Abstract
Context: Consumption of medicinal mushrooms for disease prevention and maintaining health has a very long history in Asia. Grifola frondosa (Fr) S.F. Gray (GF) (Meripilaceae) is a medicinal fungus popularly used for enhancing immune systems, lowering blood glucose, and improving spleen, stomach, and nerve functions.
Objective: This study examines the hypoglycemic effects of GF in vitro and in vivo, and analyzes the chemical profiles of its bioactive components.
Materials and methods: In vitro hypoglycemic effects of GF was evaluated enzymatically using α-amylase and α-glucosidase inhibition assays, whereas in vivo study was conducted on high-fat diet fed and streptozotocin (HFD + STZ)-induced hyperglycemic mice. GC-MS was used to determine the chemical profiles of bioactive components.
Results: The non-polar fraction of GF exhibited a stronger anti-α-glucosidase activity (IC50: 0.0332 mg/ml) than acarbose, but its anti-α-amylase activity (IC50: 0.671 mg/ml) was weaker. Oral administration of GF at 600 mg/kg (GF600) significantly lowered the blood glucose, HbA1c, average blood glucose, and serum total cholesterol levels in hyperglycemic mice. Although GF was found to contain mainly oleic acid and linoleic acid, their levels in the fungus were low, suggesting that the effects of GF on HFD + STZ-induced hyperglycemic mice could be due to factors other than these fatty acids.
Conclusion: These results conclude that GF possesses anti-α-glucosidase activity, and hypoglycemic effect in HFD + STZ-induced hyperglycemic mice.
Introduction
Consumption of medicinal mushrooms for disease prevention and maintaining health has a very long history in Asia. Grifola frondosa (Fr) S.F. Gray (GF) (Meripilaceae), a species of Basidiomycetes, is a medicinal fungus commonly known as Wu-gu or Hui-shu-hua. In mainland China, Japan, and other Asian countries, it is popularly consumed as traditional medicines or health foods. Regular consumption of GF is believed to enhance immune systems, to lower blood glucose, and to improve the spleen, stomach, and nerve functions. Extracts and polysaccharides of liquid cultured GF have been shown to possess immunomodulatory (Kodama et al., Citation2004), anticarcinogenic (Kodama et al., Citation2002), antihyperglycemic (Horio & Ohtsuru, Citation2001; Kubo et al., Citation1994; Manohar et al., Citation2002), antihypertensive (Talpur et al., Citation2002), antihyperlipidemic (Fukushima et al., Citation2001), and antioxidative (Zhang et al., Citation2002) activities. α-Glucan of GF fruiting bodies was demonstrated to have antidiabetic activity in type 2 diabetic animals (Lei et al., Citation2007).
Diabetes mellitus is a disease of chronic metabolic disorder and is characterized by high blood glucose level. Among the many therapies, decreasing postprandial hyperglycemia is considered to be the best therapeutic approach (Park et al., Citation2013; Toeller, Citation1994). It is achieved through retarding the absorption of glucose by inhibiting activities of the carbohydrate hydrolyzing enzymes (α-amylase and α-glucosidase) in the digestive tract. At present, drugs used clinically with such action are acarbose and miglitol; however, they are known to cause adverse effects (Chakrabarti & Reeba, Citation2002). Therefore, the search for better and safer hypoglycemic agents from natural resources remains an important area of active research.
In many cultures, diabetes has been treated with extracts of medicinal plants based on the knowledge of folk medicine. Studies have shown that plant-based bioactive components possessed significant anti-diabetic activity (Lui et al., Citation1999; Takahashi & Miyazawa, Citation2012; Zheng et al., Citation2011), and some of them were even more effective than clinically hypoglycemic agents (Malviya et al., Citation2010). In this study, the main objective was to examine the hypoglycemic effects of G. frondosa in vitro and in vivo, as well as analyzing the chemical profiles of its bioactive components.
Materials and methods
Chemicals
Acarbose, streptozotocin (STZ), fatty acid standards [(palmitic acid (C16:0), palmitoleic acid (C16:1n-7), heptadecanoic acid (C17:0), stearic acid (C18:0), oleic acid (C18:1n-9), linoleic acid (C18:2n-6) and α-linolenic acid (C18:3n-3) of purity ≥99%], α-amylase (E.C. 3.2.1.1), and α-glucosidase (EC 3.2.1.20) were purchased from Sigma-Aldrich Co. (St Louis, MO). All other chemicals used were of analytical grade.
Preparation of G. frondosa extract
Grifola frondosa (GF) fruiting bodies were obtained from Kang Jian Biotech Co., Ltd. (Nantou, Taiwan). The authenticity of the species was confirmed by Prof. Airong Song, Qingdao Agricultural University, Shandong, China. The culture specimen (No.: KJ-GF-10-1) was deposited at Kang Jian Biotech Co., Ltd. The GF materials were dried and then ground into powdered form, followed by subjecting to n-hexane extraction. In brief, 3 kg of dried GF powdered materials were extracted with 10 l of n-hexane under ambient temperature for 2 weeks. The filtrates were filtered and concentrated with a rotary evaporator under reduced pressure at 40 °C. The yield of concentrated n-hexane extract, non-polar fraction of GF, was 1.87%. It was collected in a brown bottle and kept at 4 °C until use.
Assays of digestive enzyme inhibition
The α-amylase and α-glucosidase inhibition assays were conducted according to Apostolidis and Lee (Citation2010). In each of the assay, 50–500 μl of GF were taken to react with the respective reagents, followed by measuring the absorbance of the reaction mixtures at 540 nm for anti-α-amylase activity, and at 405 nm for anti-α-glucosidase activity. Acarbose was used as a positive control.
Animals
Male mice (strain C57BL/6J) were obtained from the National Laboratory of Animal Breeding and Research Center (Taipei, Taiwan). They were housed under constant environmental conditions of temperature and illumination (light between 7:30 a.m. and 7:30 p.m.). Water and standard chow diet were made available ad libitum.
Experimental design
After a week of adaptation, the 6 week old mice were randomly divided into five groups, with eight animals per group. The control group was fed on standard chow diet containing 5% fat (Young Li Co., Ltd., Taipei, Taiwan), whereas the hyperglycemia groups (a total of four groups) were fed on high-fat diet (HFD) containing 20% fat supplemented with 0.5% cholesterol and 0.1% cholic acid for 2 weeks. After receiving a single intraperitoneal injection of freshly prepared STZ (35 mg/kg), animals were further given HFD. Among the four STZ treated groups, one group received no treatment (HFD), one treated with 40 mg/kg acarbose (Acar), one received 300 mg/kg of GF (GF300) and the other given 600 mg/kg of GF (GF600); these treatments were carried out for 1 week. Food intake was recorded every day and the body weight was measured weekly.
At the end of experiment, all animals were fasted for 12 h, followed by subjecting to blood collection from the posterior vena cava under anesthetized condition. After blood sampling, liver was immediately excised, weighed, and stored at −80 °C for further biochemical analyses. The animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the University.
Preparation of tissue homogenate
To prepare the tissue homogenate, frozen tissues were homogenized in four volumes of ice-cold 20 mM Tris-HCl (pH 7.4) containing 0.15 M KCl using the Potter–Elvehjem homogenizer with a Teflon pestle. The homogenates were centrifuged at 3200 × g for 20 min at 4 °C to obtain the supernatant for use in biochemical analyses.
Measurement of biochemical parameters
Blood glucose levels were determined by the glucose oxidized method (Sigma glucose assay kit 510-A; Sigma-Aldrich Co., St Louis, MO). Glycated hemoglobins (HbA1c) were estimated by Diagnostic kits (BioSystems, Costa Brava, Barcelona, Spain). The average blood glucose (ABG) was estimated using the standard technique of linear interpolation (DCCT, Citation1987). Blood insulin levels were determined by an Insulin ELISA kit (Seikagaku Industries, Tokyo, Japan). The serum triglyceride (TG) and total cholesterol (TC) levels were determined using Randox biochemical assay kits (Laboratories Ltd., Lakewood, CA,). The level of malondialdehyde (MDA) indicating of lipid peroxidation in serum was measured by the thiobarbituric acid-reactive method (Kimura et al., Citation1981) whereas activity of liver glutathione peroxidase (GPx) was measured by assay kits (Enzo Life Science, Farmingdale, NY). The protein concentration of tissue homogenates was measured by the method of Lowry et al. (Citation1951).
Gas chromatography–mass spectrometry (GC–MS)
GC–MS analysis was performed using an Agilent 5890 gas chromatograph interfaced with an Agilent 5972 mass spectrometer (Agilent, Palo Alto, CA). Helium was used as the carrier gas with flow rate set at 1 ml/min. A split injector (the split ratio being 1:15) at 220 °C was used to inject the sample (1.0 μl) into a capillary column (DB-5MS, 30 m × 0.25 mm I.D., 0.25 μm film thickness, Agilent, Palo Alto, CA). Trimethylsilyl (TMS) esters of fatty acids were separated with the following oven program: (a) initially 100 °C for 1 min; (b) temperature was increased at a rate of 40 °C/min up to 180 °C; (c) increased at a rate of 2 °C/min up to 200 °C; (d) maintained at 200 °C for 6 min; (e) increased at a rate of 20 °C/min up to 280 °C; (e) maintained at 280 °C for 15 min. The transfer line was maintained at 220 °C. Mass spectra of m/z 50–550 were collected by full scan mode. The solvent delay time was 6 min. The ion source temperature was 220 °C with the electron energy at 70 eV.
Statistical analysis
Results were expressed as mean ± standard error of mean (SE). Statistical differences between treatments were determined by Student’s t-tests. p < 0.05 was considered as statistical significance.
Results
Inhibitory effects on digestive enzymes
Based on IC50 values, results showed that GF possessed weaker anti-α-amylase activity (IC50: 0.671 mg/ml) but stronger anti-α-glucosidase activity (IC50: 0.0332 mg/ml) than acarbose ().
Table 1. Inhibitory concentrations (IC50) of non-polar fraction of G. frondosa (GF) and acarbose on α-glucosidase and α-amylase activities.
Body weight gain
Results showed that over the 3-week period, no obvious difference was noted on the body weight gain between mice fed standard diet and HFD + STZ (). However, animals receiving treatments appeared to have a lower weight gain. Compared with the HFD + STZ group, the change in body weight of hyperglycemic animals receiving GF300 and GF600 was 5.4% and −4%, respectively; a significant lesser body weight gain was noted on the HFD + STZ + GF600 animals. A similar trend in weight gain was also noted in HFD + STZ + Acar animals.
Table 2. Effects of non-polar fraction of G. frondosa (GF) and streptozotocin on the body weight gain.
Blood glucose, HbA1c, insulin, and average blood glucose levels
Compared with other treatments, animals receiving HFD + STZ had a significant higher blood glucose level (). However, it was significantly reduced after GF600 treatment but not the others. Besides GF600 treatment, there was no difference in the levels of HbA1c and ABG between groups. Acarbose and GF treated groups showed an increase level of insulin.
Table 3. Effects of non-polar fraction of G. frondosa (GF) on lipid profile and hyperglycemic indicators in high-fat diet fed and streptozotocin-induced mice.
Serum TG and TC levels
The effect of GF on serum TG and TC in hyperglycemic animals is summarized in . Results showed that at the end of experiment, HFD + STZ group had a 1.7-fold higher TC level than the control group. Although a trend of decrease in the TC level was noted in animals receiving GF and acarbose, only GF600 showed a significant reduction in the TC level.
Serum MDA contents and liver GPx activity
Compared with the control group, HFD + STZ treatment showed a significant lower GPx activity and a higher MDA concentration (). However, treatment with either GF or acarbose did not improve the effects of HFD + STZ-induced oxidative stress markers, suggesting that these test samples possessed poor antioxidant activity.
Chemical profiles of G. frondosa extract
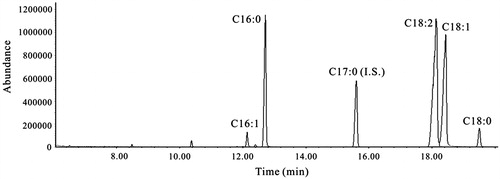
GC–MS analysis showed that GF contained mainly oleic acid and linoleic acid (), suggesting that factors other than these unsaturated fatty may have contributed to the hypoglycemic effect of GF.
Figure 1. A representative total ion chromatogram of non-polar fraction of G. frondosa. C16:0: palmitic acid (71.3 ± 2.2 mg/ml), C16:1: palmitoleic acid (5.57 ± 0.48 mg/ml), C17:0 (internal standard): heptadecenoic acid, C18:0: stearic acid (5.71 ± 0.08 mg/ml), C18:1: oleic acid (388 ± 5.8 mg/ml), C18:2: linoleic acid (327 ± 6.1 mg/ml). Values are mean ± SE (n = 3).
Discussion
This study demonstrated that GF possessed a stronger anti-α-glucosidase activity than acarbose, but its anti-α-amylase activity was weaker. HFD + STZ-treated animals showed a significant heavier body weight than HFD + STZ-GF600 animals. Administration of GF600 led to a significant decrease in the blood glucose, HbA1c, ABG, and TC levels in HFD + STZ-induced hyperglycemic mice. However, no difference was noted in the TG level between treatments. These results indicate that GF600 treatment was able to ameliorate the HFD + STZ-induced body weight gain, hyperglycemia, and hypercholesterolemia. As GF showed potent anti-α-glucosidase activity, suggesting that the possible mechanism by which GF exerts its hypoglycemic action in hyperglycemic mice could be through the inhibition of starch digestion and decrease in glucose absorption, and thus result in reduced blood glucose level.
STZ is widely used to induce both insulin-dependent and non-insulin-dependent diabetes mellitus (Szkudelski, Citation2001). Although high-dose STZ severely impairs insulin secretion mimicking type 1 diabetes, low-dose STZ is known to induce a mild impairment of insulin secretion, which is similar to the condition of the later stage of type 2 diabetes. The combination of HFD and low doses of STZ model has been suggested to be a better model mimicking the characteristic of type 2 diabetes mellitus (Flanagan et al., Citation2008; Srinivasan et al., Citation2005). In this study, a single dose of STZ at 35 mg/kg body weight was injected to animals fed on HFD. As the blood insulin level was not significantly increased, it could be due to the short STZ stimulating period that was insufficient to effectively induced type 2 diabetes; furthermore, the feeding period on HFD for 2 weeks could be too short to boast the STZ-induced diabetes. Hence, only hyperglycemia was observed in the experimental mice. Compared with the control group, both acarbose and GF had an increased level of insulin, however, only GF600 decreased the blood glucose level. This observation suggests that the anti-hyperglycemic activity of GF600 was not related to increasing insulin level, but could be through the inhibition of digestive enzyme-related to type 2 diabetes, i.e. α-glucosidase activity.
Anti-diabetic activity of GF was reported to be from the peptidoglycan fraction, and its effect was not related to the inhibition of glucose absorption in the enteron, but resulted from the process of metabolism of absorbed glucose (Horio & Ohtsuru, Citation2001; Kubo et al., Citation1994). In another study, Lei et al. (Citation2007) showed that maitake-α-glucan has anti-diabetic activity in KK-Ay mice, a model of type 2 diabetes, of which the mechanism of action was mediated through the amelioration of peripheral insulin resistance and enhancement of insulin sensitivity. In this study, our results indicate that GF effectively lowered the blood glucose and cholesterol levels in HFD + STZ-induced hyperglycemic mice; of which the possible mechanism of action could be related to lengthening of carbohydrate digestion, and thus reduced the glucose absorption and the cholesterol biosynthesis.
Studies have shown that oral supplementation with oils rich in eicosapentaenoic acid, docosahexaenoic acid, γ-linolenic acid, and arachidonic acid was able to protect Wistar albino rats from alloxan-induced diabetes mellitus (Mohan & Das, Citation2001). According to Ide et al. (Citation2001), dietary γ-linolenic acid increased glucose metabolism in isolated rat adipocytes. Substituting dietary linoleic acid with α-linolenic acid resulted in lowered blood lipid level and improved insulin sensitivity in sucrose fed rats (Ghafoorunissa et al., Citation2005). Zhou et al. (Citation2008) reported that dietary conjugated linoleic acid increased peroxisomal proliferator activated receptor-γ (PPAR-γ) gene expression in adipose tissue of obese rat, and also improved insulin resistance in vivo. Fatty acids such as palmitic, linoleic, and linolenic acid, major compounds in subfractions of Juniperus oxycedrus L. ssp. oxycedrus L. (Cupressaceae) leaf extract, were reported to raise the sensitivity of PPAR-γ receptors and the release of insulin from β-cells (Orhan et al., Citation2012). Oleic acid and linoleic acid were shown to possess potent anti-α-glucosidase activity in vitro (Su et al., Citation2013). Given GF was shown to contain mainly oleic acid and linoleic acid, but their levels in the fungus were low; this suggests that the hypoglycemic properties of GF could be contributed by factors other than these unsaturated fatty acids through their inhibitory action on α-glucosidase activity.
In conclusion, this study has demonstrated that the non-polar fraction of GF possessed potent anti-α-glucosidase activity. Compared with the HFD + STZ group, GF600 treatment significantly lowered the blood glucose, HbAlc, ABG, and TC levels in hyperglycemic animals. These results have provided some experimental justifications on the traditional use of this mushroom for preventing hyperglycemia.
Declaration of interest
The authors would like to thank the National Science Council of Taiwan for funding the study under Grant number NSC 98-2313-B-002-055-MY3.
References
- Apostolidis E, Lee CM. (2010). In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated α-glucosidase and α-amylase inhibition. J Food Sci 75:H97–102
- Chakrabarti R, Reeba K. (2002). Antidiabetic and hypolipidemic activity of Helicteres isora in animal models. J Ethnopharmacol 81:343–9
- DCCT (Diabetes Control and Complications Trial). (1987). Results of feasibility study. The DCCT research group. Diabetes Care 10:1–19
- Flanagan AM, Brown JL, Santiago CA, et al. (2008). High-fat diets promote insulin resistance through cytokine gene expression in growing female rats. J Nutr Biochem 19:505–13
- Fukushima M, Ohashi T, Fujiwara Y, et al. (2001). Cholesterol-lowering effects of Maitake (Grifola frondosa) fiber, Shiitake (Lentinus edodes) fiber, and Enokitake (Flammulina velutipes) fiber in rats. Exp Biol Med 226:758–65
- Ghafoorunissa, Ibrahim A, Natarajan S. (2005). Substituting diatery linoleic acid with α-linolenic acid improves insulin sensitivity in sucrose fed rats. Biochim Biophys Acta 1733:67–75
- Horio H, Ohtsuru M. (2001). Maitake (Grifola frondosa) improve glucose tolerance of experimental diabetic rats. J Nutr Sci Vitaminol 47:57–63
- Ide T, Kushiro M, Takahashi Y. (2001). Dietary mold oil rich in gamma linolenic acid increases insulin-dependent glucose utilization in isolated rat adipocytes. Comp Biochem Physiol B 130:401–9
- Kimura YM, Kubo M, Tani T, et al. (1981). Studies on Scutellariae radix IV: Effects on lipid peroxidation in rat liver. Chem Pharm Bull 29:2610–17
- Kodama N, Komuta K, Sakai N, Nanba H. (2002). Effects of D-fraction, a polysaccharide from Grifola frondosa on tumor growth involve activation of NK cells. Biol Pharm Bull 25:1647–50
- Kodama N, Murata Y, Nanba H. (2004). Administration of a polysaccharide from Grifola frondosa stimulates immune function of normal mice. J Med Food 7:141–5
- Kubo K, Aoki H, Nanba H. (1994). Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). Biol Pharm Bull 17:1106–10
- Lei H, Ma X, Wu W. (2007). Anti-diabetic effect of an α-glucan from fruit body of Maitake (Grifola frondosa) on KK-Ay mice. J Pharm Pharmacol 59:575–82
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Lui IM, Chi TC, Hsu FL, et al. (1999). Isoferulic acid as active principle from the rhizoma of Cimicifuga dahurica to lower plasma glucose in diabetic rats. Planta Med 65:712–14
- Malviya N, Jain S, Malviya S. (2010). Antidiabetic potential of medicinal plants. Acta Pol Pharm 67:113–18
- Manohar V, Talpur NA, Echard BW, et al. (2002). Effects of a water-soluble extract of Maitake mushroom on circulating glucose/insulin concentrations in KK mice. Diabetes Obes Metab 4:43–8
- Mohan IK, Das UN. (2001). Prevention of chemically induced diabetes mellitus in experimental animals by polyunsaturated fatty acids. Nutrition 17:126–51
- Orhan N, Aslan M, Demirci B, Ergun F. (2012). A bioactivity guided study on the antidiabetic activity of Juniperus oxycedrus subsp. oxycedrus L. leaves. J Ethnopharmacol 140:409–15
- Park MH, Ju JW, Park MJ, Han JS. (2013). Daidzein inhibits carbohydrate digestive enzymes in vitro and alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol 12:48–52
- Srinivasan K, Viswanad B, Asrat L, et al. (2005). Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: A model for type 2 diabetes and pharmacological screening. Pharmacol Res 52:313–20
- Su CH, Hsu CH, Ng LT. (2013). Inhibitory potential of fatty acids on key enzymes related to type 2 diabetes. BioFactors 39:415–21
- Szkudelski T. (2001). The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 50:537–46
- Takahashi T, Miyazawa M. (2012). Potent α-glucosidase inhibitors from safflower (Carthamus tinctorius L.) seed. Phytother Res 26:722–76
- Talpur N, Echard B, Dadgar A, et al. (2002). Effects of Maitake mushroom fractions on blood pressure of Zucker fatty rats. Res Comm Mol Pathol Pharmacol 112:68–82
- Toeller M. (1994). α-Glucosidase inhibitors in diabetes: Efficacy in NIDDM subjects. Eur J Clin Invest 24:31–5
- Zhang Y, Mills GL, Nair MG. (2002). Cyclooxygenase inhibitory and antioxidant compounds from the mycelia of the edible mushroom Grifola frondosa. J Agric Food Chem 50:7581–5
- Zheng XK, Zhang L, Wang WW, et al. (2011). Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J Ethnopharmacol 137:662–8
- Zhou XR, Sun CH, Liu JR, Zhao D. (2008). Dietary conjugated linoleic acid increases PPAR-γ gene expression in adipose tissue of obese rat, and improves insulin resistance. Growth Horm IGF Res 18:361–8
