Abstract
Context: Zingiber officinale Roscoe (Zingiberaceae), or ginger, used in traditional Chinese medicine, has antioxidant activity and neuroprotective effects. The effects of this plant on clonic seizure have not yet been studied.
Objective: The present study evaluated the anticonvulsant effect of ginger in a model of clonic seizures induced with pentylenetetrazole (PTZ) in male mice.
Materials and methods: The anticonvulsant effect of Z. officinale was investigated using i.v. PTZ-induced seizure models in mice. Different doses of the hydroethanolic extract of Z. officinale (25, 50, and 100 mg/kg) were administered intraperitonal (i.p.), daily for 1 week before induction of PTZ. Phenobarbital sodium (30 mg/kg), a reference standard, was also tested for comparison. The effect of ginger on to the appearance of three separate seizure endpoints, e.g., myoclonic, generalized clonic, and tonic extension phase, was recorded.
Results: Hydroethanolic extract of Z. officinale significantly increased the onset time of myoclonic seizure at doses of 25–100 mg/kg (55.33 ± 1.91 versus 24.47 ± 1.33 mg/kg, p < 0.001) and significantly prevented generalized clonic (74.64 ± 3.52 versus 47.72 ± 2.31 mg/kg, p < 0.001) and increased the threshold for the forelimb tonic extension (102.6 ± 5.39 versus 71.82 ± 7.82 mg/kg, p < 0.01) seizure induced by PTZ compared with the control group.
Discussion and conclusion: Based on the results, the hydroethanolic extract of ginger has anticonvulsant effects, possibly through an interaction with inhibitory and excitatory systems, antioxidant mechanisms, and oxidative stress inhibition.
Introduction
Epilepsy is a common neurological disorder characterized by recurrent, unpredictable seizures with a prevalence rate of about 1% (McNamara, Citation1986). A great deal of research is currently being conducted to reveal the mechanisms underlying epilepsy and to find more effective drugs for epilepsy treatment. The available antiepileptic medications can be effective in the seizures in only about 40% of cases, and they merely reduce the frequency of convulsions in other cases (Delgado-Escueta et al., Citation1999). Unfortunately, despite the availability of a diverse array of anti-epileptic drugs (AEDs), approximately half of the patients treated with modern AEDs continue to experience seizures (Pitkanen, Citation2002). Furthermore, undesirable side effects of the drugs which are used clinically often render treatment difficult; a demand for new types of anticonvulsants exists. One of the approaches to search for new antiepileptic drugs is the investigation of naturally occurring compounds belonging to new structural classes (Sayyah et al., Citation2011).
There is evidence that dietary enrichment with nutritional antioxidants could improve brain damage and cognitive function (Bisson et al., Citation2008; Head, Citation2009). Zingiber officinale Roscoe (Zingiberaceae) or ginger is widely used as a spice. Indians and Chinese are believed to have produced ginger as a tonic root for over 5000 years to treat many ailments, and this plant is now cultivated throughout the humid tropics, with India being the largest producer (Benzie & Sissi, Citation2011). The oleoresin from the rhizomes of ginger contains many bioactive components, such as 6-gingerol, which is the primary pungent ingredient that is believed to exert a variety of remarkable pharmacological and physiological activities. Ginger has been used for thousands of years for the treatment of numerous ailments, such as colds, arthritis, migraines, and hypertension (Benzie & Sissi, Citation2011). It is used in traditional Asian medicine for the treatment of stomachaches (Mascolo et al., Citation1989), nausea, diarrhea, and joint and muscle pain (Ojewole, Citation2006). Recently, several research groups have demonstrated that ginger has anti-inflammatory effects (Chang et al., Citation1995; Lantz et al., Citation2007), antioxidant activity (Kuo et al., Citation2005; Nanjundaiah et al., Citation2011), and a neuroprotective effects (Shanmugam et al., Citation2011; Waggas, Citation2009). Antioxidants in ginger include gingerols, shogaols, and some phenolic ketone derivatives. The anti-inflammatory and antioxidant properties in ginger help to relieve various inflammatory disorders such as gout, osteoarthritis, and rheumatoid arthritis. It provides substantial relief in pain caused by inflammation and helps to decrease swelling and morning stiffness (Habib et al., Citation2008). Another study suggests that ginger can reduce cell death and restore motor function in a rat spinal cord injury (Kyung et al., Citation2006).
Laboratory models for epilepsy induction make it possible to analyze the mechanisms and predisposing factors of epilepsy and to evaluate anticonvulsive drugs and treatment modalities. One of these models is an intravenous PTZ (i.v. PTZ) infusion with a constant flow rate through the tail vein in mice (Nutt et al., Citation1986; Orloff et al., Citation1949) or the jugular vein catheter in rats (Pollack & Shen, Citation1985) elicits seizure response in a reliable, reproducible, and rapid manner. Depending on the dosage, PTZ can produce myoclonic jerking movements, clonic convulsions, or forelimbs/hindlimbs tonic extensor (Snead, Citation1992; Swinyard et al., Citation1989). The objective of the present study was to evaluate the anticonvulsant activity of a hydroethanolic extract of Z. officinale in the timed i.v. PTZ seizure test in mice.
Materials and methods
Plant material and preparation of the extract
The fresh rhizomes of Z. officinale (herbarium code no. 1483) were purchased from the Institute of Medicinal Plants, Tehran, Iran, in October 2012 and authenticate by Dr. R. Kalvandi, at the Botanical Laboratory, Biology, Hamedan, Iran. A sample of the rhizome was deposited in the local herbarium of the Department of Biology, Faculty of Science, Bu-Ali Sina University, Iran. Approximately 200 g of the air-dried rhizome powder from Z. officinale was extracted with 3 L of 80% aqueous ethanol using the percolation method at room temperature. The extracts were filtered through filter paper and evaporated to dryness under reduced pressure at a maximum of 45 °C using a rotary evaporator. Zingiber officinale yielded 33.28% dried extract. Zingiber officinale extract (ZOE) was dissolved in normal saline to a stock concentration of 50% w/v and then stored at 4 °C. The dosage calculations were based on the body weight of animals.
Drugs
Drugs used were PTZ (purchased from the Sigma, Bristol, UK) and phenobarbital (PB) Na (purchased from the Chemidaru Industrial Company, Tehran, Iran). PTZ was prepared in saline as 1% w/v solution. Based on the previous study on dose-response of PB (Markowitz et al., Citation2011), the dose of 30 mg/kg (dissolved in physiologic saline solution) was chosen as a suitable dose for this investigation.
Experimental animals
Adult male Swiss mice that weighed 30 ± 5 g (Razi Institute, Karadj, Iran) were used in the study. The animals were housed in standard polycarbonate cages at a temperature of 22 ± 2 °C on a 12-h light/dark cycle with free access to food and water and were acclimated at least 1 week before experiments. The experiments were conducted between 9:00 AM and 3:00 PM. Animals were handled in accordance with the criteria outlined in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health (NIH) publication 86-23; revised 1985). All the protocols were also approved by the institutional ethics committee of Bu-Ali Sina University. Each mouse was used only once and each treatment group consisted of at least six animals. The animals were randomly assigned to the following groups:
Group I: mice received vehicle (normal saline 1 ml/kg, i.p.) as normal control, daily for 1 week before PTZ injection. Group II: mice received PB Na (30 mg/kg, i.p.), daily for 1 week before PTZ injection. Group III: mice were treated with ginger extract (25 mg/kg, i.p.), daily for 1 week before PTZ injection. Group IV: mice were treated with ginger extract (50 mg/kg, i.p.), daily for 1 week before PTZ injection. Group V: mice were treated with ginger extract (100 mg/kg, i.p.), daily for 1 week before PTZ injection.
The timed i.v. PTZ infusion test in mice
The test was conducted in mice based on a previously reported method (Mandhane et al., Citation2007). A butterfly cannula (needle size 30G, DENTSPLY MPL Technologies, England) attached to an insulin syringe prefilled with PTZ solution was used. For the purpose of infusion, the animal was restrained and needle was inserted into the tail vein. The needle was connected by polyethylene tubing with a plastic syringe that was placed in the syringe pump (GMS Syringe Pump, JMS Singapore Pvt Ltd., Singapore). The accuracy of needle placement in the vein was confirmed by the appearance of blood in the cannula. The needle was secured to the tail by a special tape. The animal was kept in a transparent Perspex box with holes for ventilation. In this way, the animal could move freely in the box without strain on the attached cannula with no severe struggling. The syringe contained 1% solution of PTZ in saline, which was administered into the vein of unrestrained animal at a constant rate of 1 ml/min. The time intervals from the start of infusion of PTZ solution to the appearance of three separate endpoints, i.e., first myoclonic twitch, generalized clonus with loss of righting reflex and forelimb/hindlimbs tonus, were recorded. The thresholds were calculated separately for each endpoint according to the following formula: threshold dose of PTZ (mg/kg) = (infusion duration (s) × infusion rate (ml/s) × PTZ concentration (mg/ml) × 1000)/body weight kg.
Statistical analysis
Statistical analysis was performed using SPSS software (Version 21; SPSS Inc., Chicago, IL). Seizure thresholds were expressed as the amount of PTZ (in mg/kg) ± SEM (standard error of the mean) needed to produce the first apparent sign of each endpoint, and analyzed with the one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. p < 0.05 was considered statistically significant.
Results
An intravenous infusion of PTZ with a constant flow rate in mice elicited stereotyped seizure response in the following sequence: myoclonic twitch, generalized clonus with the loss of righting reflex, and forelimb tonus. The seizure manifestation progressed from one stage to the other, as the infusion progressed. It was possible to limit the seizure activity by controlling the infusion. The concentration of PTZ solution and its rate of infusion were constant. Therefore, the body weight of animal and the time duration of infusion were the only variables required to be used in the calculation of threshold dose for an individual animal.
Effect of different doses of ginger on the threshold for the myoclonic seizures
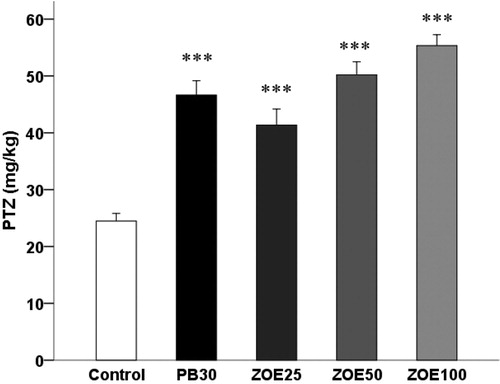
The effect of ginger on seizure thresholds for the first myoclonic twitch in the i.v. PTZ seizure threshold test in mice is shown in (F(4, 29) = 26.992, p < 0.001). Ginger at doses ranging from 25 to 100 mg/kg influenced thresholds for myoclonic () seizures in the timed i.v. PTZ infusion test in mice (41.36 ± 2.81 versus 24.47 ± 1.34, 50.19 ± 2.31 versus 24.47 ± 1.34, and 55.33 ± 1.91 versus 24.47 ± 1.34 mg/kg, p < 0.001).
Figure 1. The effect of different doses of ginger (25–100 mg/kg) on the threshold for the first myoclonic twitch in the i.v. PTZ seizure threshold test in mice. Data are presented as mean ± SEM of six mice in each group. One-way ANOVA followed by the Tukey's post hoc multiple comparison test was used to analyze the data (PB, phenobarbital; ZOE: Zingiber officinale extract). ***p < 0.001 versus vehicle control (saline).
Effect of different doses of ginger on the threshold for the generalized clonic seizures
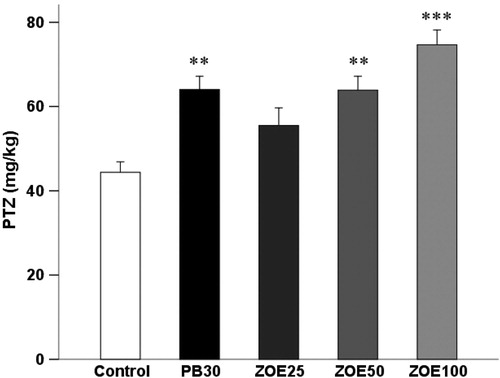
As shown in , ginger affects thresholds for the generalized clonic seizures (F(4, 29) = 9.416, p < 0.001) provoked by i.v. PTZ infusion in mice. Statistically significant raise in the threshold for clonic seizures was also observed in groups of animals treated with ginger at the dose of 50 (63.89 ± 3.29 versus 47.72 ± 2.32 mg/kg, p < 0.01) and 100 mg/kg (74.64 ± 3.52 versus 47.72 ± 2.31 mg/kg, p < 0.001) compared with the control group.
Figure 2. The effect of different doses of ginger (25–100 mg/kg) on the threshold for the generalized clonic seizures in the i.v. PTZ seizure threshold test in mice. Data are presented as mean ± SEM of six mice in each group. One-way ANOVA followed by the Tukey's post hoc multiple comparison test was used to analyze the data (PB, phenobarbital; ZOE: Zingiber officinale extract). **p < 0.05 and ***p < 0.001 versus vehicle control (saline).
Effect of different doses of ginger on the threshold for the forelimb/hindlimbs tonic extension
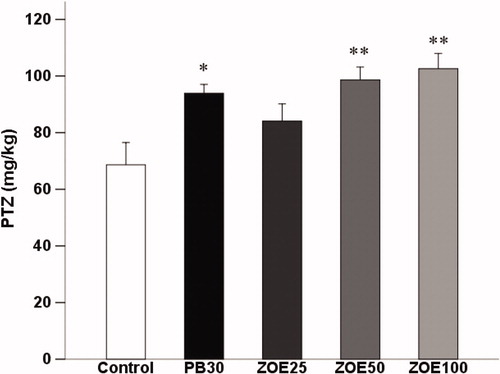
In , ginger significantly increased threshold in comparison with the control (saline-treated) group for the forelimb/hindlimbs tonic extension (F(4, 29) = 5.209, p < 0.01) in the timed i.v. PTZ infusion test in mice. One-way ANOVA revealed that ginger statistically significant raised the threshold for tonic seizures and post hoc analysis showed significant anticonvulsant effect for the dose of 50 (98.59 ± 4.56 versus 71.82 ± 7.82 mg/kg, p < 0.01) and 100 mg/kg (102.6 ± 5.39 versus 71.82 ± 7.82 mg/kg, p < 0.01) compared with the control group.
Figure 3. The effect of different doses of ginger (25–100 mg/kg) on the threshold for the forelimb/hindlimbs tonic extension in the i.v. PTZ seizure threshold test in mice. Data are presented as mean ± SEM of six mice in each group. One-way ANOVA followed by the Tukey's post hoc multiple comparison test was used to analyze the data (PB, phenobarbital; ZOE: Zingiber officinale extract). *p < 0.05 and **p < 0.01 versus vehicle control (saline).
Discussion
The present study investigated the anticonvulsant activity of Z. officinale in the timed PTZ infusion test in mice. This test is considered as a very sensitive model of acute seizures which enables screening of various agents with different mechanisms of action, both pro- and anticonvulsants. Additionally, it makes possible to assess the influence of the investigated compounds on separate components of seizure behavior. Although PTZ-induced seizures are one of the most common experimental models of seizures, mechanism(s) by which PTZ causes convulsions is not entirely known. It is widely accepted that its proconvulsant activity is at least partially mediated by interactions with the chloride ion channel in the complex of γ-aminobutyric acid (GABA) type A receptors (Mandhane et al., Citation2007). Moreover, experimental evidence demonstrated that PTZ increased the level of cGMP in many brain regions including cerebral cortex, hippocampus, striatum, and cerebellum (Ferrendelli et al., Citation1980).
High antioxidant activity of ginger and its compounds has been demonstrated in numerous reports (Ghasemzadeh et al., Citation2010; Rehman et al., Citation2011). 6-Gingerol, a bioactive component of ginger, was reported to dose-dependently inhibit nitric oxide (NO) production and reduce inducible nitric oxide synthase (iNOS) in lipopolysaccharide (LPS)-stimulated mouse macrophages (Ippoushi et al., Citation2003). Reactive nitrogen species, such as NO, influence signal transduction and cause DNA damage, which contributes to disease processes such as seizure. Nitric oxide is produced by iNOS, which is stimulated in response to various stresses. In addition to the above findings, NO also increases the level of cyclic guanosine monophosphate (cGMP) through the activation of soluble guanylyl cyclase (sGC), which influences a wide range of physiological functions including regulation of seizure threshold (Ni Dhi et al., Citation1999). Numerous experimental studies and clinical observations indicate that ginger influences some of the central nervous system effects (Felipe et al., Citation2008), but accurate mechanisms of its action are not precisely known. It is highly possible that the observed effects result from the elevated intracellular cGMP level. It was noted that cGMP and its downstream targets, including PDEs, cGMP-dependent channels and PKG, regulate neurotransmission, long-term potentiation, gene expression, neurotoxicity, and neurodegenerative processes (Wang & Robinson, Citation1997). Components of the NO/cGMP pathway modulate release of both excitatory and inhibitory amino acids in the central nervous system (Prast & Philippu, Citation2001; Yu & Eldred, Citation2005). Because imbalance between the excitatory and the inhibitory neurotransmission is the main reason of epileptic discharges, cGMP may affect convulsant activity in brain (Garthwaite & Boulton, Citation1995). Therefore, it seems that antioxidant property of ginger and its nitric oxide synthase inhibitory effect can be, at least in part, responsible for such inhibiting response in this study.
Most AEDs act by three main mechanisms of pharmacological action. They intensify inhibitory potential of GABAergic system, reduce excitatory neurotransmission, and/or affect voltage-dependent ion channels, mainly calcium, sodium, and potassium (Lasoń et al., Citation2011). Pharmacological action of the AEDs, like PB, is above all connected to the influence on the GABAergic system. PB is an agonist of GABA and its development was strictly associated with GABAergic hypothesis of epilepsy. In addition to the effects on GABAA receptors, barbiturates block AMPA receptors, and they inhibit glutamate release through an effect on high-voltage-activated calcium channels. These channels are categorized into several subtypes-L, N, P/Q, and R-type, according to their electrophysiological properties (Armijo et al., Citation2005). In addition to the effects on GABAA receptors, barbiturates block AMPA/kainate receptors, and they inhibit glutamate release through an effect on P/Q-type high-voltage-activated calcium channels and in this way reduce calcium influx into the neurons, which subsequently diminishes generation of action potentials in neuronal cells (Czapiński et al., Citation2005; Lasoń et al., Citation2011). Blockage of Ca2+ channels, which has been demonstrated in a series of in vivo and in vitro studies, is an important pharmacological action of ginger (Ghayur et al., Citation2008a,Citationb; Heinemann et al., Citation1977). Not surprisingly, plants such as ginger with Ca2+ channel blocking properties could exert such observed effects. Thus, the data indicate that the mechanism underlying the prevention of PTZ-induced seizure by ginger extract involves mediation, at least in part, by attenuating the effect of seizure threshold on calcium channel inhibition and calcium homeostasis disturbance.
Data presented in our study showed that ginger increased the threshold for both myoclonic and generalized clonic seizures induced by the i.v. injection of PTZ in mice. Increase in NO following PTZ-induced seizures leads to the raise in the intracellular cGMP level and subsequently might activate PKG, a major intracellular receptor for cGMP. Although PKG was noted in the brain only at low levels, it plays a significant role in the regulation of calcium concentration in neurons, generation of action potentials, and neurotransmitters release. These results might be mediated by the impact of PKG on some ion channels, including calcium channels. Phosphorylation of calcium channels by this enzyme leads to their blockade, decrease in the intracellular calcium level, and reduction of the neuronal excitability (Wang & Robinson, Citation1997). The NO/cGMP/PKG pathway was also reported to inhibit activity of N- and P/Q-type voltage-dependent calcium channels which might decrease neurotransmitter release in neurons (Yu & Eldred, Citation2005). Moreover, PKG was also reported to modulate calcium-activated potassium channels. These channels are the major constituents regulating membrane potentials by the modulation of potassium efflux and their phosphorylation consequently leads to hyperpolarization of neurons and inhibition of voltage-gated calcium channels (Wang & Robinson, Citation1997). It has been reported that ginger bioactive components such as 6-shogaol, 1-dehydro-10-gingerdione, and 10-gingerdione also decreased LPS-induced NO production, and 6-shogaol and 1-dehydro-10-gingerdione were reported to effectively reduce iNOS expression (Koh et al., Citation2009). These evidence seem to suggest that ginger and some of its components are effective antioxidants in vitro and may be interfering with the NO/cGMP/PKG pathway due to anticonvulsant effects and thereby elevation of the seizure threshold.
Glutamate in high doses produced neuroendocrine abnormalities (Moreno et al., Citation2005), neurodegeneration, neurotoxicity (Chapano-Hue et al., Citation2002), and oxidative damage in different organs (Farombi & Onyema, Citation2006; Pavlovic et al., Citation2007). Glutamate receptors known as mGluRI were strongly suggestive of the initiation of an epileptogenic process, one in which normal neuronal cortex is converted into a persistently hyperexcise state with a lowered threshold for the production of seizure discharges. It was proposed that this form of epileptogenesis was likely to be clinically relevant because the instigating agent was acting at glutamate receptors, and glutamate was long recognized as the key excitatory transmitter in the CNS underlying the expression of seizure discharges (Anwyl, Citation2009; Merlin & Wong, Citation1997). It has been shown that ginger has neuroprotective and inhibitory effects on glutamate receptors, which may be responsible for the anticonvulsant activity and for the prevention of seizure discharges (Shanmugam et al., Citation2011; Waggas, Citation2009).
Conclusion
In conclusion, in the present study, we demonstrated that ginger extract has an anticonvulsant effect in the timed PTZ infusion test in mice. Summing up, we can hypothesize that the anticonvulsant effect of ginger may have been mediated by antioxidant mechanisms, oxidative stress inhibition, and simultaneous influence on different kinds of calcium channels and both excitatory and inhibitory systems of neurotransmission. The precise molecular mechanism of interactions between the AEDs and ginger should be investigated in further experiments. Our results might support new treatment methods using ginger in patients with epileptic seizure. Due to favorable pharmacodynamic characteristics and lack of acute side effects, the tested interactions might be beneficial and worthy of consideration for testing in clinical trials.
Acknowledgements
We would like to thank Mr. Ali Gomar for assistance in this research project.
Declaration of interest
The authors declare that they have no competing interests.
References
- Anwyl R. (2009). Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology 56:735–40
- Armijo JA, Shushtarian M, Valdizan EM, et al. (2005). Ion channels and epilepsy. Curr Pharm Des 11:1975–2003
- Benzie IFF, Sissi W-G. (2011). Herbal Medicine Biomolecular Clinical Aspects, 2nd ed. New York, USA: Taylor & Francis Group, LLC
- Bisson JF, Nejdi A, Rozan P, et al. (2008). Effects of long-term administration of a cocoa polyphenolic extract (Acticoa powder) on cognitive performances in aged rats. Br J Nutr 100:94–101
- Chang CP, Chang JY, Wang FY, Chang JG. (1995). The effect of Chinese medicinal herb Zingiberis rhizoma extract on cytokine secretion by human peripheral blood mononuclear cells. J Ethnopharmacol 48:13–19
- Chapano-Hue V, Rwera-Cemantes MC, Tomes-Mendoza BM, Beas-Zarate C. (2002). Neuronal death and tumor necrosis factor-alpha response to glutamate-induced excitotoxicity in the cerebral cortex of neonatal rats. Neurosci Lett 333:95–8
- Czapiński P, Błaszczyk B, Czuczwar SJ. (2005). Mechanisms of action of antiepileptic drugs. Curr Top Med Chem 5:3–14
- Delgado-Escueta AV, Wilson WA, Olsen RW, Porter RJ. (1999). New waves of research in the epilepsies: Crossing into the third millennium. Adv Neurol 79:3–58
- Farombi EO, Onyema OO. (2006). Monosodium glutamate-induced oxidative damage and genotoxicity in the rat: Modulatory role of vitamin C, vitamin E and quercetin. Hum Exp Toxicol 25:251–9
- Felipe CFB, Fonsêca KS, dos Reis Barbosa AL, et al. (2008). Alterations in behavior and memory induced by the essential oil of Zingiber officinale Roscoe (ginger) in mice are cholinergic-dependent. J Med Plants Res 2:163–70
- Ferrendelli JA, Blank AC, Gross RA. (1980). Relationships between seizure activity and cyclic nucleotide levels in brain. Brain Res 200:93–103
- Garthwaite J, Boulton CL. (1995). Nitric oxide signaling in the central nervous system. Annu Rev Physiol 57:683–706
- Ghasemzadeh A, Jaafar HZ, Rahmat A. (2010). Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 15:4324–33
- Ghayur MN, Gilani AH, Ahmed T, et al. (2008a). Muscarinic, Ca2+ antagonist and specific butyrylcholinesterase inhibitory activity of dried ginger extract might explain its use in dementia. J Pharm Pharmacol 60:1375–83
- Ghayur MN, Gilani AH, Janssen LJ. (2008b). Ginger attenuates acetylcholineinduced contraction and Ca2+ signaling in murine airway smooth muscle cells. Can J Physiol Pharmacol 86:264–71
- Habib SH, Makpol S, Abdul Hamid NA, et al. (2008). Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 63:807–13
- Head E. (2009). Oxidative damage and cognitive dysfunction: Antioxidant treatments to promote healthy brain aging. Neurochem Res 34:670–8
- Heinemann U, Lux HD, Gutnick MJ. (1977). Extracellular free calcium and potassium during paroxsmal activity in the cerebral cortex of the cat. Exp Brain Res 27:237–43
- Ippoushi K, Azuma K, Ito H, et al. (2003). [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci 73:3427–37
- Koh EM, Kim HJ, Kim S, et al. (2009). Modulation of macrophage functions by compounds isolated from Zingiber officinale. Planta Med 75:148–51
- Kuo PC, Damu AG, Cherng CY, et al. (2005). Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Arch Pharm Res 28:518–28
- Kyung KS, Gon JH, Geun KY, et al. (2006). 6-Shogaol, a natural product, reduces cell death and restores motor function in rat spinal cord injury. Eur J Neurosci 24:1042–52
- Lantz RC, Chen GJ, Sarihan M, et al. (2007). The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine 14:123–8
- Lasoń W, Dudra-Jastrzębska M, Rejdak K, Czuczwar SJ. (2011). Basic mechanisms of antiepileptic drugs and their pharmacokinetic/pharmacodynamic interactions: An update. Pharmacol Rep 63:271–92
- Mandhane SN, Aavula K, Rajamannar T. (2007). Timed pentylenetetrazol infusion test: A comparative analysis with s.c.PTZ and MES models of anticonvulsant screening in mice. Seizure 16:636–44
- Markowitz GJ, Kadam SD, Smith DR, et al. (2011). Different effects of high- and low-dose phenobarbital on post-stroke seizure suppression and recovery in immature CD1 mice. Epilepsy Res 94:138–48
- Mascolo N, Jain R, Jain SC, Capasso F. (1989). Ethnopharmacologic investigation of ginger (Zingiber officinale). J Ethnopharmacol 27:129–40
- McNamara JO. (1986). Kindling model of epilepsy. Adv Neurol 44:303–18
- Merlin LR, Wong RKS. (1997). Role of group I metabotropic glutamate receptors in the patterning of epileptiform activities in vitro. J Neurophysiol 78:539–44
- Moreno G, Perello M, Gaillard RC, Spinedi E. (2005). Orexin a stimulates hypothalamic-pituitary-adrenal (HPA) axis function, but not food intake, in the absence of full hypothalamic NPY-ergic activity. Endocrine 26:99–106
- Nanjundaiah SM, Annaiah HN, Dharmesh SM. (2011). Gastroprotective effect of ginger rhizome (Zingiber officinale) extract: Role of gallic acid and cinnamic acid in H+, K+-ATPase/H. pylori inhibition and anti-oxidative mechanism. Evid Based Complement Alternat Med 2011: Article ID 249487, 13 pages
- Ni Dhi G, Balakri Shnan S, Pandhi P. (1999). Role of nitric oxide in electroshock and pentylenetetrazole seizure threshold in rats. Meth. Find. Exp Clin Pharmacol 21:609–12
- Nutt DJ, Taylor SC, Little HJ. (1986). Optimizing the pentetrazol infusion test for seizure threshold measurement. J Pharm Pharmacol 38:697–8
- Ojewole JA. (2006). Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res 20:764–72
- Orloff MJ, Williams HL, Pfeiffer CC. (1949). Timed intravenous infusion of metrazol and strychnine for testing anticonvulsant drugs. Proc Soc Exp Biol Med 70:254–7
- Pavlovic V, Pavlovic D, Kocic G, et al. (2007). Effect of monosodium glutamate on oxidative stress and apoptosis in rat thymus. Mol Cell Biochem 303:161–6
- Pitkanen A. (2002). Efficacy of current antiepileptics to prevent neurodegeneration in epilepsy models. Epilepsy Res 50:141–60
- Pollack GM, Shen DD. (1985). A timed intravenous pentylenetetrazol infusion seizure model for quantitating the anticonvulsant effect of valproic acid in the rat. J Pharmacol Methods 13:135–46
- Prast H, Philippu A. (2001). Nitric oxide as modulator of neuronal function. Prog Neurobiol 64:51–68
- Rehman R, Akram M, Akhtar N, et al. (2011). Zingiber officinale Roscoe (pharmacological activity). J Med Plants Res 5:344–8
- Sayyah M, Khodaparast A, Yazdi A, Sardari S. (2011). Screening of the anticonvulsant activity of some plants from Fabaceae family in experimental seizure models in mice. Daru 19:301–5
- Shanmugam KR, Mallikarjuna K, Kesireddy N, Reddy KS. (2011). Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol 49:893–7
- Snead OC. (1992). Pharmacological models of generalized absence seizures in rodents. J Neural Transm Suppl 35:7–19
- Swinyard EA, Woodhead JH, White HS, Franklin MR. (1989). Experimental selection, quantification and evaluation of anticonvulsants. In: Levy R, Mattson R, Meldrum B, et al., eds. Antiepileptic Drugs. 3rd ed. New York: Raven Press, 85–102
- Waggas AM. (2009). Neuroprotective evaluation of extract of ginger (Zingiber officinale) root in monosodium glutamate-induced toxicity in different brain areas male albino rats. Pak J Biol Sci 12:201–12
- Wang X, Robinson PJ. (1997). Cyclic GMP-dependent protein kinase and cellular signaling in the nervous system. J Neurochem 68:443–56
- Yu D, Eldred WD. (2005). Nitric oxide stimulates gamma-aminobutyric acid release and inhibits glycine release in retina. J Comp Neurol 483:278–91
