. Baltimore (MD): William and Wilkins Company, 139–53
