Abstract
Context: Overexpression of SIRT1 is considered to enhance the resistance of HepG2 cells to irradiation. Shikonin, a naturally occurring naphthoquinone compound, displays anticancer effects and circumvents cancer drug resistance.
Objectives: This study investigated the MDR reversal effect of shikonin induced by the overexpression of SIRT1.
Materials and methods: The overexpression of SIRT1 in HepG2 cells was established by lentivirus infection. Five days after transduction, real-time quantitative polymerase chain reaction and western blotting were used to detect the expression of SIRT1 and MDR1/P-gp. Drug resistance was also evaluated by flow cytometry after rhodamine-123 staining. On day 5, the multidrug resistance cells were treated by shikonin (10−7, 10−6, and 10−5 µmol/L) one time. The cell viability was detected by the MTT assay, and apoptosis was evaluated by Hoechst 33342 staining and caspase-3 activity 24 h after shikonin treatment.
Results: Overexpression of SIRT1 decreased rhodamine-123 staining and successfully produced the R-HepG2 cell line. Compared with HepG2, the expression of MDR1/P-gp mRNA (3.45 ± 0.35) and protein (1.40 ± 0.05) were both upregulated in R-HepG2. Shikonin inhibited cell viability (from 93.9 ± 2.1 to 66.7 ± 1.5%), induced apoptosis of R-HepG2 (apoptotic ratio from 3.5 ± 0.8 to 47.5 ± 2.7%, caspase-3 activity from 103.5 ± 1.9 to 329.2 ± 14.9%, respectively), downregulated the mRNA and protein expression of SIRT1 and MDR1/P-gp, and decreased rhodamin 123 efflux.
Discussion and conclusion: In the present study, we demonstrated that shikonin is able to overcome drug resistance in hepatocellular carcinoma cells, and the mechanism is related to the SIRT1-MDR1/P-gp signaling pathway.
Introduction
In general, multidrug resistance is the primary cause of chemotherapy failure against hepatocellular cancer (HCC) (Gottesman & Pastan, Citation1993). Although numerous resistance mechanisms are known, evidence strongly supports the role of energy-dependent efflux systems [e.g. P-glycoprotein (P-gp)] that pump anticancer agents out of cells (Ambudkar et al., Citation1999). P-gp, a 170 kD protein, belongs to the adenosine triphosphate-binding cassette superfamily of membrane transporter proteins and is encoded by the multidrug resistance 1 (MDR1) gene.
SIRT1 is a NAD+-dependent deacetylase that plays crucial roles in HCC processes. The expression of SIRT1 is significantly elevated in HCC tissues compared with that in non-tumor tissues (Choi et al., Citation2011). Overexpression of SIRT1 in hepatoma HepG2 allows the cells to become considerably more resistant to irradiation under hypoxia than under normoxia (Xie et al., Citation2012). SIRT1 is required to activate transcription factor 4 (ATF4)-induced MDR effects in gastric cancer cells (Zhu et al., Citation2012). On the basis of the evidence, we inferred that SIRT1 may induce MDR in HCC.
Shikonin, a naturally occurring naphthoquinone compound, is the primary component of the dried root Lithospermum erythrorhizon Sieb. et Zucc. (Boraginaceae) (Brigham et al., Citation1999; Yang et al., Citation2009). Shikonin and its analogues are potential pharmaceutical agents with anticancer activities in addition to anti-inflammatory properties (Lee et al., Citation2010; Wang et al., 2013; Yao & Zhou., Citation2010). Shikonin preferentially inhibits the growth of human epidermoid carcinoma cells in a concentration- and time-dependent manner (Singh et al., Citation2003). Shikonin kills cancer cells by regulating the activities of phosphorylated extracellular-regulated protein kinase, c-Jun N-terminal kinase, and protein kinase C-α. Shikonin is also inversely related to MDR. Cancer cells including K562 and MCF-7 strongly respond to shikonin treatment but fail to effectively mobilize drug resistant machineries (Wu et al., Citation2013). In addition, Shikonin circumvents cancer drug resistance in MCF-7 breast cancer cells (Han et al., Citation2007). However, the effect of shikonin on MDR in HCC has not yet been elucidated. Therefore, in this study, we investigated the MDR reversal effect of shikonin induced by the overexpression of SIRT1.
Materials and methods
Cell culture
HepG2 was obtained from ATCC (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium containing 10.0% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 mg/mL), and NaHCO3 (3.7 g/L) and grown in a humidified atmosphere of 5.0% CO2 in air at 37 °C. Cells between passages 7 and 15 were used for the experiments.
Detection of MDR1/P-gp function
Rhodamine-123 efflux was used to detect the MDR1/P-gp function of cells. After lentivirus (LV)-negative or LV-SIRT1 (Genechem, Shanghai, China) infection, HepG2 cells were cultured in 6-well plates, and when the cells reached 70–80% confluence, rhodamin-123 (Sigma, St. Louis, MO) was added to the cells at a final concentration of 0.25 μg/mL and incubated at 37 °C for 1 h. Cells were washed three times with PBS at 4 °C and resuspended at 5–10 × 105 cells/mL in PBS at 4 °C. Rhodamin-123 fluorescence was analyzed with a FACStar flow cytometer (BD Biosciences, San Jose, CA) equipped with an argon laser. The blast population was gated by forward and side scatter characteristics. Rhodamin-123 fluorescence of 10 000 cells was measured logarithmically through a 530-nm bandpass filter at an excitation wavelength of 488 nm. HepG2 cells without incubation with rhodamin-123 served as a negative control. Rhodamin-123 efflux was measured by counting cells in the M1 region of the plot and calculated as a percentage. The higher the percentage of cells in the M1 region, greater the cellular rhodamin-123 efflux and MDR1/P-gp function.
Determination of cell viability
After treating the cells with shikonin (SNK, Chengdu Biopurify Phytochemicals Ltd, Chengdu, China) or paclitaxel (PAC, Sigma, St. Louis, MO) (10 µM) for 24 h, 20 μL of MTT solution was added to each well in 200 μL of the culture medium, and cells were incubated at 37 °C for 4 h in a humidified atmosphere containing 5% CO2. After the cultured liquid was removed, 100 μL of dimethyl sulfoxide was added to each well. Absorbance at 490 nm was measured using a 96-well plate reader (Beckman, Lodi, CA).
Apoptosis analysis
Hoechst 33342 (Beyotime Institute of Biotechnology, Jiangshu, China) staining of HepG2 cells was performed in 12-well plates at 1 × 105 cells/well with 0.1 mg/mL Hoechst 33342 for 10 min at 37 °C in the dark. Hoechst-stained nuclei were observed by a fluorescence microscope (Olympus, Tokyo, Japan) at 521 nm of emission wavelength. A total of 200 cells from five random high power fields were counted. The percentage of apoptosis was expressed as a ratio of apoptotic cells to total cells.
Caspase-3 activity in HepG2 cell lysates was measured by a colorimetric caspase-3 assay kit (Beyotime Institute of Biotechnology, Jiangshu, China) and was expressed as a value of OD405.
Western blotting analysis
Cells were lysed in radioimmunoprecipitation (RIPA) [50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS)] buffer or RIPA buffer with 0.1% cetyltrimethyl ammonium bromide containing protease inhibitor mixture (Sigma, St. Louis, MO). Lysate (50 mg of protein) was resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes using a Mini Tank Transfer System (Bio-Rad Laboratories, Hercules, CA) at 80 V for 1.5 h. The membranes were blocked with 5% milk and hybridized overnight at 4 °C with anti-SIRT1 (1:1500 dilution), anti-P-gp (1:1000 dilution), and anti-β-actin (1:2000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) antibodies. HRP-anti-mouse (1:2000 dilution) and HRP-anti-rabbit (1:2000 dilution) secondary antibodies were used (Thermo Fisher, Rockford, IL). After washing, the HRP signal was developed using a chemiluminescence reagent and detected by autoradiography. Band intensities were densitometrically analyzed, and the results were expressed as the ratio of SIRT1 and P-gp to β-actin.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The mRNA expression of MDR1 and SIRT1 in HepG2 cells was analyzed by the ABI 7300 RT-PCR system (Applied Biosystem, Foster City, CA). The specific primer pairs were MDR1: 5′-CCGTGGCAAACTGGTACTTT-3′ (forward) and 5′-GACGCCAACATAGACCACCT-3′ (backward); SIRT1: 5′-GCCTCACATGCA AGCTCTAGTGAC-3′ (forward) and 5′-TTCGAGGATCTGTGCCAATCATAA-3′ (backward); and β-actin: 5′-GTGGACATCCGCAAAGAC-3′ (forward) and 5′-AAAGGGTGTAACGCAACTA-3′ (backward). The reverse transcription reaction was performed with 1 µg total RNA isolated from the cells of each group. For PCR amplification, cDNAs were amplified using SYBR Green Real-time PCR Master Mix (Takara, Frederick, MD) and 0.4 µmol/L of each primer pair. Amplification was performed starting with an initial step for 30 s at 94 °C, followed by 40 cycles of amplification (94 °C for 30 s, 60 °C for 60 s, and 72 °C for 1 min). All amplification reactions were performed in triplicate, and the averages of the threshold cycles were used to interpolate curves on 7300 System SDS Software (Applied Biosystem, Foster City, CA). Results are expressed as the ratio of SIRT1 and MDR1 to β-actin mRNA, and the value of mRNA expression levels in control conditions was set to 100%.
Statistical analysis
Results are expressed as mean ± S.E.M. Data were analyzed by one-way analysis of variance followed by the Student–Newman–Keuls test for multiple comparisons. Results were considered statistically significant at p < 0.05.
Results
Establish a R-HepG2 cell line by overexpression of SIRT1 in HepG2 cells
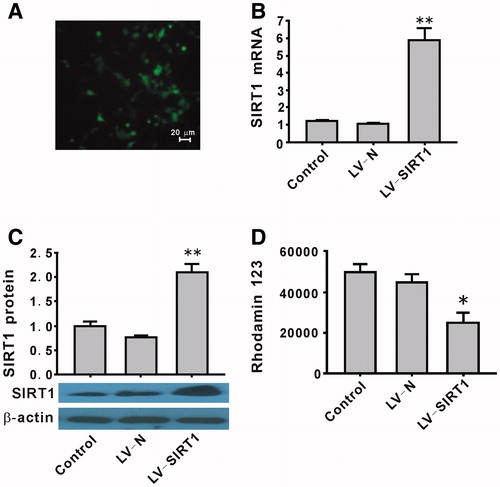
The transduction efficiency as determined by enhanced green fluorescent protein (EGFP) was approximately 80%. The representative image for EGFP is shown in . Transduction of HepG2 cells with the overexpression of LV-SIRT1 successfully upregulated the mRNA and protein expression of SIRT1 (). Multidrug resistance in cells has shown significantly increased drug efflux and decreased the drug concentration in cells. Rhodamin 123 efflux was used to detect multidrug resistance in HepG2 cells. Compared with the LV-N group, SIRT1 overexpression significantly increased rhodamin 123 efflux (). These results suggest that our R-HepG2 model has been established successfully by SIRT1 overexpression.
Figure 1. A R-HepG2 cell model produced by overexpression of SIRT1. HepG2 cells were not infected (control) or infected with control LV-negative or LV-SIRT1 for 5 d. The representative image (400×) for LV-SIRT1 transduction probed by enhanced green fluorescent proteins (EGFP) (A). The RT-qPCR (B) and western blotting (C) were performed to detect the mRNA and protein levels of SIRT1, respectively. β-actin served as a loading control. LV-SIRT1 significantly overexpressed SIRT1 at both the mRNA and protein levels (B and C). LV-SIRT1 also significantly increased the rhodamin 123 efflux (D). Values are mean ± SEM (n = 3). *p < 0.05, **p < 0.01 compared with LV-N. LV-N, lentivirus-negative; LV-SIRT1, lentivirus-SIRT1 overexpression.
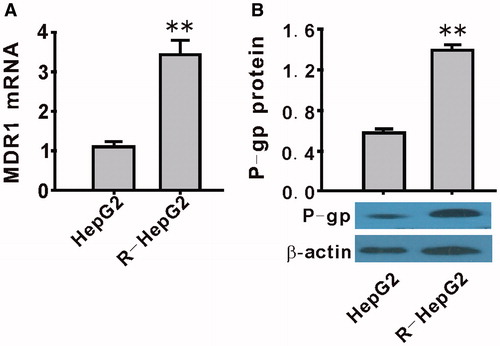
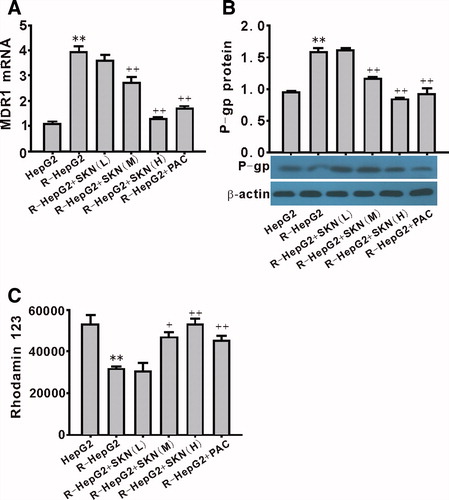
mRNA and protein expression of MDR1/P-gp in R-HepG2.
Compared with HepG2 cell line, both mRNA () and protein () of MDR1/P-gp were upregulated significantly in R-HepG2 (p < 0.01).
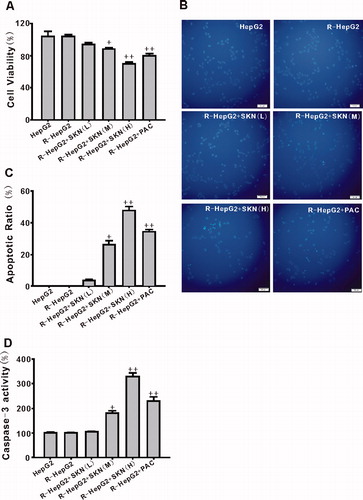
Effect of shikonin on R-HepG2 cell viability and apoptosis
MTT assay showed that R-HepG2 viability was inhibited by shikonin in a dose-dependent manner (). Hoechst 33342 staining assay and caspase-3 activity assays showed that shikonin markedly induced R-HepG2 apoptosis in a concentration-dependent manner (). The beneficial effect of shikonin (10 µmol/L) was better than that of PAC (10 µmol/L) ().
Figure 3. Effect of shikonin on the viability and apoptosis of R-HepG2 cells. MTT assay showed that shikonin decreased R-HepG2 cells viability in a concentration-dependent manner (A). Fluorescence photomicrographs of R-HepG2 cells with Hoechst 33342 staining (200×) detected the apoptotic cells (B) and shikonin increased the number of Hoechst 33342-positive cells (C) along with the caspase-3 activity in a dose-dependent manner (D). Values are mean ± SEM (n = 6). +p < 0.05, ++p < 0.01 compared with R-HepG2. R-HepG2, drug resistance-HepG2 induced by the overexpression of SIRT1; SKN (L), shikonin (10−7 mol/L); SKN (M), shikonin (10−6 mol/L); SKN (H), shikonin (10−5 mol/L); PAC, paclitaxel (10−5 mol/L).
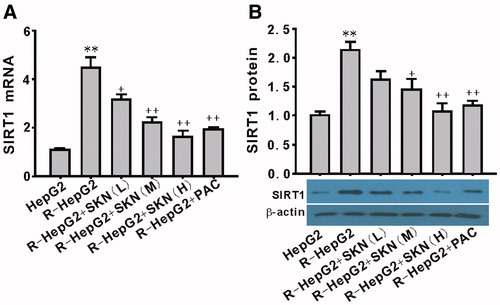
Effect of shikonin on the expression of SIRT1 in R-HepG2
To further explore the role of SIRT1 in shikonin-induced impairment of R-HepG2, the mRNA and protein expression of SIRT1 were measured. The results showed that incubation of R-HepG2 with shikonin for 24 h led to a significant decrease in the mRNA () and protein expression () of SIRT1 in a dose-dependent manner.
Figure 4. Effect of shikonin on the expression of SIRT1 in R-HepG2. Both the mRNA (A) and the protein (B) expression of SIRT1 were downregulated by shikonin. Values are mean ± SEM (n = 3). **p < 0.01 compared with HepG2; +p < 0.05, ++p < 0.01 compared with R-HepG2. R-HepG2, drug resistance-HepG2 induced by the overexpression of SIRT1; SKN (L), shikonin (10−7 mol/L); SKN (M), shikonin (10−6 mol/L); SKN (H), shikonin (10−5 mol/L); PAC, paclitaxel (10−5 mol/L).
Effect of shikonin on the expression of MDR1/P-gp and rhodamin-123 in R-HepG2
To explore the involvement of MDR1/P-gp in shikonin-induced impairment of R-HepG2, the mRNA and protein expression of MDR1/P-gp were determined. Incubation of R-HepG2 with shikonin for 24 h led to a significant decrease in MDR1/P-gp mRNA and protein expression (). Shikonin also inhibited the effect of MDR1/P-gp on drug efflux, which was detected by rhodamin-123 ().
Figure 5. Effect of shikonin on the expression of MDR1/P-gp and rhodamin 123 efflux in R-HepG2. Both the mRNA (A) and the protein (B) expression of MDR1/P-gp were downregulated by shikonin. Shikonin also significantly inhibited rhodamin 123 efflux (C). **p < 0.01 compared with HepG2; +p < 0.05 and ++p < 0.01 compared with R-HepG2. R-HepG2, drug resistant-HepG2 induced by the overexpression of SIRT1; SKN (L), shikonin (10−7 mol/L); SKN (M), shikonin (10−6 mol/L); SKN (H), shikonin (10−5 mol/L); PAC, paclitaxel (10−5 mol/L).
Discussion
In the present study, we demonstrated that overexpression of SIRT1 produced a R-HepG2 cell model mediated by MDR1/P-gp; shikonin overcame drug resistance in HepG2 in a concentration-dependent manner and the effect of shikonin on R-HepG2 was regulated by the SIRT1-MDR1/P-gp pathway.
In the past 5 years, SIRT1 has been identified as an important mediator of the HCC pathology. Clinical research confirmed that SIRT1 is upregulated in relation to regulating the expression of telomerase reverse transcriptase in a subset of HCC (Chen et al., Citation2011). SIRT1 promotes tumorigenesis of HCC through the PTEN/PI3K/AKT signaling pathway (Wang et al., Citation2012). In addition, SIRT1 takes part in the MDR in carcinoma. Reduced glucose use and altered mitochondrial metabolism mediated by SIRT1 is one of the several alterations that contribute to cellular resistance to cisplatin (Chu et al., Citation2005). Recently, researchers have identified that transactivation of SIRT1 mediating ATF4 conferred a MDR phenotype to gastric cancer cells (Zhu et al., Citation2012). In our research, HepG2 cell line was successfully transduced with a lentivirus encoding SIRT1. HepG2-overexpressed SIRT1 increased rhodamin-123 efflux. The results indeed demonstrate that SIRT1 upregulated induced multidrug resistance by increasing drug efflux and established R-HepG2 model was progressive and continuous process.
The MDR1/P-gp is an important membrane transporter that has been recognized as the most vital barrier to effective drug delivery and plays a key role in the development of MDR. Drug resistance in HCC is also related to the overexpression of MDR1/P-gp (Baldissera et al., Citation2012; Huang et al., Citation2012; Ren et al., Citation2012). The overexpression of MDR1/P-gp leads to MDR in human HCC cell lines (Huang et al., Citation1992; Shen et al., Citation1991). Interfering with the expression of MDR1/P-gp reverses the effect of MDR in human hepatoma cells (Chen et al., Citation2006). The function and expression of MDR1/P-gp are regulated by several mechanisms. Recently, researchers found that the function and expression of P-gp are regulated by NF-κB activity and MAPK/ERK pathway-mediated Y-box binding protein 1 nuclear translocation (Zhao et al., Citation2013). In breast tumor cells, the expression and chemoresistance of MDR1 gene are mediated by SIRT1 regulating beta-catenin signaling and NF-κB-specific transcriptional activity (Bourguignon et al., Citation2009). In this assay, we found that SIRT1 facilitated drug efflux which is tumor MDR phenomenon in R-HepG2 cells, resulting in the upregulation of the expression of MDR1/P-gp. These results demonstrate that the overexpression of SIRT1 induced R-HepG2, which is accompanied by an increase in the expression of MDR1/P-gp.
Shikonin is isolated from the herbal plant L. erythrorhizon that has been used clinically for thousands of years. Some pharmacological information on shikonin is readily available for the treatment of HCC (Chen et al., Citation2002; Singh et al., Citation2003; Staniforth et al., Citation2004). Shikonin can inhibit proliferation and induce apoptosis of human HepG2 cells (Yingkun et al., Citation2010). As an anticancer agent, shikonin circumvents cancer drug resistance in different cell lines (K562, MCF-7, and MDR cell K562/Adr) (Han et al., Citation2007; Wu et al., Citation2013). The results of the present study showed that shikonin inhibited proliferation and induced apoptosis of R-HepG2 cells. This suggested that shikonin still exerts a beneficial antitumor effect on multidrug resistance of HCC.
The R-HepG2 was founded by SIRT1 overexpression and the data showed that shikonin also inhibited the expression of SIRT1 as well as the expression and function of MDR1/P-gp in a dose-dependent manner. Therefore, these results demonstrated that shikonin reverse MDR of R-HepG2 may be mediated by the SIRT1-MDR1-P-pg pathway and then exert antitumor effect. However, further investigation is needed before drawing a firm conclusion.
Caspase-3 is required for cell apoptosis (Porter & Janicke, Citation1999). Pathways to activate caspase-3 have been identified that are either dependent on or independent of mitochondrial cytochrome c release and caspase-9 function (Porter & Janicke, Citation1999). It has been found that SIRT1 promotes tumor cellular survival (Wang et al., Citation2013). SIRT1 inhibits apoptosis of HepG2 by p53 deacetylation with decreasing caspase-3 signaling pathway (Zhang & Zhou, Citation2013). In this research, the results showed that shikonin inhibited caspase-3 activation with SIRT1 expression downregulated. These results suggested that SIRT1 may also be mediating the apoptotic effect of shikonin on R-HepG2.
Conclusions
In summary, we have demonstrated that shikonin is able to overcome drug resistance in hepatocellular carcinoma cells, and the mechanism is related to the SIRT1-MDR1/P-gp signaling pathway.
Acknowledgements
The authors would like to thank Enago (www.enago.cn) for the English language review.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ambudkar SV, Dey S, Hrycyna CA, et al. (1999). Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–98
- Baldissera VD, de Mattos AA, Coral GP, et al. (2012). Evaluation of the C3435T polymorphism in the MDR1 gene in patients with hepatocellular carcinoma. Ann Hepatol 11:899–906
- Bourguignon LY, Xia W, Wong G. (2009). Hyaluronan-mediated CD44 interaction with p300 and SIRT1 regulates beta-catenin signaling and NFkappaB-specific transcription activity leading to MDR1 and Bcl-xL gene expression and chemoresistance in breast tumor cells. J Biol Chem 284:2657–71
- Brigham LA, Michaels PJ, Flores HE. (1999). Cell-specific production and antimicrobial activity of naphthoquinones in roots of lithospermum erythrorhizon. Plant Physiol 119:417–28
- Chen J, Zhang B, Wong N, et al. (2011). Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res 71:4138–49
- Chen X, Yang L, Oppenheim JJ, Howard MZ. (2002). Cellular pharmacology studies of shikonin derivatives. Phytother Res: PTR 16:199–209
- Chen XP, Wang Q, Guan J, et al. (2006). Reversing multidrug resistance by RNA interference through the suppression of MDR1 gene in human hepatoma cells. World J Gastroenterol: WJG 12:3332–7
- Choi HN, Bae JS, Jamiyandorj U, et al. (2011). Expression and role of SIRT1 in hepatocellular carcinoma. Oncol Rep 26:503–10
- Chu F, Chou PM, Zheng X, et al. (2005). Control of multidrug resistance gene mdr1 and cancer resistance to chemotherapy by the longevity gene sirt1. Cancer Res 65:10183–7
- Gottesman MM, Pastan I. (1993). Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427
- Han W, Li L, Qiu S, et al. (2007). Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther 6:1641–9
- Huang C, Xu D, Xia Q, et al. (2012). Reversal of P-glycoprotein-mediated multidrug resistance of human hepatic cancer cells by Astragaloside II. J Pharm Pharmacol 64:1741–50
- Huang CC, Wu MC, Xu GW, et al. (1992). Overexpression of the MDR1 gene and P-glycoprotein in human hepatocellular carcinoma. J Natl Cancer Ins 84:262–4
- Lee CC, Wang CN, Lai YT, et al. (2010). Shikonin inhibits maturation of bone marrow-derived dendritic cells and suppresses allergic airway inflammation in a murine model of asthma. Br J Pharmacol 161:1496–511
- Porter AG, Janicke RU. (1999). Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
- Ren YQ, Han JQ, Cao JB, et al. (2012). Association of MDR1 gene polymorphisms with susceptibility to hepatocellular carcinoma in the Chinese population. Asian Pac J Cancer Prev: APJCP 13:5451–4
- Shen DW, Lu YG, Chin KV, et al. (1991). Human hepatocellular carcinoma cell lines exhibit multidrug resistance unrelated to MRD1 gene expression. J Cell Sci 98:317–22
- Singh F, Gao D, Lebwohl MG, Wei H. (2003). Shikonin modulates cell proliferation by inhibiting epidermal growth factor receptor signaling in human epidermoid carcinoma cells. Cancer Lett 200:115–21
- Staniforth V, Wang SY, Shyur LF, Yang NS. (2004). Shikonins, phytocompounds from Lithospermum erythrorhizon, inhibit the transcriptional activation of human tumor necrosis factor alpha promoter in vivo. J Biol Chem 279:5877–85
- Wang H, Liu H, Chen K, et al. (2012). SIRT1 promotes tumorigenesis of hepatocellular carcinoma through PI3K/PTEN/AKT signaling. Oncol Rep 28:311–18
- Wang H, Wu C, Wan S, et al. (2013). Shikonin attenuates lung cancer cell adhesion to extracellular matrix and metastasis by inhibiting integrin beta1 expression and the ERK1/2 signaling pathway. Toxicology 308:104–12
- Wu H, Xie J, Pan Q, et al. (2013). Anticancer agent shikonin is an incompetent inducer of cancer drug resistance. PloS One 8:e52706
- Xie Y, Zhang J, Ye S, et al. (2012). SirT1 regulates radiosensitivity of hepatoma cells differently under normoxic and hypoxic conditions. Cancer Sci 103:1238–44
- Yao Y, Zhou Q. (2010). A novel antiestrogen agent Shikonin inhibits estrogen-dependent gene transcription in human breast cancer cells. Breast Cancer Res Treat 121:233–40
- Yang H, Zhou P, Huang H, et al. (2009). Shikonin exerts antitumor activity via proteasome inhibition and cell death induction in vitro and in vivo. Int J Cancer 124:2450–9
- Yingkun N, Lvsong Z, Huimin Y. (2010). Shikonin inhibits the proliferation and induces the apoptosis of human HepG2 cells. Can J Physiol Pharmacol 88:1138–46
- Zhao BX, Sun YB, Wang SQ, et al. (2013). Grape seed procyanidin reversal of P-glycoprotein associated multi-drug resistance via down-regulation of NF-kappaB and MAPK/ERK mediated YB-1 activity in A2780/T cells. PloS One 8:e71071
- Zhang YY, Zhou LM. (2013). Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol 698:137–44
- Zhu H, Xia L, Zhang Y, et al. (2012). Activating transcription factor 4 confers a multidrug resistance phenotype to gastric cancer cells through transactivation of SIRT1 expression. PloS One 7:e31431