Abstract
Context: Phyllanthus simplex Retz. (Phyllanthaceae), Crotolaria juncea Linn. (Leguminosae), Leucas aspera Linn. (Lamiaceae), and Vitex glabrata R.Br. (Verbenaceae) are well-known Indian medicinal plants. Different parts of these plants are used for healing purposes traditionally in the treatment of psoriasis and various other disorders. This prompted us to assess the antipsoriatic activities of these plants.
Objectives: Petroleum ether and ethanol extracts of the selected plants, i.e., P. simplex (whole plant), C. juncea (seeds), L. aspera (aerial parts), and V. glabrata (leaves) were investigated for their in vitro antipsoriatic activity.
Materials and methods: Antipsoriatic activity of the extracts was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, using HaCaT cells. About 200 µl of different concentrations (25, 50, 100, 200, and 400 µg/ml) of test samples were prepared in the cell culture medium and incubated for 24 h before MTT assay to determine the viable cells. The effect of these extracts on nitric oxide (NO) production and lipid peroxidation was also evaluated.
Results: Our findings revealed that these plants showed promising skin keratinocyte antiproliferative activity. However, the petroleum ether extract of C. juncea (CJPE) and ethanol extract of L. aspera (LAEE) were found to exhibit significant activity (IC50 value = 45.45 and 55.36 µg/ml, respectively).
Discussion and conclusions: The inhibitory action against NO production and lipid peroxidation in HaCaT cells suggested that the antipsoriatic activity of the extracts was mediated by an antioxidant mechanism. These findings validate the claims of the use of these plants in the treatment of psoriasis.
Introduction
Psoriasis, a disorder of the skin, occurs when the immune system of body sends out faulty signals resulting in acceleration of the growth cycle of skin cells. It is a non-contagious, multi-factorial, autoimmune skin disorder, and is characterized by red silvery skin patches, keratinocyte hyperproliferation, increased dermal vascularity, and chronic inflammation (Lee & Cooper, Citation2006; Parisi et al., Citation2013). Such a disease is known since time immemorial and, currently, about 2–3% of the worldwide population is afflicted with it. Psoriasis has important social, psychological, and economic consequences (Aldeen & Basra, Citation2011). Its impact on the quality of life is found to be nearly similar to that associated with other chronic medical conditions such as diabetes and depression (Korte et al., Citation2004; Krueger et al., Citation2001).
Various scientific reports have demonstrated that medicinal plants, namely Radix paeoniae Alba. (Paeoniaceae), Rubia cordifolia Linn. (Rubiaceae), Coptis chinensis Franch. (Ranunculaceae), Alpinia galangal Linn. (Zingiberaceae), Annona squamosa Linn. (Annonaceae), Curcuma longa Linn. (Zingiberaceae), etc., are found to be effective for the treatment of psoriasis (Koo & Arain, Citation1998; Koo & Desai, Citation2003; Lin et al., Citation2010; Prepare et al., Citation1995; Tan et al., Citation2011; Tse et al., Citation2006; Zhou et al., Citation2012). In this study, four Indian medicinal plants, namely, Phyllanthus simplex Retz. (Phyllanthaceae), Crotolaria juncea Linn. (Leguminosae), Leucas aspera Linn. (Lamiaceae), and Vitex glabrata R.Br. (Verbenaceae) were selected to evaluate their effect on proliferation of skin keratinocytes. These plants are widely used for the treatment of chronic inflammatory disorders of skin and psoriasis like symptoms by the traditional healers. The whole plant paste of P. simplex is used to treat various inflammatory diseases (Unander et al., Citation1990). Seeds of C. juncea are used in the treatment of inflammation, chronic skin eruptions, and psoriasis (Sharma et al., Citation2001). Leaf paste and/or juice of L. aspera are reported to be useful in the treatment of inflammation, chronic skin eruption, painful swelling, and psoriasis (Kirtikar & Basu, Citation1990). Plants of genus Vitex are found to display wide range of pharmacological actions including anti-inflammatory activity and are utilized in the treatment of skin inflammatory diseases/disorders (Chouhan et al., Citation2012).
However, scientific studies to comprehend their anti-psoriatic activity are lacking in the literature. The aim of this investigation was to detect the beneficial role of these medicinal plants in the treatment of psoriasis. Cultured HaCaT cell line (spontaneously transformed and immortalized human keratinocyte cell line) was used which is commonly employed as an in vitro test model for antipsoriatic activity of new treatment because of its highly preserved differentiation capacity (Tse et al., Citation2006).
Materials and methods
Chemicals
All the chemicals and solvents, unless otherwise specified, were purchased from Merck, Mumbai, India. Dulbecco's Modified Eagel’s medium (DMEM) was purchased from Gibco, Invitrogen BioServices India Pvt. Ltd, Banglore, India. Dithranol, fetal calf serum (FCS), and trypsin-EDTA (1×) were purchased from Himedia, Mumbai, India. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich, New Delhi, India. Sterile double-distilled water was used through out the experiment.
Plant material and preparation of extracts
Collection of plant materials, their authentication, and preparation of the extracts for P. simplex (whole plant), C. juncea (seeds), and Vitex glabrata (leaves) were presented in our earlier studies (Chouhan & Singh, Citation2011a; Chouhan et al., Citation2011, Citation2012). Aerial parts of L. aspera were collected from the roadside fields of Varanasi. The plant sample was identified morphologically by Prof. N. K. Dubey, Department of Botany, Banaras Hindu University, Varanasi, and a voucher specimen (PCRL-42) was deposited in the Pharmaceutical Chemistry Research Laboratory, Department of Pharmaceutics, Indian Institute of Technology (BHU), Varanasi, for future reference. Air- and shade-dried plant materials were pulverized to coarse crude powder and stored in an airtight container at room temperature.
In general, extracts were prepared by successive soxhletion of pulverized plant material with petroleum ether (fraction collected at 60–80 °C) and ethanol. The extracts were concentrated under vacuum and stored separately in airtight containers in refrigerator.
Preparation of test materials for bioassay
In all experiments, Dulbeccos Modified Eagel’s medium (DMEM, Gibco, Mumbai, India) supplemented with 10% FCS, 1% penicillin, and 2% streptomycin was used as the cell culture medium and dithranol was used as a positive control. The extracts, namely petroleum ether extract of P. simplex (PSPE), ethanol extract of P. simplex (PSEE), petroleum ether extract of C. juncea (CJPE), ethanol extract of C. juncea (CJSE), petroleum ether extract of L. aspera (LAPE), ethanol extract of L. aspera (LAEE), and ethanol extract of V. gabrata (VGEE) were initially dissolved in DMSO/ethanol/acetone and sterilized by filtration (0.2 μm porosity). Different dilutions (25–400 μg/ml) of extracts and dithranol were prepared in the sterilized culture medium. The final concentration of solvent was kept less than 1% (v/v) in all wells as at this concentration; no toxic effect of solvent on cell growth or replication was observed.
Cell culture of HaCaT human keratinocytes
HaCaT human keratinocyte cell line was obtained from National Center for Cell Science, Pune, India, and cells were cultured in a flask containing culture medium in 5% CO2 atmosphere at 37 °C temperature. When cells were 70–90% confluent, the culture medium was aspirated out and cells were washed twice with Ca- and Mg-free phosphate buffer saline (PBS). Cells were detached from the inner surface of flask by adding 500 µl trypsin-EDTA (1×), and incubated at 37 °C for 2 min, followed by gentle horizontal force. An equal volume of culture medium was added to the flask to neutralize the trypsin. A small portion of culture medium containing free cells was pipetted out and viable cells were counted using a hemocytometer after staining with Typan blue; before carrying out the experiment, final cell density of stock was adjusted to 5 × 104 cells/ml.
Evaluation of antipsoriatic activity of extracts
The antipsoriatic activity of extracts was evaluated by an in vitro method using cultured HaCaT cell line and viable cells were determined by the MTT assay. The linearity range and seeding density of cells used in the experiment were determined by the assessment of linearity range of the experiment and from the cells growth curve, respectively.
Assessment linearity range Q
Different densities of HaCaT cells ranging from 1 × 104 to 10 × 104 were seeded in 96-well plates. Each density had three replicates on the same plate and, after 4 h of incubation, MTT assay was performed. A calibration curve was constructed by using optical density (Y-axis) and cell density (X-axis).
Growth curve assessment
HaCaT cell densities ranging from 1 × 104 to 5 × 104 were seeded in 96-well plates. Each density had three replicates on the same plate. Plates were incubated for 5 d and were monitored for the cells morphology and culture medium requirement. The culture medium was replaced on need basis. After 5 d of incubation, the MTT assay was performed. Cell growth curves were constructed using optical density (Y-axis) and time (X-axis).
Skin keratinocyte antiproliferative assessment
Cell culture stock (200 μl, 2 × 104 cells) was added to each well of the 96-well plates and was incubated for 24 h at 5% CO2 atmosphere and 37 °C. After the incubation, the medium was aspirated out and replenished with 200 μl of different concentrations (25, 50, 100, 200, and 400 µg/ml) of test samples prepared in the cell culture medium and incubated for next 48 h under the same conditions. After incubation, the MTT assay was performed to determine the number of viable cells (Saelee et al., Citation2011).
MTT assay
MTT assay is a widely employed technique to count the viable cells in in vitro bioassay. It is based on the principle of reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to a purple formazan product by mitochondria of the living cells (Saelee et al., Citation2011). Briefly, 10 μl, 0.5 mg/ml MTT was taken for cell incubation performed at 37 °C for 4 h in darkness. The media were decanted and washed with PBS. The formazan salts thus produced were dissolved in dimethylsulfoxide and the absorbance was measured at 540 nm using the microplate reader (BioRad, Hercules, CA) to estimate the formazan concentration.
Evaluation of the effect on NO production
The release of NO from HaCaT cells was assayed by measuring of nitrite accumulation in the culture medium using Griess reagent. Briefly, HaCaT cells (1 × 104 cells/well) were pretreated with extracts and dithranol. Following this, they were exposed to 10 µl of 1 µg/ml lipopolysaccharide. After incubation for 48 h, the supernatant of the cell culture medium was taken out in another 96-well plate and was used for the estimation of accumulated NO radicals by the Griess reagent (Nakai et al., Citation2003).
Evaluation of effect on lipid peroxidation
Lipid peroxidation was evaluated as described previously (Chouhan & Singh, Citation2011a). Cells were collected and resuspended in cold PBS. Cell homogenates were prepared with a homogenizer. After centrifugation, the supernatant was removed and MDA, a terminal product of lipid peroxidation (LPO) was measured to estimate the extent of lipid peroxidation. Levels of MDA were determined by adding thiobarbituric acid (TBA). Briefly, the supernatant of each sample (0.15 ml) was mixed with 3% sodium dodecylsulfate (SDS), 0.1 N HCl, 10% phosphotungstic acid, and 0.7% TBA, and incubated at 95 °C for 45 min. After centrifugation at 3500 rpm for 10 min, the supernatant was removed and the absorbance was measured by a microplate reader at 485 and 535 nm.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analysis of group differences was performed using Student’s t-test. Values of p < 0.05 were considered statistically significant.
Results and discussion
Skin is the largest exposed organ of body and is easily targeted for allergic and immunological reactions. Skin ailments, namely dermatitis, urticaria, angioedema, psoriasis, etc., are immune mediated disorders and are chronic, inflammatory, and proliferative in nature (Meeuwis et al., Citation2011). Psoriasis has become an important area of scientific study due to its severe effect on the quality of life, cost of treatment and toxicity, and/or the side effects of available medication (Korte et al., Citation2004; Krueger et al., Citation2001). Medicinal plants are known to be safe for human health and are widely employed by the traditional healers for the treatment of various diseases including psoriasis. Medicinal plants are known to be a rich citadel of a variety of chemical compounds and have attracted attention to find new treatments for various diseases including psoriasis (Kaur & Kumar, Citation2012).
Inhibition of hyperproliferation of epidermal keratinoocytes is one of the key mechanisms by which most of the available antipsoriatic drugs act. HaCaT cells are human spontaneous immortal keratinocyte cells and are often used as an effective model instead of primary-cultured keratinocytes and the data obtained by this model have shown a good correlation with in vivo skin irritation (Tse et al., Citation2006). Hence, we investigated antipsoriatic activity of selected medicinal plants using cultured HaCaT cells and estimated cell viability by the MTT assay. In MTT assay, metabolic activity of viable cells is measured by determining the reduction of yellow 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) into an insoluble, colored (dark purple) formazan product by the mitochondrial succinate dehydrogenase enzyme. As the reduction of MTT can only occur in metabolically active cells, hence measurement of color intensity is used to determine the number of viable cells (Loosdrecht et al., Citation1994).
The calibration curve was plotted between absorbance and cell numbers to obtain linear equation for the MTT assay and was found to be linear (y = 0.050x + 0.090) in the range of 1 × 104–15 × 104 cells/ml with a regression coefficient value of r2 = 0.996 (). Therefore, it can be inferred that MTT assay can be used to evaluate the proliferation rate of cultured HaCaT cells with high accuracy of results for the measurement of cells population up to 15 × 104 cells/ml, and hence is suitable for the present study.
Figure 1. (a) Calibration curve of MTT assay and (b) growth curve of different implant HaCaT cells densities in 96-well plates. All values are expressed as mean ± SEM of three replicates from the same plate.
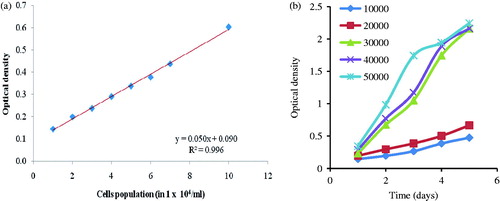
The seeding density of HaCaT cells used in the experiment was selected from the growth curve (). The different counts (density) of HaCaT cells ranging from 1 × 104–5 × 104 cells per well were implanted and their experimental growth was observed for a period of 5 d incubation at 5% CO2 atmosphere and 37 ± 0.5 °C using DMEM as the culture medium. The culture medium of well of 96-well plates after the 48 h of incubation was required to replenished with fresh medium on every 24 h time interval where implanted cell density was found to be greater than 3 × 104 cells per well. However, implanted cell density of 2 × 104 per well did not require repletion of medium until the third day of incubation and also produced a linear growth curve. Therefore, HaCaT cell density of 2 × 104 cells per well and a 3 d incubation time period were selected for the experiments to achieve a high degree of accuracy and also to avoid operating troubles during replenishment of culture medium.
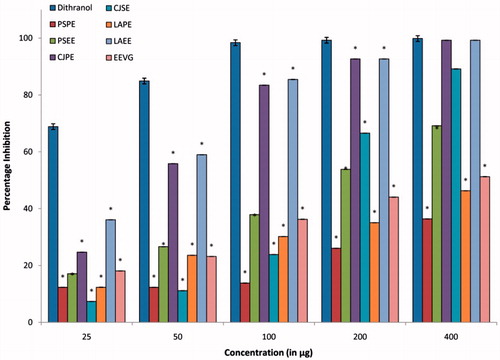
Our findings demonstrated that extracts of all plants selected for the study showed appreciable keratinocytes antiproliferant activity in the HaCaT cell line (). The activity was found to be in the following order: CJPE (IC50 value = 45.45 µg/ml) > LAEE (IC50 value = 55.36 µg/ml) > CJSE (IC50 value = 138.65 µg/ml) > PSEE (IC50 value = 161.33 µg/ml) > EEVG (IC50 value = 356.13 µg/ml) > LAPE (IC50 value = 598.4.79 µg/ml) > PSPE (IC50 value = 2824.79 µg/ml). The pet ether extract (obtained as crude oil) from seed of C. juncea (CJPE) and ethanol extract of aerial parts of L. aspera (LAEE) exhibited potent antiproliferative activity. It may be suggested that the fatty acids present in the CJPE and the phenolics in LAEE may be primarily responsible for the activity. Dithranol (IC50 value = 2.58 µg/ml) was used as a positive control to validate the results. The antiproliferative activity of CJPE was comparable with that exhibited by the Caesalpinia bonduc (L.) Roxb. hydroalcoholic extract (IC50 value = 77.5 µg/ml), ethanolic extract of yellow birch obtained by 48 h maceration (IC50 value = 94.80 µg/ml), and was better than black spruce extract obtained by hot water extraction (IC50 value = 94.80 µg/ml); however, it possessed less activity than the root extract of R. cordifolia (IC50 value = 1.4 µg/ml), Realgar (IC50 value = 6.6 µg/ml), and the rhizome of C. chinensis (IC50 value = 23.4 µg/ml) (Lin et al., Citation2010; Prepare et al., Citation1995; Tan et al., Citation2011; Tse et al., Citation2006; Zhou et al., Citation2012).
Figure 2. Antiproliferative activity of different extractives of P. simplex, C. juncea, L. aspera. and V. glabrata. All values are expressed as mean ± SEM of three replicates from the same plate. *p < 0.05 when compared with dithranol.
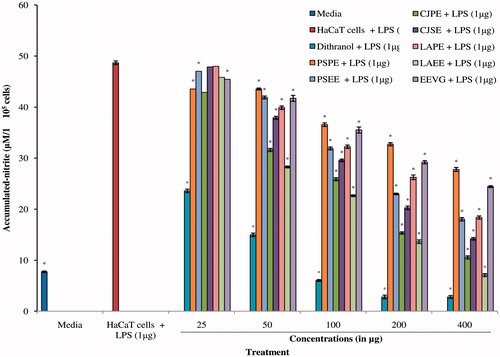
Free radicals are the key indicators of activity of keratinocytic proliferation in psoriasis (Grashin et al., Citation2010). Nitric oxide, a free radical, acts as an important intracellular messenger and plays an important role in autoimmunity and inflammation. Excessive production of the radical has been reported in the pathogenesis of many inflammatory disorders including psoriasis. However, the role of NO radical as pro- and anti-inflammatory agent is the function of its cellular concentration (Cirino et al., Citation2006; Henderson, Citation1994; Kolodziej et al., Citation2008; Miller & Grisham, Citation1995). Therefore, in this study, we also evaluated the effect of these plant extracts on NO production in skin keratinocyte cells by colorimetric assay using the Griess reagent. Results, presented in , revealed that extracts exhibited significant inhibition of NO production. The inhibitory activity was found to be in the order of PSPE (IC50 value = 775.106 µg/ml) > EEVG (IC50 value = 399.814 µg/ml) > LAPE (IC50 value = 227.011 µg/ml) > PSEE (IC50 value = 206.232 µg/ml) > CJSE (IC50 value = 158.38 µg/ml) > CJPE (IC50 value = 108.31 µg/ml) > LAEE (IC50 value = 93.785 µg/ml). Dithranol (IC50 value = 33.616 µg/ml) was used as a positive control to validate results. The findings suggested that the extracts exhibited their action by inhibiting NO production in the cells.
Figure 3. Effect of different extractives of P. simplex, C. juncea, L. aspera, and V. glabrata on nitric oxide production in LPS-stimulated HaCaT cell culture. All values were expressed as mean ± SEM of three replicates from the same plate, *p < 0.05 when compared with dithranol.
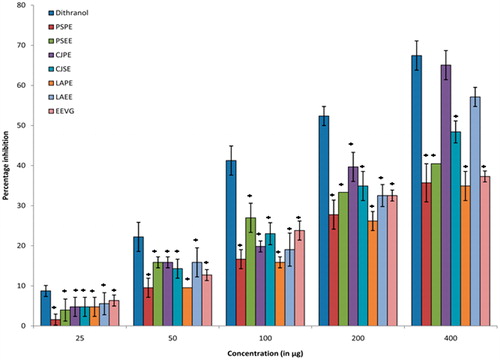
An increased level of lipid peroxidation is reported in psoriasis which is mediated by lipoxygenases (LOXs) enzymes utilizing the arachidonic acid pathway. The LOXs-catalyzed oxygenation products are apparently involved in the development of psoriasis (Young, Citation1999) and hence inhibiting the lipoxygenase pathway may be helpful in psoriasis. Therefore, we evaluated the extracts for lipoxygenase inhibition activity and results are presented in . It was observed that LAEE and CJPE produced maximum inhibition activity of the enzyme and the IC50 values were found to be 52.71 and 49.32 µg/ml, respectively. The findings suggested that CJPE and LAEE were able to significantly inhibit the lipid peroxidation and protect the cellular morphology of skin keratinocyte cells.
Figure 4. Effect of different extractives of P. simplex, C. juncea, L. aspera, and V. glabrata on lipid peroxidation in HaCaT cell culture. All values were expressed as mean ± SEM of three replicates from the same plate, *p < 0.05 when compared with dithranol.
Earlier, we reported the presence of abundance of unsaturated fatty acids in CJPE with major component as linoleic acid which was found to play a pivotal role in its anti-inflammatory effect (Chouhan et al., Citation2011). Scientific studies have revealed that linoleic acid in its conjugate form exhibited beneficial effect to human health by regulation of body fat gain, enhanced immunity, reduced inflammation, and minimized adverse reactions due to enhanced body immunity (Calder, Citation2001, Citation2006). Further, phenolics, steroids, triterpenes, fatty acids, aliphatic, and nitrogenous compounds were largely reported from L. aspera plant. The plant was found to display anti-inflammatory activity and phenolics were predominantly considered to be responsible for the activity (Chouhan & Singh, Citation2011b). Therefore, it may be suggested that fatty acids, namely, linoleic acid and plant phenolics of C. juncea and L. aspera, may be largely responsible antipsoriatic activity of the plants.
Conclusions
This investigation establishes the promising skin keratinocyte antiproliferative activity of selected Indian medicinal plants. Further, their inhibitory action against NO production and lipid peroxidation in HaCaT cells suggests that the antipsoriatic activity is mediated by an antioxidant mechanism. Significant antipsoriatic activity has been observed in crude oil (CJPE) obtained from the pet ether extracts of C. juncea and ethanolic extracts of L. aspera (LAEE). These results validate the claims of the use of these plants in the treatment of psoriasis by traditional healers.
Declaration of interest
The authors report that there are no declarations of interest. Authors are thankful to NMPB, AYUSH, New Delhi, for Grant (GO/UP-02/2007). H. S. C. is also thankful to UGC, New Delhi, for the award of research fellowship (SRF).
References
- Aldeen T, Basra M. (2011). Management of psoriasis and its comorbidities in primary care. Br J Nurs 20:1186–92
- Calder PC. (2001). Polyunsaturated fatty acids, inflammation, and immunity. Lipids 36:1007–24
- Calder PC. (2006). n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83:1505S–19S
- Chouhan HS, Sahu AN, Singh SK. (2011). Fatty acid composition, antioxidant, anti-inflammatory and antibacterial activities of seed oil from Crotalaria juncea Linn. J Med Plants Res 5:984–91
- Chouhan HS, Singh SK. (2011a). Phytochemical analysis, antioxidant and anti-inflammatory activities of Phyllanthus simplex. J Ethnopharmacol 137:1337–44
- Chouhan HS, Singh SK. (2011b). A review of plants of genus Leucas. J Pharmacog Phytother 3:13–26
- Chouhan HS, Sridevi VK, Singh NK, Singh SK. (2012). Anti-inflammatory activity of ethanol extract of Vitex glabrata leaves. Pak J Pharm Sci 25:131–4
- Cirino G, Distrutti E, Wallace JL. (2006). Nitric oxide and inflammation. Inflamm Allergy Drug Targets 5:115–19
- Grashin RA, Antonov VG, Karpishchenko AI, Khaĭrutdinov VR. (2010). The free radical oxidation and antioxidant defense systems as indicators of the activity of keratinocytic proliferation in psoriasis. Klin Lab Diagn 1:18–24
- Henderson WR Jr. (1994). The role of leukotrienes in inflammation. Ann Intern Med 121:684–97
- Kaur A, Kumar S. (2012). Plants and plant products with potential antipsoriatic activity – A review. Pharm Biol 50:1573–91
- Kirtikar KR, Basu BD. (1990). Indian Medicinal Plants. Allahabad, India: Lalit Mohan Basu Publishers
- Kolodziej H, Radtke OA, Kiderlen AF. (2008). Stimulus (polyphenol, IFNgamma, LPS)-dependent nitric oxide production and antileishmanial effects in RAW 264.7 macrophages. Phytochemistry 69:3103–10
- Koo J, Arain S. (1998). Traditional Chinese medicine for the treatment of dermatologic disorders. Arch Dermatol 134:1388–93
- Koo J, Desai R. (2003). Traditional Chinese medicine in dermatology. Dermatol Ther 16:98–105
- Korte DJ, Sprangers MA, Mombers FM, Bos JD. (2004). Quality of life in patients with psoriasis: A systematic literature review. J Invest Derm Symp P 9:140–7
- Krueger G, Koo J, Lebwohl M, et al. (2001). The impact of psoriasis on quality of life: Results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol 137:280–4
- Lee MR, Cooper AJ. (2006). Immunopathogenesis of psoriasis. Aust J Dermatol 47:151–9
- Lin ZX, Jiao BW, Che CT. (2010). Ethyl acetate fraction of the root of Rubia cordifolia L. inhibits keratinocyte proliferation in vitro and promotes keratinocyte differentiation in vivo: Potential application for psoriasis treatment. Phytother Res 24:1056–64
- Loosdrecht VDAA, Beelen RH, Ossenkoppele GJ, et al. (1994). A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 174:311–20
- Meeuwis KA, de Hullu JA, Massuger LF, et al. (2011). Genital psoriasis: A systematic literature review on this hidden skin disease. Acta Derm-Venereol 91:5–11
- Miller MJS, Grisham MB. (1995). Nitric oxide as a mediator of inflammation? – You had better believe it. Mediat Inflamm 4:387–96
- Nakai K, Fujii S, Yamamoto A, et al. (2003). Effects of high glucose on NO synthesis in human keratinocyte cell line (HaCaT). J Dermatol Sci 31:211–18
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. (2013). Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Derm 133:377–85
- Prepare L, Shaw D, Leon C. (1995). Possible association of liver damage with the use of Chinese herbal medicine for skin disease. Vet Hum Toxicol 37:562–6
- Saelee C, Thongrakard V, Tencomnao T. (2011). Effects of Thai medicinal herb extracts with antipsoriatic activity on the expression on NF-κB signaling biomarkers in HaCaT keratinocytes. Molecules 16:3908–32
- Sharma HK, Chhangte L, Dolu AK. (2001). Traditional medicinal plants in Mizoram, India. Fitoterapia 72:146–61
- Tan YQ, Liu JL, Bai YP, Zhang LX. (2011). Literature research of Chinese medicine recipes for the treatment of psoriasis vulgaris with blood-heat syndrome type. Chin J Integr Med 17:150–3
- Tse WP, Che CT, Liu K, Lin ZX. (2006). Evaluation of the anti-proliferative properties of selected psoriasis-treating Chinese medicines on cultured HaCaT cells. J Ethnopharmacol 108:133–41
- Unander DW, Webster GL, Blumberg BS. (1990). Records of usage or assays in Phyllanthus (Euphorbiaceae) I. Subgenera Isocladus, Kirginelia, Cicca and Emblica. J Ethnopharmacol 30:233–64
- Young RN. (1999). Inhibitors of 5-lipoxygenase: A therapeutic potential yet to be fully realized? Eur J Med Chem 34:671–85.
- Zhou LL, Lin ZX, Fung KP. (2012). Ethyl acetate fraction of Radix rubiae inhibits cell growth and promotes terminal differentiation in cultured human keratinocytes. J Ethnopharmacol 142:241–7
