Abstract
Context: Vismia cauliflora A.C.Sm. [Hypericaceae (Clusiaceae)] is a plant from Amazonian forest. It is used by Amerindians to treat dermatosis and inflammatory processes in the skin and has been considered an interesting source of bioactive compounds.
Objective: We evaluated the scavenging capacity of extracts from V. cauliflora (leaf, branch, stem bark, flower, and whole fruit) against reactive oxygen (ROS) and nitrogen species (RNS), namely, superoxide radical (), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), singlet oxygen (1O2), nitric oxide (•NO), and peroxynitrite (ONOO−). In addition, for the first time, the profile of phenolic compounds and carotenoids was determined.
Materials and methods: The scavenging capacities of each extract were determined using specific probes (fluorescent, colorimetric, and chemiluminescent) to detect different reactive species (1O2, HOCl, H2O2, , •NO, and ONOO−). The identification and the quantification of phenolic compounds and carotenoids were carried out by HPLC–DAD–ESI–MS/MS and HPLC–DAD, respectively.
Results: (−)-Epicatechin and proanthocyanidin dimers and trimer were the major phenolic compounds tentatively identified in leaf, branch, stem bark, and flower extracts, while dihydroxybenzoic acids were the major compounds in whole fruit extracts. All-trans-zeinoxanthin and all-trans-β-carotene were the major carotenoids tentatively identified in leaf extracts. All extracts of V. cauliflora showed high efficiency against all tested ROS and RNS, although flower and stem bark extracts exhibited the most remarkable scavenging capacity, especially for •NO and ONOO−.
Discussion and conclusion: Vismia cauliflora has great potential to be used in the development of phytopharmaceutical products due to its characteristic of being a promising source of bioactive compounds with high antioxidant properties.
Introduction
Some regions, such as Amazonian forest, possess an enormous diverse flora, with unique ecological and physiological conditions where many medicinal plants can be found. The interest for plants in South America has sharply increased in recent years, following an uncountable number of medicinal plants that have been used by local population, namely Amerindians, to treat many diseases. The World Health Organization (WHO) recognizes the importance of plant species used by the Amerindian to improve health, thus, recommending their efficacies to be evaluated through pharmacological and toxicological studies.
Among the plants used in Brazilian folk medicine, the genus Vismia may be highlighted. Vismia belongs to Hypericaceae family (Clusiaceae), widely distributed in the Americas, especially in the Central and South America. The importance of some Vismia species is due to its yellow-orange color latex, exuded after small incisions or cuts in several of its parts. Some Amazon tribes use this latex for the treatment of wounds, herpes, and fungal infections on the skin (Pasqua et al., Citation1995), as a potent laxative (Lorenzi & Matos, Citation2002), while its leaves are used for tonic (Macfoy & Sama, Citation1983), antipyretic, and anti-rheumatic properties (Lorenzi & Matos, Citation2002). In other countries, species such as Vismia guineensis (Choiay) Deuce. have been traditionally used to treat various skin diseases and malaria.
The typical metabolites of Vismia species are a special group of secondary metabolites named anthraquinones and prenylated anthraquinones, as well as other components, such as triterpenoids diantraquinonas, benzophenones, and lignans, have been found in Vismia genera (Hussain et al., Citation2012). Considering the activity of their chemical compounds, this species has been considered to be active against certain tumors (ovarian carcinoma and melanocarcinoma) (Cassinelli et al., Citation1986), showing lethal activity on human breast tumor cells (MCF-7) and lethality over a line of cancer cells of colon adenocarcinoma (KM-12) (Suffredini et al., Citation2007).
Although the therapeutic potential of some Vismia species can be thoroughly found in the literature, V. cauliflora A.C.Sm continues to be scientifically unexploited. This species is frequently used in traditional Brazilian medicine for the treatment of dermatosis and inflammatory processes in the skin. Although reactive oxygen (ROS) and nitrogen species (RNS) are known to be associated in both processes, there are no studies available in the literature about its antioxidant potential. Furthermore, to the best of our knowledge, there is no information about the identification and the quantification of bioactive compounds, such as phenolic compounds and carotenoids, of V. cauliflora plant.
Considering the phytopharmaceutical potential of V. cauliflora, this study was designed to determine the scavenging capacity of five extracts from different parts of the V. cauliflora plant (leaf, branch, stem bark, flower, and whole fruit) against the most physiologically relevant reactive oxygen (ROS) and nitrogen species (RNS). In addition, for the first time, the phenolic compounds and carotenoids of each extract were tentatively identified and quantified by HPLC–DAD–MS/MS and HPLC–DAD, respectively.
Materials and methods
Chemicals
The following reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO): dihydrorhodamine 123 (DHR), 4,5-diaminofluorescein (DAF-2), 30% hydrogen peroxide, sodium hypochlorite solution with 4% available chlorine, 3-(aminopropyl)-1-hydroxy-3-isopropyl-2-oxo-1-triazene (NOC-5), β-nicotinamide adenine dinucleotide (NADH), phenazine methosulfate (PMS), nitroblue tetrazolium chloride (NBT), lucigenin, l-ascorbic acid, chlorogenic acid, p-coumaric acid, ellagic acid, ferulic acid, quercetin, gallic acid, catechin, (−)-epicatechin, taxifolin, luteolin, apigenin, myricetin, kaempferol, xanthone, anthraquinone, methanol, methyl tert-butyl ether (MTBE), acetonitrile, and all other chemical salts and solvents of analytical grade. All-trans-lutein, all-trans-zeaxanthin, all-trans-β-cryptoxanthin, and all-trans-β-carotene were acquired from Extrasynthèse (Genay, France). Ultrapure water was obtained from the Arium® pro system (Sartorius, Göttingen, Germany). All phenolic compounds and carotenoids standards showed at least 95% purity, as determined by HPLC–DAD.
Vismia cauliflora samples
The samples (10 kg, including all parts) of Vismia cauliflora were collected in the “Reserva Florestal Adolpho Ducke”, Manaus, Amazonas, Brazil. The previously selected individuals were properly marked and botanically identified and a voucher specimen (No. 212450) is deposited at the Herbarium “Instituto Nacional de Pesquisas da Amazônia” (INPA, Brazil). Plant parts (leaves, branches, stem barks, flowers, and whole fruits) were separated, washed, fragmented, and dried in an oven with forced air circulation (<40 °C) for a week.
Preparation of Vismia cauliflora extracts
Each extract from different parts of V. cauliflora plant was ground separately in a Wiley mill (Biothec/BT 602) and the extracts were prepared by maceration with absolute ethanol in the proportion of mass:solvent of 1:10 (w/v) during 7 d, with frequent stirring (80 rpm) at room temperature (25 °C) without light exposure. The extracts were filtered (Whatman filter paper No. 4) and the liquid extracts were subjected to evaporation at reduced pressure in a rotary evaporator (<38 °C) and each dried extracts were stored at −20 °C for further analysis.
HPLC–DAD–MS/MS analysis of phenolic compounds and carotenoids
HPLC–DAD analysis of phenolic compounds was performed in an Accela LC system (Thermo Fisher Scientific, San Jose, CA) equipped with quaternary pumps (Accela 600), a DAD detector, and an auto-sampler cooled to 5 °C. This equipment was also connected in series to a LTQ ObritrapTM XL mass spectrometer (MS/MS) (Thermo Fisher Scientific, San Jose, CA) with electrospray ionization source (ESI), and a hybrid system combining a linear ion-trap and the Orbitrap mass analyzer. HPLC–DAD analysis of carotenoids was carried out in a LaChrom system (D-700, Merck Hitachi Ltd., Tokyo, Japan) equipped with quaternary pumps (L-7100) and DAD detector (L-7455). For chromatographic analysis, samples and solvents were filtered using, respectively, membranes of 0.22 and 0.45 μm, both from Millipore (Billerica, MA).
The phenolic compounds from all parts of V. cauliflora were analyzed after solubilizing 5 mg of each extract in 2 mL of ethanol, and filtering using membranes of 0.22 μm. Both identification and quantification of phenolic compounds by HPLC–DAD–ESI–MS/MS were carried out on a C18 Synergy Hydro column (4 μm, 250 × 4.6 mm, Phenomenex) (Chisté & Mercadante, Citation2012). The mass spectra were acquired with a scan range from m/z 100 to 1000; the MS parameters were set as follows: ESI source in the negative ion mode; the capillary temperature was 275 °C; and the capillary voltage was set at 2.5 kV. The flow rates of the sheath gas and the auxiliary gas were set to be 40 and 10, respectively (arbitrary unit as provided by the software settings) and the normalized collision energy (CID) for MS/MS experiments was 35%. The phenolic compounds were tentatively identified based on the following information: elution order, retention time of peaks, and UV–visible and mass spectra features as compared with authentic standards (data not shown) analyzed under the same conditions and data available in the literature (Bilia et al., Citation2000; Chisté & Mercadante, Citation2012; Cuyckens & Claeys, Citation2004; Lizcano et al., Citation2012). Phenolic compounds were quantified by comparison with external standards using six-point analytical curves (0.6–20 μg/mL, in duplicate, r2 ≥ 0.99) of gallic acid, quercetin, catechin, (−)-epicatechin, taxifolin, luteolin, apigenin, xanthone, and anthraquinone.
The carotenoids of each extract (15 mg) from different parts of V. cauliflora were extracted (Chisté et al., Citation2012) and then, separated on a C30 YMC column (5 µm, 250 mm × 4.6 mm) using a linear gradient of methanol/MTBE as a mobile phase, at a flow rate of 0.9 mL/min and the column temperature was set at 29 °C (Chisté & Mercadante, Citation2012). The carotenoids were tentatively identified according to the following combined information: elution order on C30 column, co-chromatography with authentic standards, UV–visible spectrum (λmax, spectral fine structure (%III/II), peak cis intensity (%AB/AII)) compared with the data available in the literature (Britton et al., Citation2004; Chisté & Mercadante, Citation2012; de Rosso & Mercadante, Citation2007). The final characterization of each cis-isomers of carotenoids was based on the observation of decreasing %III/II and by the %AB/AII values (≈10% = 9-cis; ≈45% = 13-cis and ≈56% = 15-cis carotenoid) (Briton et al., 2014). The carotenoids were quantified by HPLC-DAD, using external seven-point analytical curves (0.4–30 μg/mL, in duplicate, r2 > 0.99) of all-trans-lutein, all-trans-β-cryptoxanthin, and all-trans-β-carotene.
The limits of detection (LOD) and quantification (LOQ) for each compound were calculated using the parameters of the analytical curves (standard deviation and the slope). The contents of carotenoids and phenolic compounds, determined by HPLC-DAD, were expressed as mg/g of extract (dry basis), considering three independent extraction procedures (n = 3).
ROS- and RNS-scavenging assays
General
To perform all ROS and RNS-scavenging assays, the extracts of V. cauliflora were dissolved in DMSO, except for the extracts in the hyphoclorous acid (HOCl) assay (dissolved in ethanol). The positive controls, quercetin, gallic acid, and ascorbic acid, were dissolved in ethanol (0.03–2 mg/mL). A microplate reader (Synergy HT, Biotek, Winooski, VT) was used for all ROS- and RNS assays, for fluorescence, UV/Vis, and chemiluminescence measurements. In each assay, four independent experiments were performed, using 5–7 concentrations in duplicate, to obtain the IC50 values, which was calculated from the curves of percentage of inhibition versus antioxidant concentration, using the GraphPad Prism 5 software (GraphPad Inc., La Jolla, CA) and the comparison graphs were plotted using OriginPro 8 software (GraphPad Inc., La Jolla, CA).
Superoxide radical-scavenging assay
The non-enzymatic NADH/PMS/O2 system produces and these radicals reduce NBT into a purple colored formazan. Therefore, the effect of the extracts and standards on the
-induced reduction of NBT at 560 nm was determined spectrophotometrically (Gomes et al., Citation2007) and the results are expressed as the inhibition, in percentage, of the NBT reduction to formazan.
Hydrogen peroxide-scavenging assay
The H2O2-induced oxidation of lucigenin system was used to measure the scavenging capacity of V. cauliflora extracts and standards by a chemiluminescence methodology (Chisté et al., Citation2011) and the results are expressed as the inhibition, in percentage, of the H2O2-induced oxidation of lucigenin.
Hypochlorous acid-scavenging assay
The ability of each extract to scavenge HOCl was determined according to Gomes et al. (Citation2007). The assay verifies the effect of the extracts and standards on HOCl-induced oxidation of DHR to rhodamine 123. The results are expressed as percentage inhibition of HOCl-induced oxidation of DHR.
Singlet oxygen-scavenging assay
The 1O2-scavenging capacity was determined by a fluorescence method described by Gomes et al. (Citation2007). It consists in monitoring the oxidation of the non-fluorescent DHR to the fluorescent rhodamine 123 by the reaction with 1O2 generated by thermal decomposition (37 °C) of a previously synthesized water-soluble endoperoxide (disodium 3,3′-(1,4-naphthalene) bispropionate, NDPO2. The results are expressed as percentage inhibition of 1O2-induced oxidation of DHR.
Nitric oxide-scavenging assay
The •NO-scavenging capacity of V. cauliflora extracts and standards was determined according to Chisté et al. (Citation2011). •NO was generated by decomposition of NOC-5, and the •NO− induced oxidation of non-fluorescent DAF-2 to the fluorescent triazolofluorescein (DAF-2T). The results are expressed as the percentage inhibition of •NO-induced oxidation of DAF-2.
Peroxynitrite-scavenging assay
The ONOO−-scavenging capacity of each V. cauliflora extract and standards was determined by monitoring the ONOO−-induced oxidation of non-fluorescent DHR to the fluorescent rhodamine 123 (Chisté et al., Citation2011). In a parallel set of experiments, the assays were performed in the presence of 25 mM NaHCO3, in order to simulate the physiological CO2 concentrations. The results are expressed as percentage inhibition of ONOO− induced oxidation of DHR.
Results and discussion
Phenolic compounds and carotenoids from Vismia cauliflora extracts
The applied HPLC–DAD–ESI–MS/MS methodology allowed the separation (), quantification, and tentative identification of 32 phenolic compounds () in the five extracts obtained from V. cauliflora plant. ESI in the negative ion mode and the hybrid m/z analyzer (linear ion-trap with Orbitrap) provided a very sensitive method and produced the most characteristic data for the identification of each compound in these extracts. In a recent review, Hussain et al. (Citation2012) gathered literature information from 130 compounds already reported in extracts from 15 different species of Vismia, but not for V. cauliflora, and these compounds are mostly xanthones, anthraquinones, and its derivative compounds. Although in our study we focused on the identification of the most incident phenolic compounds families, two xanthones and one anthraquinone could also be tentatively identified. Therefore, to the best of our knowledge, this is the first study where the extensive profile of phenolic compounds in extracts of different parts of V. cauliflora plant is reported.
Figure 1. Chromatogram obtained by HPLC–DAD (270 nm) of phenolic compounds from extracts of Vismia cauliflora. Peak characterization is given in .
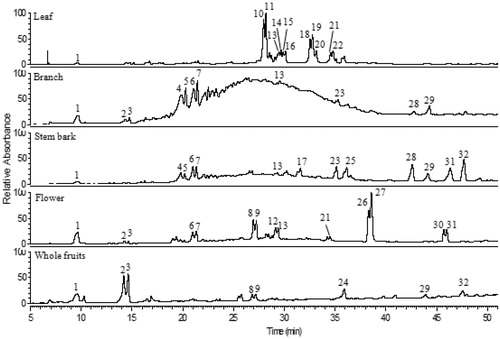
Table 1. Chromatographic, spectroscopic characteristics and contents of phenolic compounds from extracts of Vismia cauliflora plant.
Gallic acid, (−)-epicatechin, and quercetin were positively identified, based on comparisons with UV–Vis and MS spectrum characteristics of authentic standards and by co-chromatography. Peaks 2 and 3 () showed a deprotonated molecule at m/z 153 [M–H]−, and an intense fragment at m/z 109 [M–H-44]−, which correspond to a CO2 loss, and were tentatively identified as dihydroxybenzoic acid molecules. Two proanthocyanindin dimers (peaks 4 and 5) and one proanthocyanindin trimer (peak 6) were tentatively identified, based on the [M–H]−at m/z 577 (dimers) and m/z 865 (trimer), with fragment ions in the MS/MS spectrum at m/z 425 [M–H-152]− (dimers) and m/z 713 [M–H-152]− (trimer) and both showed fragments at m/z 289, corresponding to the characteristic loss of 152 u due to retro-Diels–Alder fission, and of a molecule of catechin or epicatechin, respectively (Cuyckens & Claeys, Citation2004; Kondo et al., Citation2000). Peaks 8, 9, and 12–16 were assigned as quercetin glycosides due to the similar UV–Vis spectra and the presence of intense MS/MS fragment at m/z 301 (quercetin), after respective losses of 162 u (hexose, peaks 8 and 9), 132 u (pentose, peaks 12, 13, and 14), and 146 u (deoxyhexose, peaks 15 and 16). The fragmentation patterns exhibited by these compounds were the same observed for quercetin (peak 27 and also for the authentic standard). Peaks 10 and 11 were tentatively identified as taxifolin deoxyhexosides with [M–H]− at m/z 449 and a deoxyhexose loss at m/z 303 [M–H-146]−. The presence of fragments at m/z 285 [M–H-146-18]− and at m/z 151 [M–H-146-152]−, both derived from taxifolin moiety, corresponds to the loss of a hydroxyl group (18 u) and a characteristic fragment due to retro-Diels–Alder fission (152 u) was also observed in the taxifolin standard. The glycosylation position of each sugar moiety was not assigned in this study, since, in principle, any of the hydroxyl groups in phenolic compounds can be glycosylated, but certain positions are favored: for example, the 7-hydroxyl group in flavones, flavanones, and isoflavones; the 3- and 7-hydroxyls in flavonols and flavanols; and the 3- and 5-hydroxyls in anthocyanidins are common glycosylation sites (Cuyckens & Claeys, Citation2004). Peaks 18, 19, and 20 were tentatively identified as luteolin derivatives due to the presence of intense MS/MS fragments at m/z 285 [M–H-206]−, which correspond to luteolin aglycone after the neutral loss of 206 u. Peaks 23 and 24 were assigned as phloretin hexoside with [M–H]− at m/z 435, followed by a loss of 162 u (hexose moiety) at m/z 273 (phloretin aglycone). Peak 25, tentatively identified as epigallocatechin methyl gallate, presented deprotonated molecule at m/z 471 [M–H]− and an intense MS/MS fragment at m/z 183 [M–H-288]−, corresponding to a loss of catechin or epicatechin, and these patterns match those previously reported (Dou et al., Citation2007). Peaks 28 and 29 presented [M–H]− at m/z 287 and 257, respectively, with UV–Vis and fragmentation pattern similar to those already reported for xanthones (dos Santos et al., Citation2000; Du et al., Citation2012), and were assigned as xanthone derivative compounds. Peaks 30 and 31 were tentatively identified as apigenin derivatives due to similar UV–Vis and MS spectra acquired for apigenin standard and, in both peaks, the ion at m/z 269 was present, as well as the well-known losses of small neutral molecules, such as H2O (-18 u) and CO2 (-44 u) (Cuyckens & Claeys, Citation2004). Finally, an anthraquinone derivative was tentatively identified with [M–H]− at m/z 661 and intense MS/MS fragment at m/z 269, probably emodin moiety, which was already well described in the Vismia genus (Bilia et al., Citation2000; Noungoue et al., Citation2008).
The highest phenolic contents were found in stem bark extract (106 mg/g extract), followed by leaf (103 mg/g), flower (85 mg/g), and branch (68 mg/g), while the least content was found in the whole fruit extract (2.7 mg/g) (). In general, (−)-epicatechin and proanthocyanidin dimers and trimer were the major compounds identified in leaf, branch, stem bark, and flower extracts, while dihydroxybenzoic acids were the major compounds identified in whole fruit extract. Another study also reported that catechin and epicatechin derivatives (proanthocyanidin dimers and trimers) were found as the major compounds in leaf and stem extracts of V. baccifera (Lizcano et al., Citation2012). Additionally, in our study, quercetin was present in high amounts (12 mg/g extract) in flower extracts, as well as epigallocatechin methyl gallate in stem bark extracts (17 mg/g extract).
Regarding carotenoid composition of V. cauliflora extracts, in general, nine compounds were separated () and tentatively identified. It is important to highlight that this is the first report concerning the tentative identification and the quantification of carotenoids from Vismia genus. The extract of leaves presented the highest carotenoid contents (0.6 mg/g extract) () and the major compound tentatively identified was all-trans-zeinoxanthin (0.26 mg/g extract), followed by all-trans-β-carotene (0.17 mg/g extract) (). The other extracts exhibited very low carotenoid contents (below the LOQ), but the presence of some compounds could still be observed ().
Figure 2. Chromatogram obtained by HPLC–DAD (450 nm) of carotenoids from extracts of different parts of Vismia cauliflora plant. Peak characterization is given in .
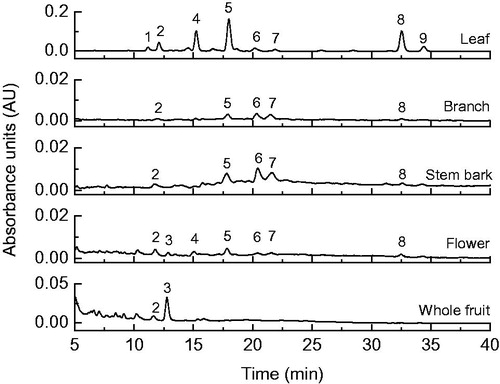
Table 2. Chromatographic, UV–Vis characteristics (HPLC-DAD) and contents of carotenoids from extracts of Vismia cauliflora plant.
All-trans-lutein (peak 3) and all-trans-β-carotene (peak 8) were positively confirmed after comparison with authentic standards. The assignment of all-trans-zeinoxanthin was based on the same chromatographic and spectroscopic behaviors (retention time on the C30 column, λmax, %III/II) at the same analysis condition described by de Rosso and Mercadante (Citation2007). Differentiation between zeinoxanthin and α-cryptoxanthin is difficult because they both have the same chemical formula (C40H56O) and, therefore, the same chromophore; however, the retention time in a C30 column, in a linear gradient of methanol/MTBE, is different and zeinoxanthin elute almost 3 min earlier than α-cryptoxanthin (de Rosso & Mercadante, Citation2007). The epoxy-carotenoid (peak 1), di-cis-lutein (peak 2) and 9-cis-lutein (peak 4) showed similar spectroscopic behavior to those reported in the literature (2004, Updike & Schwartz, Citation2003). Peaks 6 and 7 were assigned as a mixture of di-cis-carotenoid, since both UV–vis spectra were clearly distorted by the presence of mixed compounds and due to the increase of cis-peak (%AB/AII), probably a mixture of cis-compounds derived from the major compounds (zeinoxanthin and β-carotene). The 9-cis isomer of β-carotene (peak 9) was identified considering that the spectral fine structure (%III/II) decreases and intensity of %AB/AII increases as the cis-double bond is getting closer to the centre of the molecule, when compared with β-carotene.
Scavenging of ROS and RNS by Vismia cauliflora extracts
It is well known that ROS and RNS exert beneficial physiological effects; however, at high levels, free radicals and oxidants generate oxidative stress producing damage in cellular macromolecules and furthermore, the reactive species are related to a several human pathologies (Valko et al., Citation2007). It is interesting to highlight that the overproduction of reactive species seems to be associated to allergic and inflammatory skin diseases like atopic dermatitis, urticarial, and psoriasis (Okayama, Citation2005). To neutralize the excess of free radicals and minimize the occurrence of diseases related to oxidative stress, the body possesses various defense mechanisms. However, in the most cases, the endogenous antioxidants are not enough, thus, it is necessary to supply antioxidants from an exogenous source. In this context, to use consciously, a food/plant/extract as an antioxidant exogenous source is mandatory to prove its capacity through chemical and cellular assays and, then, in vivo tests.
The results summarized in show that all parts of V. cauliflora presented high effectiveness against all reactive species in a concentration-dependent manner (). Furthermore, the scavenging capacity of the most extracts of V. caulifora seemed to be closely dependent on the phenolic compound contents. For example, the stem bark extracts, which presented the highest phenolic content (106 mg/g extract), exhibited excellent efficiency as a scavenger of ROS and RNS in most assays (). Despite the presence of carotenoids in all extracts (0.6 mg/g extract in leaf extract and a very low amount for the others), in this study, the carotenoids probably did not contribute to the scavenging capacity against all the tested ROS and RNS.
Figure 3. Scavenging capacity of extracts of different parts from Vismia cauliflora plant against (a) superoxide radical (), (b) hydrogen peroxide (H2O2), (c) hypochlorous acid (HOCl), (d) singlet oxygen (1O2), (e) nitric oxide (•NO), and peroxynitrite (ONOO−) in the absence (f) and presence (g) of NaHCO3. Each point shows the standard error bars and represents the values obtained from four independent experiments, in five to seven concentrations.
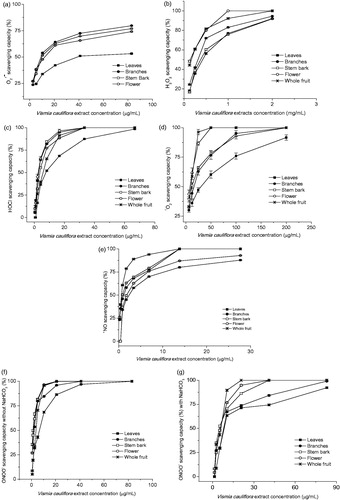
Table 3. Scavenging capacity of Vismia cauliflora extracts against superoxide radical ( ), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), singlet oxygen (1O2), nitric oxide (•NO), and peroxynitrite (ONOO−).
), hydrogen peroxide (H2O2), hypochlorous acid (HOCl), singlet oxygen (1O2), nitric oxide (•NO), and peroxynitrite (ONOO−).
The IC50 values were under 54 µg/mL for all species tested, except for H2O2. Besides the high antioxidant efficiency found for all extracts against ROS, all parts of V. cauliflora and standard compounds were more effective to scavenge RNS, namely •NO and ONOO−. These results are in agreement to results described to quercetin and luteolin (Gomes et al., Citation2007) Castanea sativa and Quercus robur leaf extracts (Almeida et al., Citation2008a).
All extracts of V. cauliflora effectively scavenged at concentrations in the range of 10.3–53.8 µg/mL. The branch, stem bark, and flower extracts presented similar results and higher efficiency than quercetin (14 ± 1 µg/mL) () and Caryocar villosum, an Amazonian fruit (15 µg/mL) (Chisté et al., Citation2012). Considering that
may be dismutated by the superoxide dismutase (SOD) to H2O2 or by metal-catalyzed processes producing •OH, the extracts of V. cauliflora may be very useful to avoid the cascade of ROS formation (H2O2, •OH, and 1O2), inhibiting oxidative damage in lipids, proteins, and DNA and the diseases arising from those reactive species (Gulcin et al., Citation2006).
H2O2 is a potent non-free radical species, able to cross cell membranes, and oxidize a number of cell compounds originating many of its toxic effects (Gulcin et al., Citation2006). Therefore, the extracts that are able to eliminate hydrogen peroxide may be significant for human health and have the potential to be used to protect pharmaceutical and food system (Sen et al., Citation2013). Concerning the scavenging capacity of V. cauliflora against H2O2, the flower extract was more effective than all other parts and also showed higher efficiency than standards of gallic acid, quercetin, ascorbic acid (), ellagic acid (108.3 µg/mL) (Chisté et al., Citation2012), Juglans regia leaf extracts (383 µg/mL) (Almeida et al., Citation2008b), and Meyna spinosa leaf extracts, a traditional medicinal plant from India (126.8 µg/mL) (Sen et al., Citation2013). In general, the IC50 values to scavenge H2O2 are higher in comparison with other ROS and RNS and sometimes the IC50 could be not achieved in the maximum concentration tested (Chisté et al., Citation2012; Sen et al., Citation2013).
The HOCl is the strongest oxidant produced by neutrophils and is considered as a potent pro-inflammatory agent, and can also react with to generate another microbicidal species •OH (Aruoma, Citation1997). The present results suggest that all parts of V. cauliflora have a great potential to scavenge HOCl (). The branch and flower extracts demonstrated higher capacity to scavenge HOCl than other V. cauliflora extracts and also than extracts of Caryocar villosum pulp (3.6–299 µg/mL) (Chisté et al., Citation2012) and Solanum sessiliflorum pulp (13 µg/mL) (Rodrigues et al., Citation2013). However, gallic acid, quercetin, and ascorbic acid were even more efficient than V. cauliflora extracts ().
The stem bark, flower, and whole fruit extracts showed the highest scavenging capacity against 1O2 with IC50 varying from 8.1 ± 0.4 to 11.5 ± 0.5 µg/mL. Its scavenging efficiencies was similar to Quercus robur and Castanea sativa leaf extracts (IC50 values of 7.9 and 12.3 µg/mL, respectively) (Almeida et al., Citation2008a), but lower than gallic acid, ascorbic acid, and quercetin (). 1O2 can generate peroxides and cause protein damage, thus, plants/extracts that present high capacity to scavenge this reactive specie is highly desirable.
The production of •NO at low physiological levels exerts fundamental activity on physiological processes. However, the sustained •NO generation can cause deleterious effect in tissues and it is involved in the pathogenesis of disease states like endotoxin shock and inflammation (Gomes et al., Citation2006). Moreover, •NO and , produced by cells of the immune system during the oxidative burst, may react with each other, generating significant amounts of ONOO−, which is a strong oxidative molecule (Carr et al., Citation2000). In this study, all extracts of V. cauliflora were very efficient scavengers of •NO, with low IC50 values (varying from 0.9 to 2.2 µg/mL). The whole fruit extract exhibited the lower efficiency (3.6 ± 0.2 µg/mL) than other parts; nevertheless, its IC50 value was similar to bixin (3 µg/mL), considered as a good scavenger of •NO (Chisté et al., Citation2011).
In relation to ONOO−, this RNS has been shown as a strong oxidizing and nitrating compound capable to oxidize a variety of biomolecules including lipids, proteins, carbohydrates, and DNA (Carr et al., Citation2000). Consequently, this RNS has been implicated in neurodegenerative disorders, Alzheimer’s, Parkinson’s, and cardiovascular diseases (Gomes et al., Citation2006). Vismia cauliflora extracts demonstrated high capacity to scavenge ONOO− and all parts presented IC50 values lower than 5.8 µg/mL (without NaHCO3) and 9.8 µg/mL (in the presence of 25 mM NaHCO3) (). The flower and stem bark extracts of V. cauliflora exhibit slight higher efficiency than Juglans regia leaf extract (1.66 µg/mL), a plant used in folk medicine for the treatment of several disorders as skin inflammations and ulcers (Almeida et al., Citation2008b).
In this work, the phenolic and carotenoid profiles of V. cauliflora extracts, as well as its scavenging capacities against the most important physiologic ROS and RNS, were reported for the first time. The flower and stem bark extracts exhibited the most remarkable scavenging capacity against all ROS and RNS, especially for •NO and ONOO−, which may be related to the structural characteristics and the high amount of phenolic constituents present in these extracts.
In conclusion, the results presented in this study suggest that V. cauliflora, a scientific unknown Amazonian plant, has great potential to be used in the development of phytopharmaceutical products due to its characteristic to be a promising source of bioactive compounds with antioxidant properties. Furthermore, after this important screening in relation to the antioxidant potential of extracts from different parts of V. cauliflora plant, additional studies should be carried out to fully understand the effect of this specie in cellular systems when oxidative stress is considered, namely concerning its indigenous use to treat dermatosis and inflammatory processes in the skin.
Declaration of interest
This work received financial support from the European Union (FEDER funds through COMPETE) and National Funds (FCT, Fundação para a Ciência e Tecnologia) through project Pest-C/EQB/LA0006/2013. The work also received financial support from the European Union (FEDER funds) under the framework of QREN through Project NORTE-07-0124-FEDER-000066. Alessandra Braga Ribeiro acknowledges CAPES Foundation (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Ministry of Education of Brazil, the financial support for the PDSE grant n. 2262-13-4. Marisa Freitas acknowledges FCT the financial support for the Post-doc Grant (SFRH/BPD/76909/2011) in the ambit of “POPH-QREN – Tipologia 4.1-Formação Avançada” co-sponsored by FSE and national funds of MCTES. The authors report that they have no conflicts of interest.
References
- Almeida IF, Fernandes E, Lima JLFC, et al. (2008a). Protective effect of Castanea sativa and Quercus robur leaf extracts against oxygen and nitrogen reactive species. J Photochem Photobiol B 91:87–95
- Almeida IF, Fernandes E, Lima JLFC, et al. (2008b). Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem 106:1014–20
- Aruoma OI. (1997). Scavenging of hypochlorous acid by carvedilol and ebselen in vitro. Gen Pharmacol 28:269–72
- Bilia AR, Yusuf AW, Braca A, et al. (2000). New prenylated anthraquinones and xanthones from Vismia guineensis. J Nat Prod 63:16–21
- Britton G, Liaaen-Jensen S, Pfander H. (2004). Carotenoids Handbook. Switzerland: Birkhauser Publishing
- Carr AC, Mccall MR, Frei B. (2000). Oxidation of LDL by myeloperoxidase and reactive nitrogen species – Reaction pathways and antioxidant protection. Arterioscl Throm Vas 20:1716–23
- Cassinelli G, Geroni C, Botta B, et al. (1986). Cytotoxic and antitumor-activity of vismiones isolated from Vismieae. J Nat Prod 49:929–31
- Chisté RC, Mercadante AZ. (2012). Identification and quantification, by HPLC-DAD-MS/MS, of carotenoids and phenolic compounds from the Amazonian fruit Caryocar villosum. J Agric Food Chem 60:5884–92
- Chisté RC, Freitas M, Mercadante AZ, Fernandes E. (2012). The potential of extracts of Caryocar villosum pulp to scavenge reactive oxygen and nitrogen species. Food Chem 135:1740–9
- Chisté RC, Mercadante AZ, Gomes A, et al. (2011). In vitro scavenging capacity of annatto seed extracts against reactive oxygen and nitrogen species. Food Chem 127:419–26
- Cuyckens F, Claeys M. (2004). Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom 39:1–15
- De Rosso VV, Mercadante AZ. (2007). Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J Agric Food Chem 55:5062–72
- Dos Santos MH, Nagem TJ, Da Silva MC, Silva LGFE. (2000). Xanthones from Vismia latifolia. J Brazil Chem Soc 11:537–9
- Dou JP, Lee VSY, Tzen JTC, Lee MR. (2007). Identification and comparison of phenolic compounds in the preparation of oolong tea manufactured by semifermentation and drying processes. J Agric Food Chem 55:7462–8
- Du XG, Wang W, Zhang QY, et al. (2012). Identification of xanthones from Swertia punicea using high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 26:2913–23
- Gomes A, Fernandes E, Lima JLFC. (2006). Use of fluorescence probes for detection of reactive nitrogen species: A review. J Fluoresc 16:119–39
- Gomes A, Fernandes E, Silva AMS, et al. (2007). 2-Styrylchromones: Novel strong scavengers of reactive oxygen and nitrogen species. Bioorgan Med Chem 15:6027–36
- Gulcin I, Mshvildadze V, Gepdiremen A, Elias R. (2006). Screening of antiradical and antioxidant activity of monodesmosides and crude extract from Leontice smirnowii tuber. Phytomedicine 13:343–51
- Hussain H, Hussain J, Al-Harrasi A, et al. (2012). Chemistry and biology of genus Vismia. Pharm Biol 50:1448–62
- Kondo K, Kurihara M, Fukuhara K, et al. (2000). Conversion of procyanidin B-type (catechin dimer) to A-type: Evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Tetrahedron Lett 41:485–8
- Lizcano LJ, Viloria-Bernal M, Vicente F, et al. (2012). Lipid oxidation inhibitory effects and phenolic composition of aqueous extracts from medicinal plants of Colombian Amazonia. Int J Mol Sci 13:5454–67
- Lorenzi H, Matos FJA. (2002). Plantas medicinais do Brasil: Nativas e exóticas cultivadas. Brazil: Instituto Plantarum
- Macfoy CA, Sama AM. (1983). Medicinal-plants in Pujehun district of Sierra Leone. J Ethnopharmacol 8:215–23
- Noungoue DT, Antheaume C, Chaabi M, et al. (2008). Anthraquinones from the fruits of Vismia laurentii. Phytochemistry 69:1024–8
- Okayama Y. (2005). Oxidative stress in allergic and inflammatory skin diseases. Curr Drug Targets Inflamm Allergy 4:517–19
- Pasqua G, Monacelli B, Cuteri A, et al. (1995). Accumulation of vismione-A in regenerated plants of Vismia guianensis Dc. Protoplasma 189:9–16
- Rodrigues E, Mariutti LRB, Mercadante AZ. (2013). Carotenoids and phenolic compounds from Solanum sessiliflorum, an unexploited Amazonian fruit, and their scavenging capacities against reactive oxygen and nitrogen species. J Agric Food Chem 61:3022–9
- Sen S, De B, Devanna N, Chalcraborty R. (2013). Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa Roxb., an Indian medicinal plant. Chin J Nat Med 11:149–57
- Suffredini IB, Paciencia MLB, Varella AD, Younes RN. (2007). In vitro cytotoxic activity of Brazilian plant extracts against human lung, colon and CNS solid cancers and leukemia. Fitoterapia 78:223–6
- Updike AA, Schwartz SJ. (2003). Thermal processing of vegetables increases cis isomers of lutein and zeaxanthin. J Agric Food Chem 51:6184–90
- Valko M, Leibfritz D, Moncol J, et al. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B 39:44–84
