Abstract
Context: Carissa edulis Vahl (Apocynaceae) is used in Nigerian folk medicine to manage a plethora of diseases including epilepsy, cancer, and inflammation; its efficacy is widely acclaimed among communities of northern Nigeria.
Objective: This study establishes anticonvulsant activities of aqueous fraction of ethanol root bark extract of Carissa edulis (RAF) and sub-fractions (S1 and S2) in animal models.
Materials and methods: We evaluated the acute toxicity of the RAF, S1 and S2, and the anticonvulsant activity using pentylenetetrazole (PTZ), picrotoxin, strychnine, N-methyl-d-aspartate (NMDA), isoniazid (INH), and aminophylline-induced seizures in mice. Their effects on maximal electroshock (MES) and kindling-induced seizures were studied in chicks and in rats, respectively, and in the electrophysiological study. The doses used for RAF were 150, 300, and 600 mg/kg while S1 and S2 were 250, 500, and 1000 mg/kg. Both RAF and sub-fractions were administered once during the experiment.
Results: The intraperitoneal LD50 of the RAF was estimated to be 2222.61 mg/kg and that of the S1 and S2 were above 5000 mg/kg. RAF protected the mice by 50% while sub-fractions by 16.67% against PTZ-induced seizures. RAF offered 33.33 and 16.67% protection against strychnine and NMDA models, respectively. However, RAF offered 66.67–33.33% protections against aminophylline-induced seizures at doses of 150 and 600 mg/kg, but RAF, S1, and S2 had no effect on MES-induced seizures.
Discussion and conclusion: Our results validate the use of the plant traditionally in the management of epilepsy, thus supporting the appraisal of biologically active components of this plant as antiepileptic agents.
Introduction
Phytotherapy still plays an important role in the management of diseases, mainly among low-income populations of African and Asian extraction. In developing countries like Nigeria, the World health Organization (WHO) recommended the initiation of programs designed to use medicinal plants more effectively in traditional health care systems (WHO, Citation1978). Traditional medicine in many parts of the world relies on the use of a wide variety of plants species (Geoffrey & Kirby, Citation1996). One of the important areas in which traditional medicine enjoys tremendous patronage is in the management of neurological and psychiatric disorders. Several plants used for the treatment of epilepsy in different systems of traditional medicine have shown activity when tested in modern bioassays for the detection of anticonvulsant activity and many such plants are yet to be scientifically investigated (Hegde et al., Citation2009). Plants are believed to be important as a source of new lead compounds with potential therapeutic benefits for the management of this debilitating neurological disorder (Eisner, 1990).
Carissa edulis Vahl (Apocynaceae), commonly called the Arabic num-num, Natal plum, or Carandas plum, has various vernacular names including Cizaki in Hausa, Kanboro in Fulfulde, Emir in Arabic, Muyunzo in Luganda, Endelkoring-neominoem in Africana, Agam in Amharic, and Mlanoa-mboo in Swahili (Msonthi, Citation1986). The plant has several synonyms including Carissa carandas (Wight) Bedd, Carissa pubescens A. D.C, Carissa pilosa Schinz, Arduina edulis Spreng, Azima pubescens Suesseng, Carissa edulis var. tomentosa (A. Rich) Stapf., Jasminonerium edule (Vahl) Kuntze, and Tasminonerium tomentosa (A. Rich) Kuntze (Forster, Citation1996).
Carissa edulis is a spiny evergreen shrub or a small tree that may reach a height of 5 ft and an equal breadth. The bark is grey and smooth with straight woody double-pronged spine often in pairs. The plant is a native of South Africa. Various plant parts are used in folk medicine for wide variety of remedies such as fever, sickle cell anemia and hernia, treatment of edema, toothache, cough, ulcer, worm infestation, management of epilepsy, and cancer.
Previous pharmacological studies revealed that extracts of C. edulis were found to induce diuresis in rats (Nedi et al., 2004), analgesic, antimicrobial activity (Ibrahim et al., Citation2005, Citation2007), hypoglycemic (El-Fiky et al, Citation1996), antiviral (Tolo, Citation2006), anticancer (Chandramu et al., Citation2003), and anticonvulsant, anxiolytic, and sedative activities (Jamilu et al., Citation2007; Ya’u et al., Citation2011, Citation2010).
The current study evaluated the acute toxicity of the aqueous fraction (RAF) and sub-fractions (S1 and S2) and the anticonvulsant activity of the RAF using in vivo (acute and chronic animal models) and in vitro (electrophysiology) studies.
Materials and methods
Plant materials
The root bark of C. edulis was collected in July 2010 at Basawa Village, Zaria, Nigeria. The plant sample was identified and authenticated by Malam Umar Gallah in the Herbarium section of the Department of Biological Sciences, Ahmadu Bello University, Zaria (voucher number 601).
The root bark samples were air-dried at room temperature under shade until a constant weight was obtained. The dried samples were size reduced with wooden pestle and mortar. The powdered sample (100 g) was extracted with 700 ml of 70% v/v ethanol in water using the cold maceration method. The filtrate was concentrated to dryness producing a brown mass with a pleasant smell.
Fractionation of the crude extract
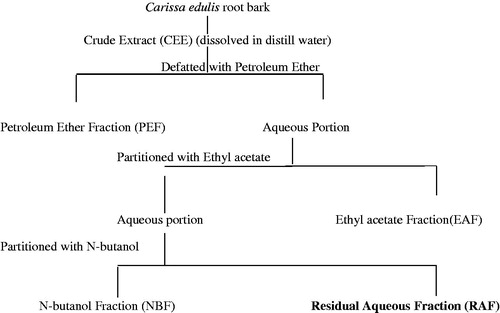
The method of Deng et al. (Citation2004) was used to fractionate the crude ethanol root bark extract of C. edulis to obtain RAF as shown in .
Preliminary phytochemical screening
The preliminary phytochemical screening of the extract fractions from C. edulis was performed according to the methods of Trease and Evans (Citation1983) and Silva et al. (Citation1998). The samples were screened for the presence or absence of alkaloids, anthraquinones, carbohydrates, flavonoids, glycosides, saponins, steroids, and tannins.
Column chromatographic separation of RAF to obtained S1 and S2
Column chromatographic separation of the RAF was conducted according to the method of Deng et al. (Citation2004). Briefly, 8 g of RAF was packed in a glass column with sintered disc at the bottom containing 400 g silica gel (60–20 mesh size). The sample was sequentially eluted with ethyl acetate, mixture of various proportions of ethyl acetate and methanol, then ethyl acetate, methanol, and water. Thirty-six (36) fractions of 50 ml each were collected and pooled into two column fractions (S1 and S2) based on their thin-layer chromatographic (TLC) profile. S1 TLC revealed three major spots with the following RF values 0.17, 0.52, and 0.7, while S2 had two major spots with RF values of 0.26 and 0.76. The anticonvulsant properties of the two fractions were also evaluated in mice.
Animals
One hundred and ninety-two male Swiss Albino mice (18–30 g) and six male Wistar albino rats (130–220 g) were obtained from Animal House Facility of the Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria, Nigeria. Fifty White Ranger Cockrels (18–35 g) were purchased from National Animal Production Research Institute, Zaria, Nigeria. They were maintained at 23.0 ± 2.0 °C, 12 h light and dark cycle, fed with standard animal feed (Feeds Masters, Ilorin, Nigeria) and water was provided ad libitum. The animals were used in compliance with the National Institute of Health Guide for the Care and use of Laboratory Animals (Publication nos. 85-23, revised 1985). The institutional approval number for the protocol was given as DAC/IW-OT/003-10.
Acute toxicity study in mice, rats, and chicks
The intraperitoneal LD50 of the fractions was determined using the method described by Lorke (Citation1983). Briefly, the method has two phases. In the first phase, three groups of three mice each were treated with the fractions at doses of 10, 100, and 1000 mg/kg and observed for signs of toxicity and death for 24 h. Based on the outcome of the first phase, three mice were injected with three more specific doses of the extract. The LD50 value was determined by calculating the geometric mean of the lowest dose that caused death and the highest dose for which the animal survived (0/1 and 1/1).
Anticonvulsant studies
Pentylenetetrazole (PTZ)-induced convulsion in mice
The method of Swinyard et al. (Citation1989) was employed to induce convulsion in mice using PTZ. Thirty mice were divided into five groups of six. The first group received normal saline 10 ml/kg body weight, the second, third, and fourth groups received 150, 300, and 600 mg/kg i.p. of RAF, while the fifth group was injected with valproate 200 mg/kg. Thirty minutes after pretreatment, the animals received PTZ 85 mg/kg subcutaneous (s.c.). Mice were observed over a period of 30 min. The absence of an episode of clonic spasm of at least 5 s duration indicated an extract’s or a compound’s ability to abolish the effect of PTZ-induced seizure threshold (Swinyard et al., Citation1989). The same procedure was repeated for the column fractions (S1 and S2) of RAF.
Maximal electroshock-induced convulsion in chicks
The method described by Swinyard and Kupferberg (Citation1985), as modified by Sayyah et al. (Citation2002), was used in the study. About 50 one-day old cockerels were randomly divided into five groups of 10 chicks per group. The first group received normal saline 10 ml/kg body weight i.p. The second, third, and fourth groups received 150, 300, and 600 mg/kg of RAF i.p. The fifth group received 20 mg/kg of phenytoin i.p. Thirty minutes later, an Ugo Basile electroconvulsive machine (model 7801, Ugo Basile, Hannover, Germany) connected to the Clande Lyons stabilizer (Claude Lyons Limited, Waltham Cross, UK) with corneal electrodes placed on the upper eyelid of the chicks was used to induce seizures in the chicks. The shock duration, frequency, current, and pulse width were set and maintained at 0.6 s, 150 pulse/s, 80 mA, and 0.6 ms, respectively, throughout the study. Seizures were manifested as hind limb tonic extension (HLTE) (Swinyard, Citation1969). The ability to prevent this feature or prolong the latency and/or onset of the HLTE was considered as an indication of anticonvulsant activity (Sayyah et al., Citation2002; Swinyard, Citation1969). The same procedure was repeated to evaluate column fractions (S1 and S2) of RAF.
Subcutaneous strychnine-induced seizure in mice
The method described by Porter et al. (Citation1984) was adopted in this study with modifications. Briefly, 30 mice were divided into five groups of six. The first group received normal saline 10 ml/kg, while the second, third, and fourth groups received 150, 300, and 600 mg/kg, i.p. of RAF, respectively. The fifth group was given phenobarbital 30 mg/kg, i.p. Thirty minutes post-treatment, mice in all the groups received 1.5 mg/kg of strychnine s.c. The proportions of mice presenting convulsions as well as the onset of tonic convulsions were recorded. Abolition of tonic extension of the hind limbs within 30 min after strychnine administration was considered as an indicator for anticonvulsant effects (Porter et al., Citation1984).
Picrotoxin-induced seizures in mice
The method described by Salih and Mustafa (Citation2008) was adopted for this study with modifications. Briefly, 30 mice were divided into five groups of six. The first group received normal saline (10 ml/kg) intraperitoneally. The second, third, and fourth groups received 150, 300, and 600 mg/kg, i.p. of RAF, while mice in the fifth group received diazepam 10 mg/kg. Thirty minutes after pretreatment, 10 mg/kg of picrotoxin was administered to each mouse, s.c. They were then observed for tonic hind limb seizures for 30 min period. The absence of tonic hind limb extension (THLE) or prolongation of the onset of THLE was considered as an indication of anticonvulsant activity (Navarro-Ruiz et al., Citation1995).
N-Methyl-d-aspartate (NMDA)-induced seizures in mice
The methods described by Ngo Bum et al. (2008) and Schmutz et al. (Citation1990) were adopted for this study with modifications. Thirty mice were divided into five groups of six. The first group received normal saline (10 ml/kg). The second, third, and fourth groups received 150, 300, and 600 mg/kg i.p. of RAF, respectively, the fifth group received diazepam 10 mg/kg body weight. Thirty minutes after treatment, 75 mg/kg of NMDA was administered to each mouse, s.c. Animals that did not elicit turning behavior within 30 min period were considered protected (Ngo Bum et al., Citation2008; Schmutz et al., Citation1990). Turning behavior was characterized by two consecutive 360° cycles by the same animal (Ngo Bum et al., Citation2008).
Aminophylline-induced seizures in mice
The method of Mamatha et al. (Citation2009) was adopted for this study with modifications. Briefly, 30 mice were divided into five groups of six. The first group received normal saline 10 ml/kg i.p. The second, third, and fourth groups received 150, 300, and 600 mg/kg of (RAF) i.p., respectively. The fifth group received phenobarbital 30 mg/kg i.p. Thirty minutes after pretreatment, mice in all groups received aminophylline 300 mg/kg s.c. The animals were observed for about 60 min, for the onset of myoclonic seizures, THLE, and mortality.
Isoniazid (INH)-induced seizures in mice
The method of Nanhakumar and Tyagi (2008) was employed for this study with modifications. Thirty mice were divided into five groups of six. The first group received normal saline 10 ml/kg i.p. The second, third, and fourth groups received 150, 300, and 600 mg/kg of (RAF) i.p., respectively. The fifth group received pyridoxine 200 mg/kg body weight i.p., while the sixth group received phenobarbital 30 mg/kg i.p. Thirty minutes after pretreatment, mice in all groups received INH 500 mg/kg s.c. The animals were observed for about 60 min for the onset of myoclonic seizures, THLE, and mortality.
Electrically induced kindling seizures in rats
Implantation of electrodes
The method described by Lothman et al. (Citation1988) was adopted for implantation of the electrodes. Briefly, with the help of stereotaxic machine, bipolar-insulated stainless steel electrodes were implanted at the left posterior ventral hippocampus of anesthetized rats. The guiding coordinates (mm) from bregma were as follows: 5.3 mm backward, 4.9 mm to the left of the midline, and 5.0 mm into the dura and secured to the skull with cranioplast (dental acrylic).
Electroencephalographic recordings
Electroencephalographic (EEG) recordings were made using hippocampal and bilateral cortical electrodes in unanesthetized, freely moving animals, as described previously by Lothman et al. (Citation1988).
Kindling procedure
Kindling was started after a postoperative period of 7 d, when the animals showed no behavioral signs of pain or discomfort. The rats were allowed to acclimatize in a Plexiglas cage, where they attached to the rig and an EEG recording was made for at least 10 trains to assess the spontaneous EEG pattern. Constant-current stimuli were delivered unilaterally to the dorsal hippocampus through a bipolar electrode (recording electrode) twice daily for 5 d then once daily for 2 d (weekend), at intervals of at least 6 h. The stimulation parameters were 50 Hz, 2 ms monophasic rectangular wave pulses for 1 s, the current intensity ranging between 60 and 200 mA. Behavior was observed and the duration of after discharge seizures was measured in the stimulated hippocampus after each stimulation for every animal. Animals were considered fully kindled when they experienced at least three consecutive stage 5 seizures according to the Racine classification (score 0: rats show no response; score 1: stop what it is doing; score 2: rats bobbling/chewing; score 3: shows unilateral shaking of the forelimb at least three times; score 4: shows bilateral shaking of the forelimbs/score 3 plus flip over or falling sidewise; score 5: shows rearing and falling backward/rearing against the wall with evidence of losing balance. Controls were implanted with electrodes, but were not electrically stimulated (referred to here as sham stimulation).
Electrophysiological studies (in vitro studies)
The method of Jones et al. (Citation2009) was employed for this study with modifications. Briefly, human embryonic kidney cells (HEK cells 293) expressing human Nav1.6 were grown in Dulbecco’s modified Eagle’s medium/F-12 media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and geneticin (G418, 500 mg/ml (2.5 ml/500 ml media); Sigma Aldrich, St. Louis, MO). Cells were grown in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Sodium currents were recorded using the whole-cell configuration of the patch-clamp recording technique with an Axopatch 200 amplifier (Molecular Devices, Sunnyvale, CA). All voltage protocols were applied using the pClamp 9 software (Molecular Devices, Sunnyvale, CA) and Digidata 1322A (Molecular Devices, Sunnyvale, CA). Currents were amplified, low pass filtered (2 kHz), and sampled at 33 kHz. Borosilicate glass pipettes were pulled using a Brown–Flaming puller (model P97; Sutter Instruments Company, Novato, CA) and heat polished to produce electrode resistances of 0.5–1.5 MΩ when filled with the following electrode solution: 130 mM CsCl, 1 mM MgCl2, 5 mM Mg ATP, 10 mM BAPTA, and 5 mM HEPES (pH adjusted to 7.4 with CsOH). Cells were plated on glass cover slips and superfused with solution containing the following composition: 130 mM NaCl, 4 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 5 mM HEPES, and 5 mM glucose (pH adjusted to 7.4 with NaOH). All sodium channel current experiments were performed at room temperature (20–22 °C). After establishing whole-cell configuration, a minimum series resistance compensation of 60% was applied, and cells were held at −60 mV for 5 min to account for any equilibrium gating shifts. After control recordings, RAF (1000 µg/ml stock concentration) of 1, 0.6, and 0.3 ml were applied and allowed for 5 min bath equilibration in 50 ml volume of extracellular solution. Tonic block was assessed by comparing peak sodium current in drug-free conditions with peak current when the drug was present.
Results
The preliminary phytochemical screening of RAF revealed the presence of anthraquinone, tannins, saponins, flavonoids, carbohydrates, and steroids (terpenoids) (). The intraperitoneal and oral the LD50 of RAF were estimated to be 2222.61 and greater than 5000 mg/kg, while the intraperitoneal and the oral LD50 of sub-fractions of the RAF were found to be >5000 mg/kg for both S1 and S2.
Table 1. Phytochemical constituents RAF.
The RAF protected 50% of the mice against PTZ-induced seizures at the highest dose (600 mg/kg) while both 150 and 300 mg/kg offered only 16.67% protection. The standard antiepileptic drugs used valproate 200 mg/kg and diazepam 1 mg/kg gave 100% protection. There were no significant differences in the mean onset of seizures between the control and the test groups, except the 600 mg/kg dose of RAF which was significant at p < 0.001. The RAF at doses tested (150–600 mg/kg) offered varied protection against mortality ranging from 16.67 to 50%. There was no significant difference on the mean onset of mortality ().
Table 2. Effects RAF on pentylenetetrazole (PTZ)-induced seizures in mice.
RAF did not protect mice against THLE and death induced by picrotoxin. Phenobarbital (30 mg/kg) offered only 16.67% seizure protection. However, RAF at doses of 150 and 300 mg/kg and the standard drug, phenobarbital, increased the mean onset of seizures/mortality significantly at p < 0.05 ().
Table 3. Effects of RAF on picrotoxin-induced seizures in mice.
The fraction produced protection against strychnine-induced seizures in mice; a low dose of 150 mg/kg gave 16.67%, 300 mg/kg, 0%, and 33.33% at the highest dose (600 mg/kg) against strychnine-induced hind limb extension and death while the standard drug used, phenobarbital (30 mg/kg), offered 100% seizure protection. There was no significant difference between the control and the test groups in the mean onset of seizures/mortality ().
Table 4. Effects of RAF on strychnine-induced seizures in mice.
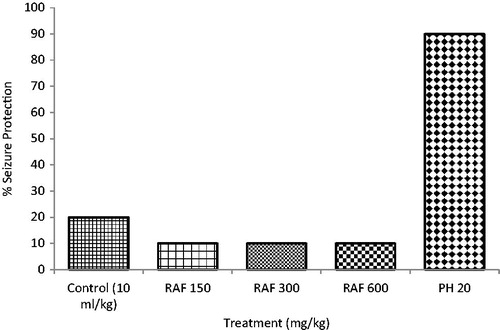
The fraction did not protect the chicks against THLE induced by maximal electroconvulsive shock while phenytoin 20 mg/kg a standard antiepileptic drug protected the animals by 90%. There was no significant reduction in the mean recovery time ().
Figure 2. Effects of residual aqueous fraction (RAF) of ethanolic extract of Carissa edulis root bark on maximal electroshock test (MEST)-induced seizures in chicks, n = 10.
The fraction offered 16.67% protection against two consecutive 360° cycles induced by NMDA at 300 and 600 mg/kg. However, the phenobarbital 30 mg/kg did not protect the mice against the seizures induced by NMDA. There was no significant increase in the mean onset of mortality between the control and the test groups. Further, the fraction protected the animal by 50% against mortality at dose of 600 mg/kg and phenobarbital 30 mg/kg ().
Table 5. Effects of RAF on N-methyl-d-aspartate (NMDA)-induced seizures in mice.
The fraction offered inversely dose-dependent protection from aminophylline-induced seizures, whereas 150 mg/kg protected the animals by 66.67% and the 600 mg/kg gave 33.33% protection. However, phenobarbital 30 mg/kg did protect the mice against the seizures 100%. There was no significant increase in the mean onset of myoclonic seizure and THLE/mortality between the control and the test groups, except 300 mg/kg of RAF that delayed the mean onset of myoclonic seizure significantly (p < 0.05) ().
Table 6. Effect of RAF on aminophylline-induced seizures in mice.
RAF did not offer any protection to mice against INH-induced seizures. Phenobarbital and pyridoxine failed to protect the mice against INH-induced seizures, but phenobarbital significantly (p < 0.005) delayed the mean onset of myoclonic seizures and mean onset of THLE and mortality (p < 0.001), RAF at dose of 600 mg/kg significantly (p < 0.05) increased the onset of THLE ().
Table 7. Effect of residual aqueous fraction (RAF) of ethanolic extract of Carissa edulis root bark on isoniazid (INH)-induced seizures in mice.
The sub-fraction S1 of RAF offered protection against PTZ-induced seizures by 16.67% at doses 500 and 1000 mg/kg, mortality onset by 33.33 and 16.67%, while valproate protected the mice 100%. There was significant increase in the mean onset of seizures across all the doses (p < 0.001). While mean onset of mortality was delayed at doses of 250 and 500 mg/kg, p < 0.05 ().
Table 8. Effects of sub-fraction S1 of RAF on pentylenetetrazole (PTZ)-induced seizures in mice.
The sub-fraction S2 of the RAF did not protect the mice against PTZ-induced seizures except 500 mg/kg, which gave 16.67% as against the standard antiepileptic drug valproate that protected the animal by 100%. However, there was significant increase in the mean onset of seizures across all doses (250–1000 mg/kg), p < 0.05. At the dose of 1000 mg/kg, a significant delay (p < 0.01) in the mortality onset was observed (). Both S1 and S2 did not offer protection against maximal electroshock-induced seizures in chicks. Hence, the data are not shown.
Table 9. Effects of sub-fraction S2 RAF on pentylenetetrazole (PTZ)-induced seizures in mice.
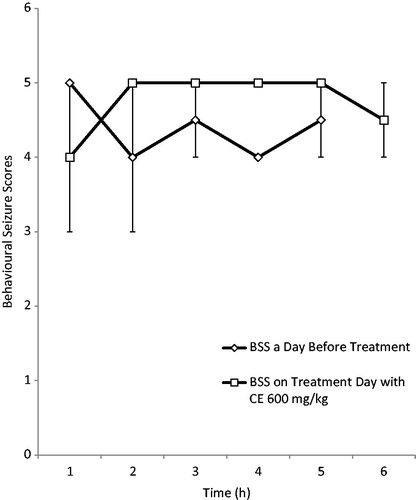
RAF at the dose of 300 mg/kg reduced both the behavioral seizure scores (BSS) and the mean seizure duration (MSD) at all the 3 h after treatment compared with the scores before treatment, while 600 mg/kg did not reduce the BSS scores, but affected the mean seizure duration. However, the reduction in the BSS and mean seizure duration was not significant in both doses used ().
Figure 3. Effect of RAF (300 mg/kg) on mean seizure duration (MSD) in electrically induced kindling in adult rats.
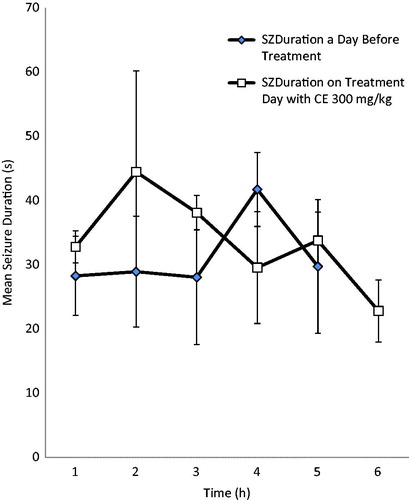
Figure 4. Effect of RAF (300 mg/kg) on behavioral seizure scores (BSS) in electrically induced kindling in adult rats.
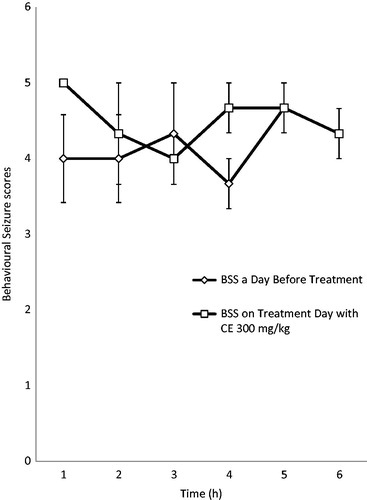
Figure 5. Effect of RAF (600 mg/kg) on mean seizure duration (MSD) in electrically induced kindling in adult rats.
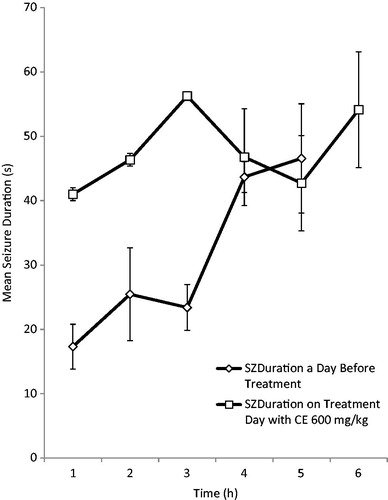
Figure 6. Effect of RAF (600 mg/kg) on behavioral seizure scores (BSS) in electrically induced kindling in adult rats.
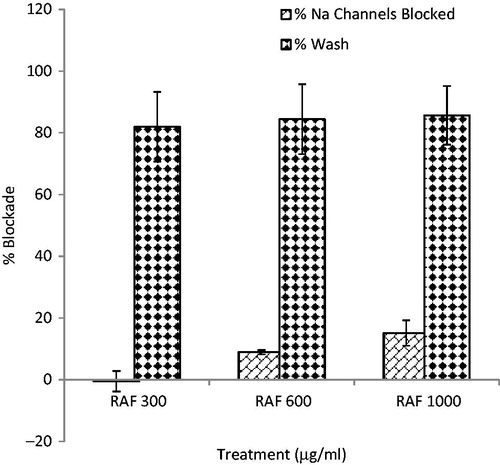
RAF showed a dose-dependent blockade for sodium channels on human embryonic kidney cells (HEK cells 293), although the percentage blockade was not significant even at high concentration ().
Discussion
Previous studies revealed that the root bark of Carissa edulis contains steroids, triterpenes, tannins, flavonoids, and cardiac glycosides (Ibrahim, Citation1997; Ibrahim et al., Citation2005, Citation2007). Among the secondary metabolites present in the crude extract of this important medicinal plant are the anthraquinones, saponins (steroids and triterpenes), cardiac glycosides (cardinolides), flavonoids, and tannins, which might be responsible for the observed pharmacological (anticonvulsant and antitumor) activities of the plant (Amos et al., Citation2001). Given that phytochemicals such as saponins, tannins, flavonoids, and cardiac glycosides are known to be biologically active (Herbalain et al., Citation1994; Ya’u et al., Citation2011), RAF has the same phytochemical constituents as that of crude ethanol extract with the exception of cardiac glycosides. The broad spectrum of the observed anticonvulsant effects in this study might be attributed to the presence of different biologically active components in the extracts. The intraperitoneal and oral LD50 of RAF was estimated to be 2222.61 mg/kg and above 5000 mg/kg, respectively, suggesting its apparent safety (Corbett et al., Citation1984; Loomis & Hayes, Citation1996; Matsumura, Citation1985). The LD50 values of column fractions of RAF (S1 and S2) were estimated to be greater than 5000 mg/kg for both i.p. and p.o. routes in mice.
The causes of epilepsy are extremely diverse, encompassing genetic, developmental defects, infective, traumatic, neoplastic, and degenerative disease processes (Roger & Brain, 2004), making it difficult for a single drug to be used to treat this debilitating neurological disorder. RAF and its sub-fractions S1 and S2 protected the mice against PTZ-induced seizures. PTZ, a well-known convulsant agent, has been shown to diminish the GABAergic tone (Macdonald & Baker, Citation1997) by the inhibition of benzodiazepine (BDZ) site of the GABA receptor channel complex (De Deyn et al., Citation1992). Gamma amino butyric acid (GABA) is a major inhibitory neurotransmitter in the brain, and the inhibition GABAergic neurotransmission has been thought to be one of the underlying factors responsible for epilepsy (Amabeoku et al., Citation1998). The enhancement of the GABAergic neurotransmission is reported to antagonize seizures (Leonard, Citation2003). Therefore, inhibition of PTZ-induced seizures suggests effects on GABAergic neurotransmission.
The PTZ test identifies compounds that can raise seizures threshold in the brain (Stables & Kupferberg, Citation1997; White et al., Citation1998) consistent with the human condition (Swinyard et al., Citation1989). The PTZ-induced seizures are similar to the symptoms observed in the absence seizures and drugs such as valproate, diazepam, and ethosuximide, which are useful in the treatment of the absence seizures and suppress PTZ-induced seizures (McNamara, Citation2006). It has been found empirically that drugs which inhibit PTZ-induced seizures and raise the threshold for production of electrically induced seizures are generally effective against absence seizures (Rang et al., Citation2003). The ability of RAF to protect the animals against PTZ-induced seizures suggests anticonvulsant activity against absence seizures (petit mal) or myoclonic seizures. Thus, RAF may be useful in the management of petit mal (absence) and/or myoclonic epilepsy.
The maximal electroshock test (MEST) is a non-mechanistic seizures model that has clearly defined end points such as inhibition of HLTE (Stables & Kupferberg, Citation1997). There is no false negative in the MES test and the currently available AEDs that are clinically effective in the management of generalized tonic–clonic and partial seizures such as carbamazepine, phenytoin, primidone, phenobarbital, valproate, and lamotrigine all suppress HLTE in MES test (Browning, Citation1992; Rho & Sankar, Citation1999). RAF and its sub-fractions (S1 and S2) did not protect the chicks against seizures induced by maximal electroshock suggesting non-activity against generalized tonic clonic and partial seizures.
Picrotoxin is a non-competitive antagonist of GABAA receptor chloride channels (Leonard, Citation2003) and its GABA inhibitory actions affect different areas of the central nervous system, thus picrotoxin produces generalized tonic–clonic seizures, which lead to death in most of the cases (Abdul-Ghani et al., Citation1980). Although RAF did not protect the animals against picrotoxin-induced THLE and death, the fraction extended the mean onset of seizures, which indicates mild anticonvulsant activity against picrotoxin.
RAF moderately protected the animals against strychnine-induced seizures. The convulsing action of strychnine is due to the interference with postsynaptic inhibition mediated by glycine, an important inhibitory transmitter of the motor neurons and interneurons in the spinal cord. Strychnine-sensitive postsynaptic inhibition in higher centers of the CNS is also mediated by glycine. Strychnine acts as a selective, competitive antagonist at all glycine receptors (Larson, Citation1969; Rajendra et al., Citation1997). The ability of RAF to prevent the strychnine-induced seizures demonstrates additional anticonvulsant effects mediated via glycine receptors (Ogbonnia et al., Citation2003).
A significant percentage (66.7%) of the animals were protected against aminophylline-induced THLE and death in an inversely dose-dependent manner. The therapeutic use of theophylline (an active constituent of aminophylline) is associated with the incidence of intractable seizures (Barnes, Citation1998). Studies suggest that seizures induced by theophylline could be due to adenosine receptor antagonism or due to inhibition of cerebral 5-nucleotidase activity (Chu, Citation1981). Apart from these findings, phosphodiesterase-3 (PDE-3) inhibition by theophylline causes transmembrane influx of Ca2+. This influx of Ca2+ is as a result of the phosphorylation processes of intracellular proteins, such as ion channels, receptors, enzymes, and transcription factors, which exhibit significant neuronal excitability and epileptic seizures (Butler et al., 1995). The release of free radicals has been implicated in many drug and chemical-induced toxicities (Lebel & Bondy, Citation1991). It is possible that increased production of reactive oxygen species could result to oxidant/anti-oxidant imbalance and thus, precipitate neurotoxicity (Gulati et al., Citation2005). The anticonvulsant activity of RAF and phenobarbital against aminophylline-induced seizure may be due to the potentiation of adenosine receptor and/or phosphodiesterase 3 activities. It may also be due to anti-oxidant activity of the RAF in addition to its GABA-mediated activity.
RAF delayed the onset of myoclonic seizure induced by INH in a dose-dependent manner. Since INH is a GABA synthesis inhibitor (Biggs et al., Citation1994), it is likely that RAF produces its anticonvulsant effect by enhancing GABAergic neurotransmission action in the brain by augmenting the synthesis of GABA (Kelly et al., Citation1990).
The kindling model was used to evaluate the effects of RAF on the chronic model of partial epilepsy. The kindling animal model is a repetitive electrical stimulation of discrete brain areas at appropriate intervals, which is well recognized as an experimental method for chronic partial epilepsy studies (Mcintyre, Citation2006). RAF at the dose of 300 mg/kg reduced both the behavioral seizure scores (BSS) and the mean seizure duration (MSD) after 3 h of treatment compared with the scores before treatment. The 600 mg/kg dose did not reduce the BSS scores, but affected the mean seizure duration. However, the reduction in the BSS and mean seizure duration was not significant in both doses used. This results suggest that RAF might not be useful in the management of temporal lobe (partial) epilepsy (Mcintyre, Citation2006) and this corroborate with the acute model of seizures where RAF was not effective against MEST-induced seizure.
RAF partially blocked voltage-gated sodium channels firing only at highest concentrations used (1000 µg/ml). This further confirms that the additional anticonvulsant activity of RAF via the voltage gated sodium channels.
Conclusion
In sum, we conclude that RAF and its sub-fractions (S1 and S2) contain biologically active principles that are sedative in nature that are relevant to the management of convulsive disorders, thus supporting the isolation and the development of the psychoactive principles as anticonvulsant agents. The multiplicity of putative mechanisms of action as reflected by the broad spectrum of activity observed in this study might be attributed to the presence of a combination of different biologically active components in the fractions.
Declaration of interest
The authors declared that there was no conflict of interest.
References
- Abdul-Ghani A, Coutinho-Netto J, Bradford HF. (1980). The action of γ-vinyl-GABA and γ-acetylenic-GABA on the resting and stimulated release of GABA in vivo. Brain Res 191:471–81
- Amabeoku GJ, Leng MJ, Syce JA. (1998). Antimicrobial and anticonvulsant activities of Viscum cupanse. J Ethnopharmacol 61:237–41
- Amos S, Kolawole E, Akah P, et al. (2001). Behavioral effects of the aqueous extract of Guiera senegalensis in mice and rats. Phytomedicine 8:356–61
- Barnes PJ. (1998). Theophylline. In: Barnes PJ, Rodger IN, Thompson NC, eds. Asthma: Basic Mechanism and Clinical Management. San Diego, USA: Academic Press, 689–706
- Biggs CS, Pearce BR, Fowler LJ, Whitton PS. (1994). Effect of isonicotinic acid hydrazide on extracellular amino acids and convulsions in the rat. Reversal of neurochemical and behavioral deficits by sodium valproate. J Neurochem 63:2197–201
- Browning R. (1992). The electroshock model, neuronal network and antiepileptic drugs. In: Faingold CL, Fromm GH, eds. Drugs for Control of Epilepsy: Actions on Neuronal Networks in Seizure Disorders. Boca Raton, FL: CRC Press, 195–211
- Butler LS, Silva AJ, Abeliovich A, et al. (1995). Limbic epilepsy in transgenic mice carrying a Ca2+/Calmodulin dependent kinase II alpha-subunit mutation. Proc Nat Acad Sci 15:6852–5
- Chandramu C, Manohar RD, Krupadanam DG, Dashavantha RV. (2003). Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother Res 17:129–34
- Chu NS. (1981). Caffeine and aminophylline-induced seizures. Epilepsia 22:85–95
- Corbett JR, Wright K, Baillie AC. (1984). The Biochemical Mode of Action of Pesticides, 2nd ed. London, New York, Tokyo: Academic Press, 382
- De Deyn PP, D’Hoope R, Marescau B, Pei YQ. (1992). Chemical model for epilepsy with some references to their applicability in the development of anticonvulsants. Epilepsy Res 12:87–110
- Deng W, Breneman C, Embrechts MJ. (2004). Predicting protein–ligand binding affinities using novel geometrical descriptors and machine-learning methods. J Chem Inf Comput Sci 44:699–703
- El-Fiky FK, Abou-Karam MA, Afify EA. (1996). Effect of Luffa aegyptiaca (seeds) and Carissa edulis (leaves) extracts on blood glucose level of normal and streptozotocin diabetic rats. J Ethnopharmacol 50:43–7
- Eisner T. (1990). Chemical prospecting: A call for action. In: Borman FH, Kellert SR, eds. Ecology, Economic and Ethics: The Broken Circle. New Haven (CT): Yale University Press Inc
- Forster PI. (1996). Flora of Australia, Vol. 28. Melbourne, Australia: CSIRO Publishing
- Geoffrey C, Kirby M. (1996). Medicinal Plants and the Control of Protozoal Disease with Reference to Malaria. London: Transaction of the Royal Society of Tropical Medicine and Hygiene, 605–9
- Gulati K, Ray A, Pal G, Vijayan VK. (2005). Possible role of free radicals in theophylline-induced seizures in mice. Pharmacol Biochem Behav 82:241–5
- Hegde KS, Thakker P, Joshi AB, et al. (2009). Anticonvulsant activity of Carissa carandas Linn. root extract in experimental mice. Trop J Pharm Res 8:117–25
- Herbalain H, Tscheirsch KP, Schazer HL. (1994). Flavanoids from Leptospermum scoparium with affinity to the benzodiazepine receptors characterized by structure activity relationship and in vivo studies of plant extract. Pharmazie 49:912–22
- Ibrahim H, Abdurrahman EM, Shok M, et al. (2007). Comparative analgesic activity of the root bark, stem bark, leaves, fruits and seed of Carissa edulis Vahl (Apocynaceae). Afr J Biotech 6:1233–5
- Ibrahim H, Bolaji RO, Abdulrahman EM, et al. (2005). Preliminary phytochemical and antimicrobial studies of the leaves of Carissa edulis. Chemclass J 2:15–18
- Ibrahim H. (1997). Pharmacognostic and biological (analgesic activity) studies of Carissa edulis Vahl [PhD thesis]. Zaria, Nigeria: Ahmadu Bello University
- Jamilu Y, Yaro AH, Abubakar MS, et al. (2007). Studies on anticonvulsant activity of fractions of hydro-alcoholic root bark extract of Carissa edulis (Vahl). Nig J Pharm Sci 6:59–64
- Jones PJ, Merrick EC, Batts TW, et al. (2009). Modulation of sodium channel inactivation gating by a novel lactam: Implications for seizure suppression in chronic limbic epilepsy. J Pharmacol Exp Ther 328:201–12
- Kelly KM, Gross RA, Macdonal RL. (1990). Valproic acid selectively reduces the low-threshold (T) calcium current in rat nodose neurones. Neurosci Lett 116:233–7
- Larson MD. (1969). An analysis of the action of strychnine on recurrent ISPS and amino acid induced inhibitions in the cat spinal cord. Brain Res 15:185–200
- Lebel CP, Bondy SC. (1991). Oxygen radicals common mediators of neurotoxicity. Neurotoxicol Teratol 13:314–16
- Leonard BE. (2003). Drug treatment of the epilepsies. In: Fundamentals of Psychopharmacology. 3rd ed. Chichester: John Wiley and Sons, 295–318
- Loomis TA, Hayes AW. (1996). Loomis’s Essentials of Toxicology, 4th ed. California: Academic Press
- Lorke D. (1983). A new approach to practical acute toxicity testing. Arch Toxicol 54:275–87
- Lothman EW, Perlin JB, Salerno RA. (1988). Response properties of rapidly recurring hippocampal seizures in rats. Epilepsy Res 2:356–66
- Macdonald RL, Baker JL. (1997). Pentylenetetrazol and penicillin are selective antagonists of GABA-mediated post-synaptic inhibition in cultured mammalian neurons. Nature 267:720–1
- Mamatha SG, John J, Preethilatha R, et al. (2009). Protective effects of graded doses of gabapentin on aminophylline-induced experimental status epilepticus in mice. Ann Neurosci 16:150–4
- Matsumura F. (1985). Toxicology of Insecticides, 2nd ed. New York: Plenum Press
- Mcintyre DC. (2006). The kindling phenomenon. In: Pitkänen A, Schwartzkroin PA, Moshé SL, eds. Models of Seizures and Epilepsy. New York: Elsevier Academic Press, 351–69
- McNamara JO. (2006). Pharmacotherapy of epilepsies. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman and Gilman’s, The Pharmacological Basic of Therapeutics, 11th ed. New York, USA: McGraw-Hill Co. Inc., 501–25
- Msonthi JD. (1986). The status of medicinal plant research and traditional medicine in Malawi. In: Sofowora A, ed. The State of Medicinal Plant Research in Nigeria. Proceedings of the Workshop Oraganized by African Biosciences Network. Nigeria: University Press Ltd. Ife, 335–50
- Nanhakumar J, Tyagi MG. (2008). Evaluation of cyclic nucleotide phosphodiesterase III inhibitors in animal models of epilepsy. Biomed Res 19:13–17
- Navarro RA, Bastidas RBE, Garcia EJ, et al. (1995). Anticonvulsant activity of Casimiroa edulis in comparison to phenytoin and phenobarbital. J Ethnopharmacol 45:199–206
- Nedi T, Mekonneni N, Urga K. (2004). Diuretic effect of the crude extract of Carissa edulis in rats. J Ethnopharmacol 95:57–61
- Ngo Bum E, Ngoupaye GT, Talla E, et al. (2008). The anticonvulsant and sedative properties of stems of Cissus quadrangularis in mice. Afr J Pharm Pharmacol 2:42–7
- Ogbonnia SO, Jager AK, Van Staden J, Coker HAB. (2003). Anticonvulsant activity of Scumanni phytonmagnificum roots extracts in mice. W Afr J Pharmacol Drug Res 19:33–6
- Porter RJ, Cereghino JJ, Gladding GD. (1984). Antiepileptic drug development program. Cleve Clin J Med 51:293–305
- Rajendra S, Lynch JW, Schofield PR. (1997). The glycine receptor. Pharmacol Ther 73:121–46
- Rang HP, Dale MM, Ritter JM, Moore PK. (2003). Antiepileptic drugs. In: Rang HP, Dale MM, Ritter JM, Moore PK, eds. Pharmacology, 5th ed. Edinburg: Churchill Livingstone, 550–60
- Rho JM, Sankar R. (1999). The pharmacologic basis of antiepileptic drug action. Epilepsia 40:1471–83
- Roger JP, Brian SM. (2004). Antiseizure drugs. In: Bertram GK, eds. Basic and Clinical Pharmacology, 9th ed. New York: Lange Medical Books/McGraw Hill, 379–85
- Salih MA, Mustafa MM. (2008). A substance in broad beans (Vicia faba) is protective against experimentally induced convulsions in mice. Epilepsy Behav 12:25–9
- Sayyah M, Valizadeh J, Kamalinejad M. (2002). Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole and maximal electroshock-induced seizures. Phytomedicine 9:212–16
- Schmutz M, Portet C, Jeker A, et al. (1990). The competitive NMDA receptor antagonists CGP 37849 and CGP 39551 are potent, orally-active anticonvulsants in rodents. Naunyn Schmiedebergs Arch Pharmacol 342:61–6
- Silva GL, Lee I, Kinghorn AD. (1998). Special problems with the extraction of plants. In: Cannell RJP, ed. Methods in Biotechnology (Natural Product Isolation). New Jersey, USA: Humana Press, 245–364
- Stables JP, Kupferberg HJ. (1997). The NIH Anticonvulsant Drug Development (ADD) program: Preclinical anticonvulsant screening project. In: Avanzini G, Regesta G, Tanganelli I, Avoli M, eds. Molecular and Cellular Target for Anti-epileptic Drugs. London: John Libbey and Company Ltd, 191–8
- Swinyard EA, Kupferberg HJ. (1985). Antiepileptic drugs: Detection, quantification and evaluation. Fed Proc 44:39–43
- Swinyard EA, Woodhead JH, White HS, Franklin MR. (1989). General principles: Experimental selection, quantification, and evaluation of anticonvulsants. In: Levy RH, Mattson B, Meldrum JK, Dreifuss FE, eds. Antiepileptic Drugs, 3rd ed. New York, USA: Raven Press, 85–103
- Swinyard EA. (1969). Laboratory evaluation of antiepileptic drugs: Review of laboratory methods. Epilepsia 10:107–19
- Tolo FM. (2006). Antiviral activity of the extracts of Kenyan medicinal plant Carissa edulis against herpes simplex virus. J Ethnopharmacol 104:92–9
- Trease GE, Evans MC. (1983). Textbook of Pharmacognosy, 12th ed. London: Balliere Tindall, 322–283
- White HS, Wolf HH, Woodhead JH, Kupferberg HJ. (1998). The National Institute of Health anticonvulsant drug development program: Screening for efficacy. In: French J, Leppik IE, Dichter MA, eds. Antiepileptic Drug Development: Advances in Neurology, vol. 76. Philadelphia, USA: Lippincott-Raven Publishers, 29–39
- WHO. (1978). Drug Policies and Management: Medicinal Plants. Geneva, Switzerland: WHO Resolution. 31–3
- Ya’u J, Abdullahi SM, Magaji MG, et al. (2010). Behavioral studies on the ethanol root bark extract of Carissa edulis. Jopatrot 1:31–4
- Ya’u J, Abdulmalik UN, Yaro AH, et al. (2011). Behavioral properties of Balanites aegyptiaca in rodents. J Ethnopharmacol 135:725–9