Abstract
Context: Three clover [Trifolium L. (Leguminosae)] species were selected on the basis of data from traditional medicine, phytochemical profiles, and agricultural significance.
Objective: The in vitro evaluations of free radical scavenging properties, ferric reducing abilities, and antioxidant effects of extracts from T. pratense L. (crude extract and phenolic fraction), T. pallidum L., and T. scabrum L. (phenolic fractions) were performed.
Materials and methods: Activities of the Trifolium extracts were determined at their final concentrations of 1.5–50 µg/ml. Free radical scavenging properties of methanol extract solutions were estimated by the reduction of DPPH• and ABTS• radicals. Measurements of the total antioxidant capacity (TAC) were carried out to assess the antioxidant activities of the extracts in human blood plasma under conditions of oxidative stress, induced by 200 μM peroxynitrite.
Results: The phenolic fraction of T. pratense displayed the strongest ABTS• and DPPH• radical scavenging effects (EC50 value of 21.69 and 12.27 µg/ml, respectively). The EC50 value for T. pallidum extract attained 29.77 and 30.06 µg/ml. The two remaining extracts were less potent scavengers (EC50 value higher than 50 µg/ml). Similar differences were obtained during evaluation of the ferric reducing abilities. Analysis of antioxidant properties of the extracts in blood plasma did not provide such evident differences in their actions, however, it indicated that the T. pratense phenolic fraction displayed the strongest effect.
Conclusions: The examined Trifolium extracts partly protected blood plasma and enhanced its non-enzymatic antioxidant defense against harmful action of peroxynitrite in vitro.
Introduction
The Trifolium L. genus (Leguminosae) consists of over 240 clover species, usually regarded as pasture plants. At least several of them (e.g., T. pratense L., T. alexandrinum L., T. repens L.) are well known herbs, used in folk medicine of various cultures. Recently, some clovers have been also recognized as a source of substances with the therapeutic effects that may be utilized in modern medicine. Most of the available literature contains reports on physiological and medicinal properties of red clover (T. pratense) (Kolodziejczyk-Czepas, Citation2012; Sabudak & Guler, Citation2009). The ethnopharmacological recommendations of T. pratense include the treatment of sore throat, fever, pneumonia and meningitis, lung illnesses, skin problems, as well as some disorders of nervous and reproductive systems (Kolodziejczyk-Czepas, Citation2012).
In contemporary medicine, herbal medications of red clover origin are a source of phytoestrogens– polyphenolic compounds with the structural similarities to 17β-estradiol and capable of binding to estrogen receptors (ERα and ERβ). The aerial parts of this plant contain at least seven additional isoflavones when compared with soybeans, which are rich in daidzein, genistein, and glycitein (Wu et al., Citation2003). On one hand, dietary supplements containing red clover extracts are mainly administered as an alternative to the conventional hormonal replacement therapy (Booth et al., Citation2006; Engelmann et al., Citation2009). Isoflavones (such as daidzein, genistein, biochanin A, and formononetin) display diverse biological activities, including antioxidant (Ruiz-Larrea et al., Citation1997), anti-inflammatory, and anti-tumor actions (Krenn & Paper, Citation2009). On the other hand, the clinical relevance of red clover-based medications is still disputable. A meta-analysis performed by Thompson Coon et al. (Citation2007) provided some evidence of marginally significant effect of T. pratense isoflavones for treating hot flushes in menopausal women. Contrary to this critical conclusion, a growing number of reports suggest the considerable medicinal value of red clover, e.g., the improvement of postmenopausal depressive and anxiety symptoms (Lipovac et al., Citation2010). Moreover, other beneficial activities of substances and extracts of T. pratense, such as cardiovascular disease-preventive effects, were also reported (Asgary et al., Citation2007). The physiological actions and biological properties of clover species other than T. pratense have not been well described; however, some evidence confirming a considerable therapeutic potential of various clovers is available (Renda et al., Citation2013). Furthermore, in our previous studies, we found an antioxidant action of T. pallidum, T. pratense, and T. scabrum in the prevention of oxidative damage to blood platelets and plasma components (Kolodziejczyk et al., Citation2011; Kolodziejczyk-Czepas et al., Citation2013a,Citationb).
The present study was intended to examine the biological activities of extracts from three Trifolium species: a common forage species (T. pratense) and two less recognized clovers (T. pallidum and T. scabrum). We compared free radical scavenging and reducing abilities of the examined extracts and we evaluated their antioxidant actions in protection of the total antioxidant capacity (TAC) of blood plasma exposed to peroxynitrite-induced oxidative stress. The plants were chosen on the basis of results from our previous studies, recommendations of traditional medicine, agricultural significance, and available data on their promising chemical profiles. A general analysis of the phytochemical profiles of 57 Trifolium species, reported by Oleszek et al. (Citation2007), indicated T. scabrum as one of the three plants containing the highest amount of isoflavones (7–9% of the dry matter). Concentrations of isoflavones in other species were higher or comparable with the amounts occurring in T. pratense (1–4% of the dry matter), whereas T. pallidum was classified to the cluster of the species rich in clovamides.
Materials and methods
Plant material
Seeds of T. pallidum Waldst. et. Kit. (TRIF 253/95) and T. scabrum L. (TRIF 12079) were obtained from GeneBank, Leibniz Institute of Plant Genetics and Crop Plant Research (Gatersleben, Germany). Both these plants and T. pratense var. Nemaro (seeds obtained from the Office of the Seed, Pulawy) were sown in the experimental plots of the Institute of Soil Science and Plant Cultivation, Pulawy, Poland. The voucher samples (58/2011, 81/2011, and 89/2011) have been deposited at the Department of Biochemistry and Crop Quality, Pulawy. The aerial parts were harvested at the beginning of flowering, frozen (−16 °C), lyophilized, powdered, and used for extraction.
Separation of extracts
The isolation of crude extract and phenolic fractions was performed according to previously developed procedures for Medicago sativa L. (Stochmal et al., Citation2001). The aerial parts of plants were extracted with 80% (v/v) MeOH for 24 h at room temperature. After filtration, the extract was concentrated under reduced pressure (35 °C). Part of the crude extract of T. pratense was used to study. The rest of the extract, as well as other species of clover extracts, was dissolved in water and applied to a C18 preparative column (60 × 100 mm; 40–63 mm, Merck, White House Station, NJ). The column was washed with water to remove sugars, and then with 40% (v/v) MeOH to elute phenolics. The obtained phenolic fractions were profiled on ultra-performance liquid chromatography (UPLC) (solvent 1% (v/v) acetic acid→40% (v/v) MeCN over 10 min; column C18 5.0 × 2.1 mm, UPLC BEH; column temperature 50 °C; flow rate 0.35 ml/min).
The phenolic fraction from T. pallidum contained four groups of compounds (phenolic acids, flavonoids, isoflavones, and clovamides, in total 35.06 mg/g), while T. pratense and T. scabrum phenolic fractions contained only three components (phenolic acids, flavonoids, and isoflavones, in total of 14.82 and 81.09 mg/g) (Kolodziejczyk-Czepas et al., Citation2013a,b). The UPLC analysis indicated that the crude extract of T. pratense contained the compounds of the same type (total 20.31 mg/g), but the content of isoflavones was two times higher than in the phenolic fraction ().
Table 1. Phytochemical composition of the examined plant extracts, derived from aerial parts of three Trifolium species (Kolodziejczyk-Czepas et al., Citation2013a,b; a compilation of data).
Evaluation of DPPH• and ABTS• radical scavenging abilities
The anti-free radical activities of the examined Trifolium extracts were assessed in the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) (Janaszewska & Bartosz, Citation2002) and ABTS• (derived from oxidation of 2,2″-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)) reduction assays (Re et al., Citation1999).
Isolation of blood plasma and preparation of samples
Blood from healthy volunteers was purchased from the Regional Centre of Blood Donation and Blood Treatment in Lodz, Poland. Plasma samples were preincubated for 5 min at 37 °C with extracts obtained from the examined Trifolium species (added to the final concentration of 1.5–50 µg/ml), and then exposed to 200 µM peroxynitrite (ONOO−). For the assessments of antioxidant effects of the extracts, samples of plasma treated with ONOO− in the absence of these extracts were also prepared. Control samples were non-treated with either the extracts or the peroxynitrite.
Measurements of the ferric-reducing ability of plasma (FRAP)
Determination of the TAC of plasma using the FRAP assay was carried out according to modified method of Benzie and Strain (Citation1996) as was described previously (Kolodziejczyk-Czepas et al., Citation2014). This method was also used for the evaluation of ferric reducing ability of different concentrations of the tested Trifolium extracts, where instead of blood plasma, solutions of these extracts were used. The obtained results were then calculated from a standard curve, ranging from 0 to 1 mM of FeSO4, and expressed as equivalents of Fe2+.
Determination of TAC of blood plasma
The evaluation of TAC was also performed by the ABTS• and DPPH• reduction assays. The ability to reduce ABTS• of the analyzed samples was measured according to the method of Re et al. (Citation1999), whereas the 1,1-diphenyl-2-picrylhydrazyl radical (DPPH•) reduction assay was performed according to a protocol described by Janaszewska and Bartosz (Citation2002).
Statistical analysis
All values were expressed as mean ± SD. The obtained results were analyzed under the account of normality with the Shapiro–Wilk test. The significance of differences between the values was assessed by the Wilcoxon signed-rank test; p < 0.05 was considered as statistically significant.
Results
Analysis of the free radical scavenging properties of the examined extracts revealed significantly higher activities of two extracts: phenolic fraction of T. pratense (the most effective) and T. pallidum (the second most effective) (). The EC50 values of T. pratense crude extract and phenolic fractions of T. pratense, T. pallidum, and T. scabrum, calculated for DPPH• radical scavenging, were 56.43, 12.27, 30.06, and 86.82 µg/ml, respectively. The EC50 values of these extracts for ABTS• radical scavenging were 51.32, 21.69, 29.77, and 55.42 µg/ml (for T. pratense crude extract and phenolic fractions of T. pratense, T. pallidum, and T. scabrum, respectively). Similarly, the ferric reducing abilities of the phenolic fraction of T. pratense and T. pallidum extract were considerably higher than those for T. pratense crude extract and phenolic fraction of T. scabrum (). Additionally, the EC50 values obtained for T. pratense, T. pallidum, and T. scabrum from measurements of free radical scavenging tests during this study were compared with results for other six clover species, derived from our earlier experiments (Kolodziejczyk-Czepas et al., Citation2014). Our comparative analysis of the radical scavenging properties of 10 extracts from Trifolium species indicated that five of them (T. alexandrinum, T. pallidum, T. pratense (phenolic fraction), T. resupinatim var. resupinatum, and T. resupinatum var. majus) were evidently stronger (EC50 ≤ 30 μg/ml) than the remaining five. The order of EC50 value in the group of stronger scavengers was as follows: T. alexandrinum < T. pratense (phenolic fraction) ≈ T. resupinatum var. resupinatum < T. resupinatum var. majus < T. pallidum ().
Table 2. The free radical scavenging activities of the examined Trifolium extracts. The results are expressed as percent of radical scavenging ability (% RSA). The table includes mean values ± SD of measurements done in triplicate.
Table 3. Comparison of the ferric reducing ability of the examined Trifolium extracts (expressed as mM of Fe2+). The table includes mean values ±SD of measurements done in triplicate.
Table 4. The comparison of the free radical scavenging effects of 10 Trifolium plant-derived extracts. The table comprises a compilation of EC50 values for either ABTS• or DPPH• scavenging measured during the present study (results for T. pratense, T. pallidum, and T. scabrum) and data from our previous work.
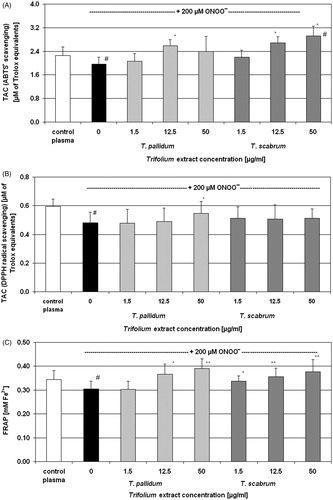
To evaluate the effectiveness of examined plant extracts in the protection of the TAC of blood plasma, we selected the three concentrations: 1.5, 12.5, and 50 µg/ml. Significant peroxynitrite-induced decrease of the TAC was observed both in the measurements of ABTS• and DPPH• radical reduction test, as well as in the FRAP assay (evaluation of the FRAP) ( and ). The comparison of protective actions of T. pratense extracts in blood plasma under ONOO−-induced oxidative stress has indicated that the phenolic fraction (at concentrations of 12.5 and 50 µg/ml) was more effective, as was estimated by measurements of the TAC performed with the use of ABTS• and in the FRAP assay (). The determination of the TAC with DPPH• radical revealed the antioxidant action of both extracts of T. pratense at all examined concentrations (). However, in the TAC assessments, the differences between phenolic fraction and the crude extract were less apparent than those from the evaluation of their free radical scavenging and reducing abilities ( and , respectively). Similarly, in blood plasma, the differences in the antioxidant effects in blood plasma of T. pallidum and T. scabrum also were not so clear, compared with their free radical scavenging and reducing properties ( and , respectively). The FRAP assay showed the protective actions of both T. pallidum and T. scabrum that were statistically significant at the concentrations of 12.5 and 50 µg/ml for T. pallidum, and at the concentration range of 1.5–50 µg/ml for T. scabrum (). In the measurements of TAC with the use of ABTS•, the antioxidant effect was statistically significant at the concentration of 12.5 µg/ml for T. pallidum, and at the concentration range of 12.5–50 µg/ml for T. scabrum. In contrast, in experiments with DPPH•, only the T. pallidum extract (50 µg/ml) had a considerable protective effect, whereas the T. scabrum extract had no significant influence ().
Figure 1. Comparison of the protective effects of Trifolium pratense extracts on the total antioxidant capacity of blood plasma under ONOO−-induced oxidative stress conditions. The TAC of human blood plasma was determined spectrophotometrically by the ABTS• (Panel A) and DPPH• (Panel B) radicals decolourization, as well as by measurements of the ferric reducing ability (the FRAP assay) (Panel C). Results are presented as means ± SD of six independent experiments (six different donors); #p < 0.0 and ##p < 0.01 for ONOO−-treated plasma (with or without extracts) versus control plasma; *p < 0.05 and **p < 0.01 for plasma treated with ONOO− in the presence of extracts versus plasma treated with ONOO− in the absence of extracts.
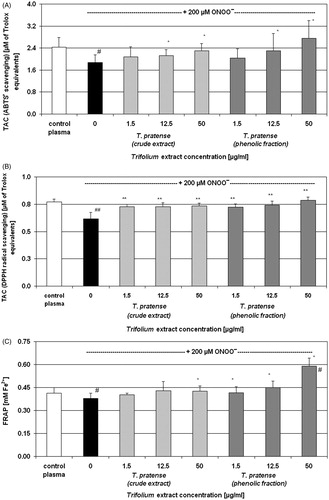
Figure 2. Evaluation of the protective effects of Trifolium pallidum and Trifolium scabrum extracts on total antioxidant capacity of blood plasma under ONOO−-induced oxidative stress conditions. The total antioxidant capacity was estimated by the reduction of ABTS• (Panel A) and DPPH• (Panel B) radicals and by the measurements of the ferric reducing ability of plasma (Panel C). Results are presented as means ± SD of six independent experiments (six different donors); #p < 0.05 for ONOO−-treated plasma (with or without extracts) versus control plasma, and *p < 0.05 and **p < 0.01 for plasma treated with ONOO− in the presence of extracts versus plasma treated with ONOO− in the absence of extracts.
Discussion
Although the generation of reactive oxygen species is associated with normal metabolism, the enhanced production of oxidants may contribute to the development of numerous pathological conditions, including cardiovascular disorders (Weseler & Bast, Citation2010) such as atherosclerosis (Tousoulis et al., Citation2011), hypertension (Schulz et al., Citation2011), and heart failure (Shah et al., Citation2011). In this in vitro study, we evaluated the biological properties of extracts from aerial parts of three clover species (T. pallidum, T. pratense, and T. scabrum) in an aspect of their possible role in the protection of TAC of blood plasma against oxidative stress. For the induction of oxidative stress, we used 200 µM peroxynitrite – one of the main oxidants formed in vivo in the cardiovascular system. Our previous studies on peroxynitrite effects on the haemostatic system revealed its harmful influence at lower concentration range (less than 10 µM) (Nowak & Wachowicz, Citation2001; Olas et al., Citation2004). However, 200 µM ONOO− induced a decrease of the TAC of blood plasma, allowing evaluation of the antioxidant activities of the examined extracts. The antioxidant action of plant polyphenolic substances may be a result of scavenging of both peroxynitrite and secondary-formed radicals. ONOO− scavenging ability of plant polyphenolics has been attributed mainly to the presence of hydroxyl groups in their structure. Furthermore, studies of Heijnen et al. (Citation2001a) indicated the importance of interactions between the aromatic –OH groups for antioxidant properties of flavonoids. The –OH group at 3 position in the C ring is crucial for deactivation of ONOO−, and its action is enhanced by –OH groups at positions 5 and 7 as well as by the double-bonded oxygen at position 4 and the ring oxygen at position 1. The comparative analysis performed by those authors (Heijnen et al., Citation2001b) showed that flavonols containing a catechol group (3′- and 4′-OH) in ring B or the AC-ring with 3-, 5- and 7-OH groups were potent peroxynitrite scavengers.
Trifolium species were originally identified as possessing estrogenic activity, and therefore, for a long time, these plants have been investigated mainly in terms of their phytoestrogenic properties. Due to the disputable effects of the conventional hormone replacement therapy (Rossouw et al., Citation2002), plant-derived supplements are of increasing interest as useful in alleviation of menopausal symptoms. Phytoestrogens may also delay the progression of atherosclerosis by reduction of pathological changes in the cardiovascular system, such as alterations in the lipid profile, inflammatory processes, and oxidative stress (Gencel et al., Citation2011). Isoflavonoid phytoestrogens significantly contribute to medicinal properties of Trifolium plants, however, it should be emphasized that in addition to isoflavones, clovers synthesize a wide range of other bioactive compounds. Our phytochemical analysis of T. pratense aerial parts (crude extract and phenolic fraction) revealed the presence of phenolic acids, flavonoids, and isoflavones. The phenolic fraction of T. scabrum contained phenolic acids, flavonoids, and isoflavones, while the T. pallidum extract was rich in phenolic acids, flavonoids, isoflavones, and clovamides (Kolodziejczyk-Czepas et al., Citation2013a,Citationb). For more detailed evaluation of the free radical scavenging and antioxidant effects, activities of clover extracts were assessed and compared at a relatively wide concentration range of 1.5–50 µg/ml. The lowest concentrations of the extracts may correspond to the physiological level of plant-derived phenolics, detected after a dietary intake. For most flavonoid and other phenolic compounds, blood plasma concentration of their metabolites attains several µmoles/l. For instance, it has been established that the single oral bolus dose of 50 mg of soy-derived either genistein or daidzein results in their plasma concentrations about 0.8 µg/ml (about 3.0 µM for genistein and 3.2 µM for daidzein) (Setchell, Citation1998). Studies on absorption of red clover isoflavones in human subjects have shown that isoflavones occurring in this plant in minor amounts such as irilone, prunetin. and pseudobaptigenin are also bioavailable (Maul & Kulling, Citation2010). The irilone content, usually provided in commercial red clover supplements, may result in physiologically relevant plasma concentrations. A single intake of 3.8 mg irilone (out of 38.8 mg isoflavones in total) yielded its plasma concentration of 0.35 µM (0.1 µg/ml), whereas plasma concentrations of the main isoflavone metabolites, daidzein and genistein, attained 0.39 µM and 0.06 µM, respectively (Maul & Kulling, Citation2010).
We assessed the efficacy of the examined extracts in scavenging of free radicals and their antioxidant action in blood plasma. The phenolic fraction of T. pratense was the most effective both in the ABTS• and DPPH• radical scavenging assays, followed by the phenolic fraction of T. pallidum. The antioxidant actions of two other extracts (the crude extract of T. pratense and phenolic fraction of T. scabrum) were significantly weaker. These findings were confirmed by the measurements of the ferric-reducing abilities of the examined extracts. Both the T. pratense extracts contained similar proportions of phenolic acids and flavonoids, but isoflavone concentration was significantly lower in the phenolic fraction – however, this fraction displayed stronger anti-free radical and antioxidant properties. Since the crude extract of red clover contained some trace elements such as saponins and oligosaccharides, we suppose that these substances might influence its activity. Despite the isoflavone content being very high (72.76 mg/g of d.m.), also anti-free radical activity of the T. scabrum extract was evidently lower than the other examined extracts, whereas in another studies, we have shown the free radical scavenging potential of 16 substances isolated from this clover, assessed by a simple benchtop thin-layer chromatography-2,2-diphenyl-1-picrylhydrazyl radical (TLC-DPPH•) bioassay (Kowalska et al., Citation2013). A strong free radical scavenging effect of T. pallidum extract seems to be easier to explain. Besides a low content of flavonoids, its antioxidant activity is significantly enhanced by the presence of considerably high concentrations of phenolic acids and clovamides. Phenolic acids are effective antioxidants, able to deactivate peroxynitrite, organic free radicals, superoxide anion, hydroxyl, and peroxyl radicals (Shahidi & Chandrasekara, Citation2010). Furthermore, a high anti-free radical activity (EC50 = 9.238 ± 1.054 µM) was also reported for clovamide (N-caffeoyl-l-DOPA) (Arlorio et al., Citation2008). The antioxidant properties of other clovamide-rich Trifolium extract have also been demonstrated in our previous studies (Kolodziejczyk et al., Citation2011; Kolodziejczyk-Czepas et al., in press). Among the examined extracts of six Trifolium species, the T. alexandrinum extract, containing 9.63 mg of clovamides/g of dry mass, was the most effective, reaching the EC50 value of 5.53 and 2.02 μg/ml in the ABTS• and DPPH• scavenging assays, respectively () (Kolodziejczyk-Czepas et al., in press).
On one hand, we have found that Trifolium-derived extracts may significantly enhance the physiological, non-enzymatic antioxidant mechanisms of blood plasma in vitro. However, the differences between the individual Trifolium extracts in the protection of the TAC (including the ferric reducing ability) of blood plasma were not so evident, when compared with their anti-free radical actions in the scavenging assays. The phenolic fraction of T. pratense seems to be the most efficient antioxidatively. On the other hand, the crude extract of T. pratense, as well as phenolic fractions of T. pallidum and T. scabrum, also protected blood plasma against oxidative action of peroxynitrite, although the antioxidant efficacy was less pronounced in comparison with the phenolic fraction of T. pratense. Similar observations were made in our previous studies. Despite the relevant differences in the radical scavenging properties of extracts from six other clover species (), their protective actions on the blood plasma TAC were mostly at a comparable level. However, species with the highest total content of phenolics – i.e., T. alexandrinum (52.55 mg/g of dry mass) and T. incarnatum (47.97 mg/g of dry mass), displayed a noticeable tendency to exhibit stronger antioxidant effects (Kolodziejczyk-Czepas et al., in press). These slight differences between antioxidant actions of the examined extracts may be an effect of a complexity of natural non-enzymatic antioxidant defense of blood plasma, as well as a result of the combination of biologically active substances of the individual extracts. The interactions between phenolic ingredients of the examined extracts as well as between plasma macromolecules and these polyphenols are also possible. Binding of flavonoids to proteins may mask their effect on the TAC of blood plasma. For instance, studies of Arts et al. (2000) demonstrated that the antioxidant capacities of several polyphenolics of green and black tea with α-, β-, and κ-casein or albumin were not additive. Moreover, these masking effects were dependent both on the properties of the protein and on the flavonoid compound.
Conclusions
A direct antioxidant effect of polyphenols in vivo is debatable, mainly because of the low concentrations that may be attained in blood. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not fully established, and the beneficial effect of polyphenol-rich diet seems to be rather an effects of their multifaceted bioactivity (Hollman et al., Citation2011). To the contrary, antioxidant properties still remain an important factor contributing to the favorable effects of polyphenolic compounds on human health. Our results indicate on a considerable anti-free radical scavenging and in vitro antioxidant effects of Trifolium species. Both this work and our previous studies have indicated that both popular species and some of less known clovers (for example, T. pallidum) contain a high content of bioactive substances. However, due to insufficient knowledge of biological effects of clovers other than T. pratense, formula of a dietary supplement or medical preparation is not predictable yet. Therefore, further studies are needed.
Declaration of interest
The authors report that there are no declaration of interest. This work was supported by Grants 506/1136, 545/464, and 545/741 from University of Lodz (Lodz, Poland), as well as by statutory activities of Institute of Soil Science and Plant Cultivation – State Research Institute (Pulawy, Poland).
Acknowledgements
Special thanks go to Dr. Michal B. Ponczek (Department of General Biochemistry, University of Lodz) for his assistance in statistical analysis.
References
- Arlorio M, Locatelli M, Travagilia F, et al. (2008). Roasting impact on the contents of clovamide (N-caffeoyl-l-DOPA) and the antioxidant activity of cocoa beans (Theobroma cacao L.). Food Chem 106:967–75
- Arts MJ, Haenen GR, Wilms LC, et al. (2002). Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. J Agric Food Chem 27:1184–7
- Asgary S, Moshtaghian J, Naderi G, et al. (2007). Effects of dietary red clover on blood factors and cardiovascular fatty streak formation in hypercholesterolemic rabbits. Phytother Res 21:768–70
- Benzie IFF, Strain JJ. (1996). The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem 239:70–76
- Booth NL, Overk CR, Yao P, et al. (2006). The chemical and biological profile of a red clover (Trifolium pratense) phase II clinical extract. J Altern Complement Med 12:133–9
- Coon JT, Pittler MH, Ernst E. (2007). Trifolium pratense isoflavones in the treatment of menopausal hot flushes: A systematic review and meta-analysis. Phytomedicine 14:153–9
- Engelmann NJ, Reppert A, Yousef G, et al. (2009). In vitro production of radiolabeled red clover (Trifolium pratense) isoflavones. Plant Cell Tiss Organ Cult 98:147–56
- Gencel VB, Benjamin MM, Bahou SN, Khalil RA. (2011). Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini Rev Med Chem 12:149–74
- Heijnen CG, Haenen GR, van Acker FA, et al. (2001a). Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol In Vitro 15:3–6
- Heijnen CG, Haenen GR, Vekemans JA, Bast A. (2001b). Peroxynitrite scavenging of flavonoids: Structure activity relationship. Environ Toxicol Pharmacol 10:199–206
- Hollman PC, Cassidy A, Comte B, et al. (2011). The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr 141:989–1009
- Janaszewska A, Bartosz G. (2002). Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand J Clin Lab Invest 62:231–6
- Kolodziejczyk-Czepas J. (2012). Trifolium species-derived substances and extracts – Biological activity and prospects for medicinal applications. J Ethnopharmacol 143:14–23
- Kolodziejczyk J, Olas B, Wachowicz B, et al. (2011). Clovamide-rich extract from Trifolium pallidum reduces oxidative stress-induced damage to blood platelets and plasma. J Physiol Biochem 67:391–9
- Kolodziejczyk-Czepas J, Nowak P, Moniuszko-Szajwaj B, et al. (2014). Biological activity of clovers – Free radical scavenging ability and antioxidant action of six Trifolium species. Pharm Biol 52:1308–14
- Kolodziejczyk-Czepas J, Olas B, Malinowska J, et al. (2013a). Trifolium pallidum and Trifolium scabrum extracts in the protection of human plasma components. J Thromb Thrombol 35:193–9
- Kolodziejczyk-Czepas J, Wachowicz B, Moniuszko-Szajwaj B, et al. (2013b). Antioxidative effects of extracts from Trifolium species on blood platelets exposed to oxidative stress. J Physiol Biochem 69:879–87
- Kowalska I, Jedrejek D, Ciesla L, et al. (2013). Isolation, chemical and free radical scavenging characterization of phenolics from Trifolium scabrum L. aerial parts. J Agric Food Chem 61:4417–23
- Krenn L, Paper DH. (2009). Inhibition of angiogenesis and inflammation by an extract of red clover (Trifolium pratense L.). Phytomedicine 16:1083–8
- Lipovac M, Chedraui P, Gruenhut C, et al. (2010). Improvement of postmenopausal depressive and anxiety symptoms after treatment with isoflavones derived from red clover extracts. Maturitas 65:258–61
- Maul R, Kulling SE. (2010). Absorption of red clover isoflavones in human subjects: Results from a pilot study. Br J Nutr 103:1569–72
- Nowak P, Wachowicz B. (2001). Studies on pig blood platelet responses to peroxynitrite action. Platelets 12:376–81
- Olas B, Nowak P, Kolodziejczyk J, Wachowicz B. (2004). The effects of antioxidants on peroxynitrite-induced changes in platelet proteins. Thromb Res 113:399–406
- Oleszek W, Stochmal A, Janda B. (2007). Concentration of isoflavones and other phenolics in the aerial parts of Trifolium species. J Agric Food Chem 55:8095–100
- Re R, Pellegrini N, Proteggente A, et al. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–7
- Renda G, Yalçın FN, Nemutlu E, et al. (2013). Comparative assessment of dermal wound healing potentials of various Trifolium L. extracts and determination of their isoflavone contents as potential active ingredients. J Ethnopharmacol 148:423–32
- Rossouw J, Anderson GL, Prentice RL, et al. ( Writing Group for the Women’s Health Initiative Investigators). (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 288:321–33
- Ruiz-Larrea MB, Mohan AR, Paganga G, et al. (1997). Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res 26:63–70
- Sabudak T, Guler N. (2009). Trifolium L. – a review on its phytochemical and pharmacological profile. Phytother Res 23:439–46
- Schulz E, Gori T, Münzel T. (2011). Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res 3:665–73
- Setchell KD. (1998). Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr 68:1333–46
- Shah A, Passacquale G, Gkaliagkousi E, et al. (2011). Platelet nitric oxide signalling in heart failure: Role of oxidative stress. Cardiovasc Res 91:625–31
- Shahidi F, Chandrasekara A. (2010). Hydroxycinnamates and their in vitro and in vivo antioxidant activities. Phytochem Rev 9:147–70
- Stochmal A, Piacente S, Pizza C, et al. (2001). Alfalfa (Medicago sativa L.) flavonoids. 1. Apigenin and luteolin glycosides from aerial parts. J Agric Food Chem 49:753–8
- Tousoulis D, Briasoulis A, Papageorgiou N, et al. (2011). Oxidative stress and endothelial function: Therapeutic interventions. Recent Pat Cardiovasc Drug Discov 6:103–14
- Weseler AR, Bast A. (2010). Oxidative stress and vascular function: Implications for pharmacologic treatments. Curr Hypertens Rep 12:154–61
- Wu Q, Wang M, Simon JE. (2003). Determination of isoflavones in red clover and related species by high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J Chromatogr A 1016:195–209
