Abstract
Context: Eugenol, an essential constituent found in plants such as Eugenia caryophyllata Thunb. (Myrtaceae) is reported to possess neuroprotective and anti-stress activities. These activities can potentially be useful in the treatment of stress-induced irritable bowel syndrome (IBS).
Objective: The protective effect of eugenol was assessed against restraint stress (RS)-induced IBS-like gastrointestinal dysfunction in rats. Further, its centrally mediated effect was evaluated in this model.
Materials and methods: Eugenol (12.5, 25, and 50 mg/kg), ondansetron (4.0 mg/kg, p.o.), and vehicle were administered to rats for 7 consecutive days before exposure to 1 h RS. One control group was not exposed to RS-induction. The effect of eugenol (50 mg/kg) with and without RS exposure was evaluated for mechanism of action and per se effect, respectively. The hypothalamic–pituitary–adrenal cortex (HPA)-axis function was evaluated by estimating the plasma corticosterone level. The levels of brain monoamines, namely serotonin, norepinephrine, dopamine, and their metabolites were estimated in stress-responsive regions such as hippocampus, hypothalamus, pre-frontal cortex (PFC), and amygdala. Oxidative damage and antioxidant defenses were also assessed in brain regions.
Results: Eugenol (50 mg/kg) reduced 80% of RS-induced increase in fecal pellets similar to that of ondansetron. Eugenol attenuated 80% of stress-induced increase in plasma corticosterone and modulated the serotonergic system in the PFC and amygdala. Eugenol attenuated stress-induced changes in norepinephrine and potentiated the antioxidant defense system in all brain regions.
Conclusion: Eugenol protected against RS-induced development of IBS-like gastrointestinal dysfunction through modulation of HPA-axis and brain monoaminergic pathways apart from its antioxidant effect.
Introduction
Eugenol is one of the major constituents of essential oils obtained from many herbal plants including Eugenia caryophyllata Thunb (Myrtaceae), Ocimum sanctum Linn (Lamiaceae), and Cinnamomum zeylanicum Blume (Lauraceae). Eugenol has been reported to have several neuropharmacological activities such as anxiolytic, antidepressant, and neuroprotection against N-methyl-d-aspartic acid-induced neurotoxicity in experimental animal models (Ardjmand et al., Citation2006; Garabadu et al., Citation2011). Eugenol has been reported to show an anti-stress effect by modulating HPA-axis and the brain monoamine system in experimental animals (Garabadu et al., Citation2011). As eugenol exhibits anti-stress and neuroprotective activities, we assumed that it may have a protective effect against the restraint stress (RS)-induced animal model of irritable bowel syndrome (IBS).
IBS is a chronic and one of the most common reasons for medical consultation in health disorders that is characterized by abdominal pain, gas excretion, and abnormal bowel movements (Spiller, Citation2007). Although the overall mechanism of IBS is still unclear, several psychological factors are responsible for development (Rey et al., Citation2009). Thus, centrally acting drugs such as selective serotonin reuptake inhibitors (SSRI's) have been shown to be effective in the treatment of IBS (Ford et al., Citation2009). Experimental studies on models exhibiting IBS symptoms have shown the engagement of emotional arousal circuitry which includes limbic regions such as hippocampus, hypothalamus, pre-frontal cortex (PFC), and amygdala (Labus et al., Citation2009). Hypothalamic–pituitary–adrenal cortex (HPA)-axis is the key component of stress response which is regulated by higher limbic brain regions. Hippocampus inhibits and amygdala activates the HPA-axis. However, PFC has dual action depending on the hemisphere activated (Jankord & Herman, Citation2008). Therefore, these limbic regions, common to both stress and IBS conditions, could be potential targets in the pharmacotherapy of stress-induced IBS.
It has been reported that acute RS significantly increased the fecal pellet output without the formation of gastrointestinal mucosal lesions in free-feeding rats, and caused diarrhea in 90–100% rats within 3 h in food-deprived rats (Miyata et al., Citation1992; Zhao et al., Citation2011). Thus, the increased colonic motility without gastrointestinal damage is considered as a representative model of IBS-like symptom in this study. Hence, the present study evaluates the centrally mediated effects of eugenol in the amelioration of gastrointestinal dysfunction in the rat-restraint model.
Materials and methods
Animals
Male adult Charles Foster strain albino rats, 5–6 weeks of age and weighing about 180–220 g, were purchased from the Central Animal House, Institute of Medical Sciences, Banaras Hindu University. The animals were housed in polypropylene cages under controlled environmental conditions of temperature of 25 ± 1 °C and 45–55% relative humidity and a 12:12 h light/dark cycle (6:00 AM–6:00 PM). All animals were randomly divided for the experiment. All animals were acclimatized for 7 d before starting the experiment. All animals had free access to water and standard diet. All experiments were conducted in accordance with the principles of laboratory animal care (NIH publication number 85-23, revised 1985) guidelines. The experimental procedures were approved by Institutional animal ethical committee, BHU (Protocol no.: Dean/11-12/328).
Drugs and chemicals
Eugenol was gifted by Prime Dental Pvt. Ltd. (Mumbai, India). It was dissolved in 40% propylene glycol in phosphate buffer (pH 6.8). All other chemicals and reagents of high-performance liquid chromatography (HPLC) and analytical grade were procured from local suppliers.
RS procedure
After 18 h of overnight fasting, the restrain stress was given to rats by restraining them in a restrainer (5 × 5 × 20 cm3) for 1 h at room temperature during the early phase of the light cycle (Miyata et al., Citation1992).
Experimental protocol
The experimental design consisted of two sets of experiments. The first experiment was designed to study the dose-dependent effect of eugenol against IBS-like symptoms in rats. The rats for this experiment were divided into six groups of five each. Eugenol was administered by oral gavage using a ball-ended feeding needle for 7 consecutive days. The dose was selected based on preliminary studies. The positive control group received ondansetron for seven consecutive days. The dose of ondansetron was 4.0 mg/kg, p.o. based on a report by Yang and Lee (Citation2008). On day 7, the animals were restrained in a restraining cage after 1 h following administration of the last dose of the drugs. The group receiving vehicle without stress exposure served as the control group, while the group receiving vehicle with stress exposure served as Sham. The number of fecal samples was counted and the dry weight was measured after lyophilization. The number of fecal pellets and weight in gram per fecal pellet were calculated.
The second set of experiments was conducted to evaluate the mechanism behind the protective effect of most effective dose of eugenol (50 mg/kg) and the per se effect in the absence of stress. This consisted of five groups of five animals each. Eugenol was administered to two groups and ondansetron (4.0 mg/kg) was administered to one group of animals for 7 d. The other two groups received vehicle for the same days in the experimental schedule. On day 7, 1 h after drug administration, the rats belonging to vehicle and eugenol-treated groups were exposed to RS procedure. Control and eugenol per se control groups were not exposed to RS procedure. After 1 h of stress, the animals were killed by decapitation. The blood and brain were collected and stored at −80°C for further analysis.
Estimation of ulcer index
The stomach was cut through greater curvature and the ulcer index was calculated by following a standard protocol by a blind observer (Sairam et al., Citation2003).
Estimation of plasma corticosterone level
The plasma level of corticosterone was estimated in control, stress control, and eugenol (50 mg/kg) group animals using HPLC with an ultraviolet detector (Garabadu et al., Citation2011; Woodward & Emery, Citation1987).
Estimation of monoamines and their metabolites
The brain was microdissected (Palkovits & Brownstein, Citation1988) into hippocampus, hypothalamus, PFC, and amygdala and, the level of neurotransmitters and their metabolites were estimated in discrete brain regions using HPLC with an electrochemical detector (Garabadu et al., Citation2011; Kim et al., Citation1987).
Estimation of malondialdehyde level
The lipid peroxidation (LPO) in terms of the malondialdehyde (MDA) content was measured colorimetrically at 532 nm (Mihara & Uchiyama, Citation1978; Sunderman et al., Citation1985) in hippocampus, hypothalamus, PFC, and amygdala. The MDA concentrations were expressed as micromoles of MDA per milligram of protein.
Estimation of superoxide dismutase activity
The activity of superoxide dismutase (SOD) was assayed by the spectrophotometric method based on the formation of NADH–phenazine methosulphate–nitro blue tetrazolium formazan measured at 560 nm against butanol as blank (Kakkar et al., Citation1984) in brain regions as mentioned earlier. A single unit of the enzyme was expressed as 50% inhibition of NBT reduction per minute per milligram of protein under the assay conditions.
Estimation of catalase activity
Decomposition of hydrogen peroxide in the presence of catalase (CAT) was estimated spectrophotometrically at 240 nm (Beers & Sizer, Citation1952) in brain regions as mentioned earlier. The results were expressed as units (U) of CAT activity/min/mg of protein.
Estimation of protein in discrete brain regions
The protein content was estimated colorimetrically based on the biuret and Folin–Ciocalteau reaction using bovin serum albumin as standard for the calibration (Lowry et al., Citation1951).
Data analysis
All statistical analysis of data was done using one-way analysis of variance (ANOVA) with the Newman–Keuls post hoc test. Groups with p < 0.05 were considered as significantly different. All the data are presented as mean ± standard error of the mean (S.E.M).
Results
Effect of eugenol on fecal pellet count and weight per fecal pellet
In dose-selection study, RS caused significant increase in both the number of fecal pellets and the weight per fecal pellet in the animals compared with vehicle-treated rats. Eugenol in doses of 12.5, 25.0, and 50.0 mg/kg attenuated RS-induced increase in the number of fecal pellets by 31, 45, and 83%, respectively. Moreover, ondansetron (4.0 mg/kg) attenuated RS-induced increase in the number of fecal pellets by 80%. These results indicate the protective effect of both eugenol and ondansetron in the animal model exhibiting IBS-like symptoms. Further, eugenol (50 mg/kg) significantly decreased the RS-induced increase in the number of fecal pellets compared with other doses of eugenol. However, neither eugenol (12.5, 25, and 50 mg/kg) nor ondansetron altered significantly the RS-induced increase in weight in gram per fecal pellet ().
Table 1, The effect of eugenol (E; 12.5, 25.0, and 50.0 mg/kg) and ondansetron (O; 4.0 mg/kg) on the number of fecal pellets and weight in gram per fecal pellet in restraint stress (RS)-exposed rats.
In a mechanistic study, eugenol (50 mg/kg) given in the absence of RS did not increase the number of fecal pellets or show any change in weight in gram per fecal pellet compared with control animals. However, eugenol and ondansetron decreased RS-induced increase in the number of fecal pellets by 82 and 77%, respectively ().
Table 2. The effect of eugenol (E; 50.0 mg/kg) on the number of fecal pellets, weight in gram per fecal pellet and plasma corticosterone level in normal and RS-exposed animals.
Effect of eugenol on gastric ulcer
One-way ANOVA of gastric ulceration (data not shown) revealed that there was no significant difference among groups.
Effect of eugenol on RS-induced elevated plasma corticosterone level
Eugenol reversed RS-induced increase in plasma corticosterone level by 84%. However, eugenol did not show any effect on corticosterone levels in normal rats. Ondansetron did not reduce RS-induced increase in the level of plasma corticosterone ().
Effect of pretreated eugenol on stress-induced changes in brain dopaminergic system
The effect of pretreatment of eugenol (50 mg/kg) on RS-induced dopamine (DA; A), 3,4-dihydroxy phenyl acetic acid (DOPAC; B) and homovanillic acid (HVA; C) levels and [(DOPAC+HVA)/DA] (D) ratio is depicted in . RS reduced the DA level in PFC and amygdala, and eugenol reversed this effect only in amygdala. RS-induced increase in the DOPAC level in hypothalamus, PFC, and amygdala compared with control was not altered by ondansetron. However, eugenol reversed the changes in DOPAC level in PFC and amygdala. RS elevated the HVA level in hypothalamus, PFC, and amygdala, and eugenol pretreatment reversed these changes only in PFC. RS increased the (DOPAC+HVA)/DA ratio in hypothalamus, PFC, and amygdala, and eugenol reversed this effect only in PFC and amygdala.
Figure 1. The effect of eugenol in the dose of 50 mg/kg (E−50) and ondansetron in the dose of 4 mg/kg (O-4) on the restraint stress (RS)-induced changes in DA (A), DOPAC (B) and HVA (C) levels, and [(DOPAC+HVA)/DA] (D) ratios in HIP, HYP, PFC, and AMY. All values are mean ± SEM (n = 5). ap < 0.05 compared with control. bp < 0.05 compared with RS. cp < 0.05 compared with E−50. dp < 0.05 compared with RS+O−4 (one-way ANOVA followed by the Student Newman–Keuls test).
![Figure 1. The effect of eugenol in the dose of 50 mg/kg (E−50) and ondansetron in the dose of 4 mg/kg (O-4) on the restraint stress (RS)-induced changes in DA (A), DOPAC (B) and HVA (C) levels, and [(DOPAC+HVA)/DA] (D) ratios in HIP, HYP, PFC, and AMY. All values are mean ± SEM (n = 5). ap < 0.05 compared with control. bp < 0.05 compared with RS. cp < 0.05 compared with E−50. dp < 0.05 compared with RS+O−4 (one-way ANOVA followed by the Student Newman–Keuls test).](/cms/asset/c712b5c3-78fe-460f-8396-f5119749618f/iphb_a_950674_f0001_b.jpg)
RS-induced alteration in serotonergic and noradrenergic system is modulated by eugenol pretreatment
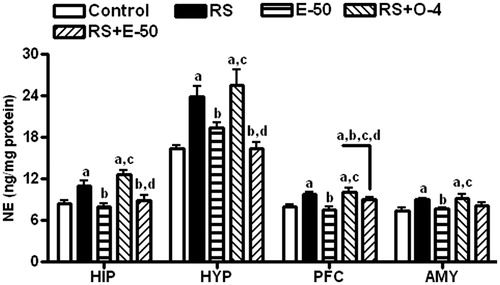
illustrates the effect of eugenol on RS-induced changes in 5-HT (A) and 5-hydroxy indole acetic acid (5-HIAA; B) level, and 5-HIAA/5-HT ratio (C) in different brain regions. The level of 5-HT was increased in the hippocampus and decreased in the hypothalamus, PFC, and amygdala of RS-exposed rats. These RS-induced changes were reversed by eugenol in PFC and amygdala and by ondansetron in the PFC. Both eugenol and ondansetron reversed the RS-induced increase in 5-HIAA level in PFC only. There was a significant increase in 5-HIAA/5-HT ratio in hypothalamus, PFC, and amygdala with RS compared with control animals. Eugenol and ondansetron reversed the RS-induced increase in 5-HIAA/5-HT ratio only in PFC. Eugenol reversed the RS-induced increase in NE level in all the brain regions ().
Figure 2. The effect of E−50 on the RS-induced changes in 5-HT (A) and 5-HIAA (B) levels and 5-HIAA/5-HT (C) ratio in HIP, HYP, PFC, and AMY. All values are mean ± SEM (n = 5). ap < 0.05 compared with control. bp < 0.05 compared with RS. cp < 0.05 compared with E-50. dp < 0.05 compared with RS+O-4 (one-way ANOVA followed by the Student Newman–Keuls test).
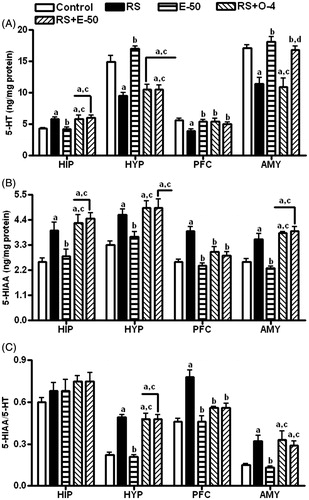
Figure 3. The effect of E−50 on the RS-induced changes in NE levels in HIP, HYP, PFC, and AMY. All values are mean ± SEM (n = 5). ap < 0.05 compared with control. bp < 0.05 compared with RS. cp < 0.05 compared with E-50. dp < 0.05 compared with RS+O−4 (one-way ANOVA followed by the Student Newman–Keuls test).
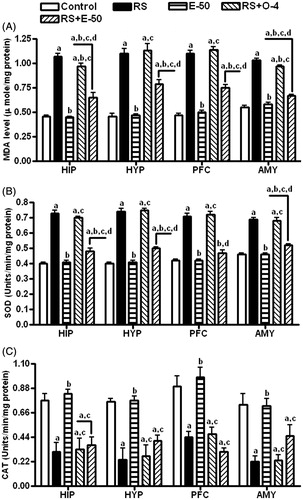
RS-induced oxidative stress is modulated by eugenol in brain tissues
The effect of eugenol (50 mg/kg) pretreatment on lipid peroxidation (LPO) in terms of MDA level (A) and on antioxidant defense enzymes such as superoxide dismutase (SOD; B) and catalase (CAT; C) in different brain regions is illustrated in . Eugenol reversed RS-induced increase in MDA and SOD levels in all the brain regions. Ondansetron reversed the RS-induced increase in the level of MDA significantly only in hippocampus and amygdala. Eugenol administration to normal animals did not change the MDA or SOD levels in any brain regions. Even though RS significantly decreased the CAT activity in all the brain regions neither eugenol nor ondansetron had any effect on this change.
Figure 4. The effect of E−50 on the RS-induced changes in LPO (A), SOD (B) and CAT (C) activities in the HIP, HYP, PFC, and AMY. All values are mean ± SEM (n = 5). ap < 0.05 compared with control. bp < 0.05 compared with RS. cp < 0.05 compared with E−50. dp < 0.05 compared with RS+O−4 (one-way ANOVA followed by the Student Newman–Keuls test).
Discussion
In the present study, we present for the first time that pretreatment with eugenol ameliorated the RS-induced gastrointestinal dysfunction in terms of changes in fecal pellet counts. Further, eugenol did not cause any change in fecal pellet count in normal rats indicating that it does not inherently change the colonic motility. Eugenol pretreatment modulated the RS-induced HPA-axis hyperactivity apart from brain serotonergic and noradrenergic activities. Eugenol also showed antioxidant effect.
The RS at room temperature is a mild and non-ulcerogenic stressor. However, it exhibits stress-related gastrointestinal dysfunction in rats similar to that of IBS-like symptom in humans (Ikeda et al., Citation1995). In the present study, eugenol ameliorated the effect of stress on fecal frequency. Although, eugenol (12.5 and 25 mg/kg) decreased RS-induced increase in the fecal pellet count, the decrease in the dose of 50 mg/kg was more significant than ondansetron. Hence, eugenol (50 mg/kg) was considered as an optimum dose for further mechanistic studies.
It has been reported that the administration of corticosterone into the central nucleus of amygdala increased colonic sensitivity suggesting a significant role of corticosterone in the pathogenesis of IBS-like symptoms (Greenwood-Van Meerveld et al., Citation2001). In this study, eugenol did not modulate the plasma corticosterone level in normal rats, but reversed RS-induced increase in the corticosterone level. This indicates its ability to regulate HPA-axis activation in a stress condition. In contrast, ondansetron did not alter the RS-induced increase in the corticosterone level in rats as that of earlier studies (Levy et al., Citation1993) indicating that the protective effect of ondansetron lacks the modulation of HPA-axis activity.
Monoamines can regulate the HPA-axis activity (Fukudo et al., Citation1992; Krishnamurthy et al., Citation2013). They are altered in different brain regions such as hippocampus, hypothalamus, PFC, and amygdala in both stress and IBS conditions (Garabadu et al., Citation2011; Greenwood-Van Meerveld et al., Citation2001). Here, eugenol per se did not cause any change in the monoaminergic system in any brain regions of normal animals. Ondansetron did not change the RS-induced alterations in the dopaminergic system in any of the brain regions tested in accordance with a previous report (Koulu et al., Citation1990). Eugenol reversed the RS-induced increase in the [(DOPAC+HVA)/DA] ratio only in PFC and amygdala. This observation is consistent with an earlier report (Garabadu et al., Citation2011).
In the present study, ondansetron reversed the RS-induced changes in the serotonergic system specifically in the PFC. Eugenol pretreatment reversed the RS-induced alterations in the 5-HT and 5-HIAA levels and the 5-HT turnover in the PFC region. The 5-HT level in amygdala was also altered by eugenol. Clinically, tricyclic antidepressants and SSRI's are used in the pharmacotherapy of IBS based on the theory in which 5-HT influences the motor function and sensitivity of the gastrointestinal tract (De Giorgio et al., Citation2007). Hence, the protective effect of eugenol against IBS-like symptoms may be due to the modulation of serotonergic activity in selective brain regions specifically in the PFC and amygdala.
Ondansetron did not change the RS-induced increase in the NE level in any of the brain regions tested. It has been reported that 5-HT3 receptor is not involved in the secretion of NE in the rat brain and thus ondansetron being a 5-HT3 antagonist may not exert any effect on central noradrenergic system (Mongeau et al., Citation1994). Eugenol reversed the stress-induced increase in the NE level in all the brain regions except amygdala. In an earlier study, eugenol reversed the 4 h of RS-induced increase in NE level in hypothalamus, PFC, and amygdala but not in hippocampus (Garabadu et al., Citation2011). The variable effect of eugenol on hippocampus and amygdala could be due to the duration of RS paradigm. Preclinical studies suggest that stress releases NE in brain regions such as amygdala and hypothalamus that stimulate colonic contractile activity, resulting in enhanced defecation in rats and mice (Habib et al., Citation2001). Therefore, it can be assumed that eugenol may exert its effect through attenuating RS-induced hyperactive noradrenergic system in rats. In accordance with the reported anti-stress effect, the protective effect of eugenol was found to be essentially mediated by the HPA-axis and noradrenergic system, while the effect of ondansetron was observed to be more of a function of the serotonergic system (Garabadu et al., Citation2011).
Corticotrophin-releasing factor (CRF) is considered to be part of the extra-hypothalamic stress system. Amygdala has numerous CRF terminals and receptors and hence is considered to be an important neuroanatomical area for the extra-hypothalamic stress system (Jankord & Herman, Citation2008). Hence, the changes in the monoaminergic system in amygdala due to eugenol pretreatment in RS rats could be due to reduced corticosterone level. Neuropathological studies suggest that the emotional arousal circuitry which plays an important role in IBS is inhibited by PFC (Labus et al., Citation2009). Therefore, we may presume that the neuroprotective effect of eugenol in RS-induced gastrointestinal dysfunction may be due to region-specific action of eugenol in PFC and amygdala which is, however, subject to further experimentation.
The involvement of oxidative stress in both stress and IBS is well documented (Atif et al., Citation2008; Radak et al., Citation2001). In the present study, ondansetron specifically decreased the activity of LPO in hippocampus without altering the activity of either SOD or CAT suggesting that there may be other mechanisms to attenuate the activity of LPO, such as inhibition of nitric oxide synthase (Bentz et al., Citation2012). Eugenol reversed the stress-induced increase in LPO and SOD activities, but did not alter the CAT activity in brain. The free radical scavenging effect of eugenol interferes with initiation as well as propagation of LPO (Nagababu & Lakshmaiah, Citation1994). Therefore, the changes afforded by eugenol in the antioxidant defense systems may be a function of its free radical scavenging activity. Hence, eugenol through attenuating RS-induced oxidative damage in brain regions may exert a neuroprotective effect in addition to modulating region-specific monoaminergic system.
Conclusion
Eugenol protects against development of IBS-like gastrointestinal symptoms in the RS model. Eugenol decreased RS-induced hyperactivity of HPA-axis. Eugenol modulated stress-induced alterations in brain serotonergic system in selected brain areas such as PFC and amygdala, and the noradrenergic system in all brain areas. Further, the stress-induced oxidative damage in all the brain regions was ameliorated by eugenol, which could be another mechanism supplementing its CNS effect. Hence, eugenol may potentially be useful in the prevention of stress-induced gastrointestinal dysfunction commonly observed in IBS.
Acknowledgements
Ankit Shah is thankful to the University Grant Commission (UGC), New Delhi, India, for the post-graduate student fellowship.
Declaration of interest
The authors declare that they have no conflict of interest.
References
- Ardjmand A, Fathollahi Y, Sayyah M, et al. (2006). Eugenol depresses synaptic transmission but does not prevent the induction of long-term potentiation in the CA1 region of rat hippocampal slices. Phytomedicine 13:146–51
- Atif F, Yousuf S, Agrawal SK. (2008). Restraint stress-induced oxidative damage and its amelioration with selenium. Eur J Pharmacol 600:59–63
- Beers RF Jr, Sizer IW. (1952). A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–40
- Bentz M, Zaouter C, Shi Q, et al. (2012). Inhibition of inducible nitric oxide synthase prevents lipid peroxidation in osteoarthritic chondrocytes. J Cell Biochem 113:2256–67
- De Giorgio R, Barbara G, Furness JB, Tonini M. (2007). Novel therapeutic targets for enteric nervous system disorders. Trends Pharmacol Sci 28:473–81
- Ford AC, Talley NJ, Schoenfeld PS, et al. (2009). Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: Systematic review and meta-analysis. Gut 58:367–78
- Fukudo S, Muranaka M, Nomura T, Satake M. (1992). Brain-gut interactions in irritable bowel syndrome: Physiological and psychological aspect. Nihon Rinsho 50:2703–11
- Garabadu D, Shah A, Ahmad A, et al. (2011). Eugenol as an anti-stress agent: Modulation of hypothalamic–pituitary–adrenal axis and brain monoaminergic systems in a rat model of stress. Stress 14:145–55
- Greenwood-Van Meerveld B, Gibson M, Gunter W, et al. (2001). Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 893:135–42
- Habib KE, Gold PW, Chrousos GP. (2001). Neuroendocrinology of stress. Endocrinol Metab Clin North Am 30:695–728
- Ikeda K, Miyata K, Orita A, et al. (1995). RP67580, a neurokinin1 receptor antagonist, decreased restraint stress-induced defecation in rat. Neurosci Lett 198:103–6
- Jankord R, Herman JP. (2008). Limbic regulation of hypothalamo–pituitary–adrenocortical function during acute and chronic stress. Ann N Y Acad Sci 1148:64–73
- Kakkar P, Das B, Viswanathan PN. (1984). A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys 21:130–2
- Kim C, Speisky MB, Kharouba SN. (1987). Rapid and sensitive method for measuring norepinephrine, dopamine, 5-hydroxytriptamine and their major metabolites in rat brain by high-performance liquid chromatography. J Chromatogr 386:25–35
- Koulu M, Lappalainen J, Hietala J, et al. (1990). 5-HT3 receptor antagonist ondansetron does not alter effects of amphetamine on DA metabolism. Neuroreport 1:126–8
- Krishnamurthy S, Garabadu D, Reddy NR. (2013). Asparagus racemosus modulates the hypothalamic–pituitary–adrenal axis and brain monoaminergic systems in rats. Nutr Neurosci 16:255–61
- Labus JS, Naliboff BD, Berman SM, et al. (2009). Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage 47:952–60
- Levy AD, Li Q, Rittenhouse PA, Van de Kar LD. (1993). Investigation of the role of 5-HT3 receptors in the secretion of prolactin, ACTH and rennin. Neuroendocrinol 58:65–70
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Mihara M, Uchiyama M. (1978). Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–8
- Miyata K, Kamato T, Nishida A, et al. (1992). Role of the serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther 261:297–303
- Mongeau R, De Montigny C, Blier P. (1994). Activation of 5-HT3 receptors enhances the electrically evoked release of [3H] noradrenaline in rat brain limbic structures. Eur J Pharmacol 256:269–79
- Nagababu E, Lakshmaiah N. (1994). Inhibition of microsomal lipid peroxidation and monooxygenase activities by eugenol. Free Radical Res 20:253–66
- Palkovits M, Brownstein MJ. (1988). Maps and Guide to Microdissection of the Rat Brain. New York: Elsevier
- Radak Z, Sasvari M, Nyakas C, et al. (2001). Single bout of exercise eliminates the immobilization-induced oxidative stress in rat brain. Neurochem Int 39:33–8
- Rey E, García-Alonso M, Moreno-Ortega M, et al. (2009). Influence of psychological distress on characteristics of symptoms in patients with GERD: The role of IBS comorbidity. Dig Dis Sci 54:321–7
- Sairam K, Priyambada S, Aryya NC, Goel RK. (2003). Gastroduodenal ulcer protective activity of Asparagus racemosus: An experimental, biochemical and histological study. J Ethnopharmacol 86:1–10
- Spiller R. (2007). Clinical update: Irritable bowel syndrome. Lancet 369:1586–8
- Sunderman FW Jr, Marzouk A, Hopfer SM, et al. (1985). Increased lipid peroxidation in tissues of nickel–chloride-treated rats. Ann Clin Lab Sci 15:229–36
- Woodward CJ, Emery PW. (1987). Determination of plasma corticosterone using high-performance liquid chromatography. J Chromatogr 419:280–4
- Yang SH, Lee MG. (2008). Dose-independent pharmacokinetics of ondansetron in rats: Contribution of hepatic and intestinal first-pass effects to low bioavailability. Biopharm Drug Dispos 29:414–26
- Zhao J, Wang J, Dong L, et al. (2011). A protease inhibitor against acute stress-induced visceral hypersensitivity and paracellular permeability in rats. Eur J Pharmacol 654:289–94
