Abstract
Context: Brazilin is a major active principle of Caesalpinia sappan L. (Leguminosae or Fabaceae). For industry aspects, brazilin-rich extract (BRE) has been prepared and standardized to contain 39% w/w brazilin. BRE may have more advantages than brazilin in term of a lower-cost production process.
Objectives: To investigate the antioxidant, antibacterial, and anti-inflammatory activities of BRE.
Material and methods: BRE was prepared by a simple one-step purification of the crude ethanol extract of C. sappan heartwood (CSE) using a Diaion® HP-20 column. The antioxidant activities were determined using three methods, including DPPH radical scavenging, reducing power, and β-carotene bleaching assays, at concentration ranges of 1–10, 10–100, and 10–100 µg/mL, respectively. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of BRE (15.6–1000 µg/mL) against Gram-positive and Gram-negative bacteria were determined by the broth microdilution method. Anti-inflammatory activity of BRE (0.1–5 µg/mL) was evaluated as anti-denaturation activity using bovine serum albumin as a substrate.
Results and discussion: On the basis of β-carotene bleaching assay, BRE showed antioxidant activity with an EC50 value of 60.5 µg/mL, which was almost equal to that of pure brazilin (52.1 µg/mL). Gram-positive bacteria were more sensitive to all tested samples than Gram-negative bacteria. BRE possessed higher antibacterial activities than CSE, but lower than brazilin. MIC/MBC values of 62.5–125/125 and 250–500/250–500 µg/mL were obtained for BRE against Gram-positive and Gram-negative bacteria, respectively. A low concentration (0.1 µg/mL) of brazilin, BRE, and CSE showed anti-inflammatory activity by inhibiting protein denaturation up to 46.8, 54.1, and 61.9%, respectively.
Introduction
Phenolic compounds from plants are known to possess antioxidant activities in terms of free radical scavenger, reducing power, and quencher of singlet oxygen formation (Duh et al., Citation2001). It is well known that antioxidant compounds can protect against several degenerative diseases, and have potential use as a dietary supplement. In addition, many food or functional food producers are interested in a natural antioxidant to improve the nutritional quality of processes foods or to prepare functional food (Wijeratne et al., Citation2006).
Caesalpinia sappan L. (Leguminosae or Fabaceae) heartwood has been traditionally used as a natural coloring agent in beverages, food, cosmetics, and garments. The heartwood contains various types of phenolic components, such as dibenzoxocins, flavones, homoisoflavonoids, chalcones, xanthone, and brazilin (Chen et al., Citation2008). A decoction of the heartwood has been traditionally used as an emmenagogue, anti-inflammatory, and hemostatic agent in Southeast Asia (Zhao et al., Citation2008). The wood extract has also been used as a natural preservative in chili paste (Saraya et al., Citation2009).
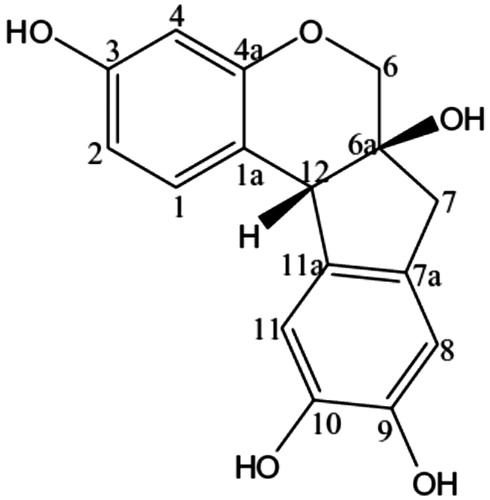
Brazilin [(6aS,11bR)-7,11b-dihydro-6H-indeno[2,1-c]chromene-3,6a,9,10-tetrol] () is a major active compound of C. sappan that has antibacterial, anti-inflammatory, and antioxidant activities (Bae et al., Citation2005; Batubara et al., Citation2010; Hu et al., Citation2009; Sasaki et al., Citation2007; Xu & Lee, Citation2004). However, the major limitation of the use of pure brazilin is its high cost of production, due to the number of steps and time involved for purification. Brazilin, therefore, is used as an indicative marker for preparation and standardization of brazilin-rich extract (BRE) from C. sappan heartwood (Nirmal & Panichayupakaranant, Citation2014).
In order to investigate the full potential of the standardized BRE as natural food preservatives and nutraceuticals, we now report on the antioxidant, antibacterial, and anti-inflammatory activities of BRE containing 39% w/w brazilin.
Materials and methods
Chemicals and reagents
Folin–Ciocalteu reagent, 2,2-diphenyl-1-picryl hydrazyl (DPPH), sodium carbonate, sodium chloride, trichloroacetic acid, and potassium ferricyanide were from Fluka Chemie AG (Buchs, Switzerland). Ferric chloride, quercetin, ascorbic acid, β-carotene, linoleic acid, and BSA were from Sigma-Aldrich (St. Louis, MO). Tween-40 was from E. Merck (Darmstadt, Germany). All solvents were HPLC and analytical grade and from Labscan Asia (Bangkok, Thailand).
Plant material
Caesalpinia sappan heartwoods were collected from Chonburi province, Thailand, in February 2012. A voucher specimen (specimen no. SKP 098 03 19 01) was identified by Associate Professor Pharkphoom Panichayupakaranant and has been deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. The plant material was washed and dried at 60 °C for 24 h in a hot air oven, and was reduced to powder using a grinder, and the powder passed through a sieve no. 45.
Preparation of BRE
BRE was prepared and standardized to contain 39% w/w brazilin. The dried CSE (25 g) was dissolved in 35% ethanol (3 L) to obtain a solution after filtering. The solution was loaded on a Diaion® HP-20 column (12 × 50 cm, 1 g CSE per 60 g resin) and eluted with 35% ethanol. The first 2 L fraction was discarded and another fraction (4 L) that contained brazilin was collected. The collected fractions were evaporated to dryness to obtain a reddish extract of BRE.
Determination of the total phenolic content
The total phenolic content was determined with the Folin–Ciocalteu reagent according to the method of Nirmal and Benjakul (Citation2011). Appropriately diluted extract (0.1–5.0 µg/mL; 1 mL) was added with 0.2 mL of two-fold diluted Folin–Ciocalteu reagent and mixed thoroughly. After 3 min, 3 mL of 2% sodium carbonate solution was added. After standing for 30 min at room temperature (25 ± 2 °C), the absorbance was measured at 760 nm using a UV-spectrophotometer (Genesys-6, Thermo-Scientific, Madison, WI). The concentration of total phenolic compound in CSE and BRE was calculated from the standard curve of quercetin with the range of 0.01–0.1 mg/mL and expressed as g quercetin equivalent (QE)/ 100 g dry extract powder.
DPPH radical scavenging assay
DPPH radical scavenging activity was determined according to the method of Nirmal and Benjakul (Citation2011) with some modifications. Samples (1.5 mL) at concentration ranges of 1–10 µg/mL were added to 1.5 mL of 0.15 mM DPPH in 95% ethanol. The reaction mixtures were allowed to stand for 30 min at room temperature (25 ± 2 °C) in the dark and the absorbance was measured at 517 nm using the UV-spectrophotometer. Quercetin (1–7 µg/mL) was used as a standard. The sample blank at each concentration was prepared in the same manner except that ethanol was used instead of the DPPH solution. Control was prepared with DPPH solution and ethanol without any sample. The percentage inhibition of DPPH radical was calculated from following equation:
Radical scavenging potential was expressed as IC50 values which represents the sample concentration at which 50% of the radicals are scavenged.
Determination of reducing power
The reducing power was determined as described by Duh et al. (Citation2001). The samples (0.01–0.1 mg) in phosphate buffer (1.0 mL, 0.2 M, pH 6.6) were mixed with 1% potassium ferric cyanide (1 mL), and the mixtures were incubated at 50 °C for 20 min. After incubation, 1 mL of 10% trichloroacetic acid was added and the mixtures were centrifuged at 2500 rpm for 10 min. The upper layer of the solution (1 mL) was mixed with distilled water (1 mL) and 0.1% ferric chloride (0.2 mL) and the absorbance was read at 700 nm using the UV-spectrophotometer. Higher absorbance of the reaction mixture indicated greater reducing power. Ascorbic acid and quercetin were used as the standard and subjected to the same procedure mentioned above instead of extract.
β-Carotene bleaching assays
The antioxidant activity of extracts was evaluated by using the β-carotene–inoleate model system (Panichayupakaranant et al., Citation2010). β-Carotene (10 mg) was dissolved in 10 mL of chloroform. The carotene–chloroform solution (0.2 mL) was transferred into a flask containing linoleic acid (20 mg) and Tween-40 (200 mg). Chloroform was removed under vacuum using a rotary evaporator at 40 °C for 5 min. The resulting mixture was slowly diluted with 50 mL of distilled water and mixed thoroughly with vigorous shaking to form an emulsion. Aliquots (5 mL) of the emulsion were transferred into a series of tubes containing 0.2 mL of samples solution. Immediately after the addition of the emulsion to each tube, the zero time absorbance was measured at 470 nm. The tubes were placed in a water bath at 50 °C and monitored spectrophotometrically by measuring the absorbance at 470 nm over a 60-min period. Quercetin was used as the reference antioxidant. Blank samples devoid of β-carotene were prepared for background subtraction. A control sample contained 0.2 mL of distilled water instead of sample solution was prepared. All tests were performed in triplicate. The antioxidant activity was expressed as inhibition percentage with reference to the control after 60 min incubation using the following equation.
where AA is the antioxidant activity; BRC is the bleaching rate of the control = (ln(a/b)/60); BRS is the bleaching rate in the presence of sample = (ln(a/b)/60); a is the absorbance at time 0; b is the absorbance at 60 min.
Anti-inflammatory assay
Anti-inflammatory activity of BRE was evaluated as anti-denaturation activity using bovine serum albumin as a substrate. Anti-denaturation activities of the samples were determined according to the method of Williams et al. (Citation2008) with slight modification. A stock solution of 0.2% (w/v) BSA was prepared in the Tris-acetate buffer, pH 6.8. The test compounds (0.1–5 µg/mL) were prepared in methanol. The reaction mixture consists of 150 µL of test samples and 2850 µL of BSA solution was incubated at room temperature for 15 min. Test tubes were heated at 72 °C for 4 min and then cooled at room temperature for 20 min. The absorbance of these solutions was determined by the UV-spectrophotometer at a wavelength of 660 nm. The percentage inhibition of denaturation or precipitation of the BSA from the sample solution was calculated from following equation:
The control consists of 2850 µL of BSA solution with 150 µL of methanol. Diclofenac was used as the standard drug.
Microorganisms and media
Pseudomonas aeruginosa (DMST 15442) and Salmonella typhimurium (DMST 562) were obtained from the Department of Medical Science Center, Thailand. Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), and S. epidermidis (ATCC 14990) were obtained from the Department of Microbiology, Faculty of Medicine, Prince of Songkhla University, Thailand. Bacillus subtilis was from Faculty of Science, Prince of Songkhla University. Muller–Hinton agar (MHA), Muller–Hinton broth, Brain–Heart infusion (BHI), and agar were from Becton Dickinson (Franklin Lakes, NJ).
Antibacterial assay
All bacteria were incubated in BHA at 37 °C for 24 h. Inocula were prepared by mixing a few bacteria colonies with sterile ringer solution (0.85% NaCl) and comparing the turbidity with the standard 0.5 McFarland solution, which were equivalent to 108 CFU/mL.
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined by the broth microdilution assay (NCCLS, Citation2008). Ampicillin and DMSO were used as positive and negative controls, respectively. The MIC was defined as the lowest concentration of the compound to inhibit the growth of microorganisms and the MBC was defined as the lowest concentration of the compound required to kill the microorganisms.
Statistical analysis
All analyses were performed in triplicate and a completely randomized design (CRD) was used. Analysis of variance (ANOVA) was performed, and mean comparisons were done by Duncan's multiple range tests. For pair comparisons, a t-test was used. P values less than 0.05 were considered to be statistically significant.
Results and discussion
The antioxidant activities of a compound have been attributed to various mechanisms, including prevention of chain initiation, binding of transition metal ion catalyst, decomposition of peroxides, scavenging of free radicals, and prevention of hydrogen abstraction. In this study, the antioxidant activities of BRE, CSE, and the pure brazilin were determined using three different assay systems, including DPPH radical scavenging, reducing power, and β-carotene bleaching assays.
On the basis of DPPH radical scavenging method, increase of concentrations of all samples resulted in increase of DPPH radical scavenging activity (). BRE showed higher DPPH radical scavenging activity than CSE, but lower than brazilin and quercetin. The IC50 values of 6.2, 7.3, 5.6, and 3.5 µg/mL were calculated for BRE, CSE, brazilin, and quercetin, respectively. These results agree with the results from β-carotene bleaching assay. BRE showed almost equal radical scavenging activity to brazilin, but higher activity than that of CSE (). Although BRE contained only 39% w/w brazilin, it showed comparable radical scavenging activities to brazilin. This implies that BRE contains other radical scavenging compounds besides brazilin.
Figure 2. Percentage inhibitions of DPPH radicals by BRE, CSE, and brazilin at different concentrations, n = 3 (Nirmal & Benjakul, Citation2011).
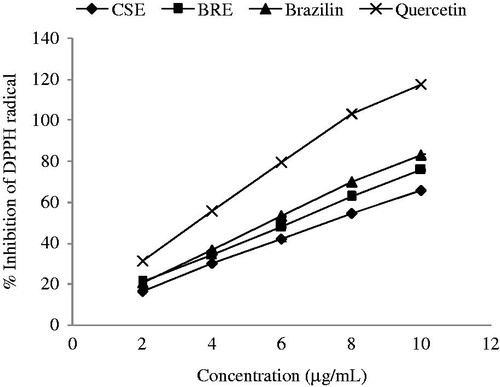
Table 1. Radical scavenging activity of BRE, CSE, and brazilin determined by β-carotene bleaching assay (Panichayupakaranant et al., Citation2010).
Determination of the total phenolic content of BRE and CSE found that BRE contained a lower total phenolic content (50.3 ± 1.0 g QE/100 g of extract powder) than that of CSE (97.8 ± 3.3 g QE/100 g of extract powder). This implied that about half of the phenolic compounds in the crude extract had been eliminated by treatment with the Diaion® HP-20 column without any significant reduction of the antioxidant and reducing activities. This also implied that brazilin contributed more radical scavenging activities than any other phenolic compounds in the BRE. In addition, CSE may contain a greater content of polar phenolic compounds than BRE that were removed, so that the radical scavenging activity of CSE was markedly lower than that of BRE, especially in the β-carotene–linoleic acid assay system. These results indicated that BRE and brazilin have more advantages than CSE for their application as the antioxidant in the products containing oil–water emulsion systems.
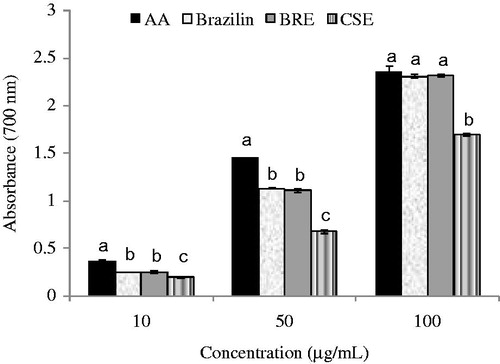
The reducing capacity of a compound may also serve as a significant indicator of its potential antioxidant activity (Duh et al., Citation2001). The reducing properties associated with the presence of reductones such as ascorbic acid, which is capable of breaking the free radical chain by donating a hydrogen atom. Reductones are also reported to react with certain precursor of peroxide, thus preventing peroxide formation (Jayaprakasha et al., 2001). Determination of reducing powers of BRE CSE and brazilin found that the reducing powers of all samples were in a dose-dependent manner (). In all tested concentrations, BRE possessed equal reducing power to brazilin, but higher than CSE. At low concentrations (10 and 50 µg/mL), both BRE and brazilin exhibited lower reducing power than the ascorbic acid. However, at high concentration of 100 µg/mL, they showed comparable activity to ascorbic acid. The reducing powers of BRE and CSE were also not related to the total phenolic content of the extracts. All antioxidant activities suggested that BRE possessed the comparable activities to the pure brazilin and higher than CSE. BRE can be, therefore, considered as an alternative natural antioxidant agent.
Figure 3. Reducing power of ascorbic acid, brazilin, BRE, and CSE at different concentrations. Small letters within the same concentration of different samples indicate significant difference (p < 0.05). AA, ascorbic acid; BRE, brazilin-rich extract; CSE, crude ethanol extract of C. sappan heartwood.
Protein denaturation is a process in which proteins lose their tertiary structure and secondary structure by the application of external stress during food processing and post-harvest treatments (Adarsh et al., Citation2011). Most biological proteins lose their biological function when denatured. The compounds inhibiting denaturation greater than 20% over the range of concentration were considered as having anti-inflammatory properties (Williams et al., Citation2008). In this study, BRE, CSE, and brazilin, at a concentration of 0.1 µg/mL, were capable of inhibiting denaturation of albumin by 54.1, 61.9, and 46.8%, respectively. Surprisingly, an increase of sample concentrations resulted in decrease anti-inflammatory property (). However, this phenomenon was coincident with the previous reports (Adarsh et al., Citation2011; Duganath et al., Citation2010; Williams et al., Citation2008), which demonstrated that the relationships between concentrations and anti-denaturation effects of most plant extracts were controversial. The results suggested BRE, CSE, and brazilin may have the anti-inflammatory property approximately by 50% inhibition, if they are used at low concentration as 0.1 µg/mL.
Table 2. Anti-denaturation activity of BRE, CSE, and brazilin (Williams et al., Citation2008).
Evaluation of antibacterial activities of BRE, CSE, and brazilin found that all of them possessed antibacterial activity against both Gram-positive and Gram-negative bacteria (). Gram-positive bacteria were more sensitive to all tested compounds than Gram negative bacteria. The antibacterial activities of BRE and CSE were related to the content of brazilin in the extracts. BRE and CSE contained only 39% and 20% w/w brazilin, respectively. Thus, brazilin showed the highest antibacterial activity with MICs/MBCs values of 31.3–250/62.5–250 µg/mL, followed by BRE (MICs/MBCs values of 62.5–500/125–500 µg/mL), and CSE (MICs/MBCs values of 125–1000/125–1000 µg/mL), respectively (). This indicated that brazilin plays an important role in antibacterial activity of CSE or BRE. The bactericidal effect of brazilin could be attributed to the ability to inhibit DNA and protein synthesis in cells (Xu & Lee, Citation2004).
Table 3. Antibacterial activity of BRE, CSE, and brazilin against Gram-positive and Gram-negative bacteria (NCCLS, Citation2008).
Conclusion
The results from this study support the potential use of BRE for antioxidant, anti-inflammatory, and antibacterial proposes in food and nutraceutical applications. Regarding an industrial application, BRE has more advantages than the use of pure brazilin in terms of a simple and low-cost production process.
Acknowledgements
The authors thank Dr. Brian Hodgson for assistance with the English language.
Declaration of interest
The authors have declared that there are no conflicts of interest. This work was supported by Prince of Songkla University, Thailand under postdoctoral fellowship program and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission.
References
- Adarsh VM, Ajay KP, Kavitha D, Anurag KB. (2011). Antidenaturation and antioxidant activities of Annona cherimola in vitro. Int J Pharm Biol Sci 2:1–6
- Bae IK, Min HY, Han AR, et al. (2005). Suppression of lipopolysacchride-induced expression of inducible nitric oxide synthase by brazilin in RAW 264.7 macrophage cells. Eur J Pharmacol 513:237–42
- Batubara I, Mitsunaga T, Ohashi H. (2010). Brazilin from Caesalpinia sappan wood as an antiacne agent. J Wood Sci 56:77–81
- Chen YP, Liu L, Zhou YH, et al. (2008). Chemical constituents from Sappan Lignum. J Chin Pharm Sci 17:82–6
- Duganath N, Kumar SR, Kumanan R, Jayaveera KN. (2010). Evaluation of anti-denaturation property and antioxidant activity of traditionally used medicinal plants. Int J Pharm Biol Sci 1:1–7
- Duh PD, Yen GC, Yen WJ, Chang LW. (2001). Antioxidant effects of water extracts from barley (Hordeum vulgare L.) prepared under different roasting temperatures. J Agric Food Chem 49:1455–63
- Hu CM, Liu YH, Cheah KP, et al. (2009). Heme oxygenase-1 mediates the inhibitory actions of brazilin in RAW264.7 macrophages stimulated with lipopolysaccharide. J Ethnopharmacol 121:79–85
- Jayaprakasha GK, Singh RP, Sakariah KK. (2001). Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem 73:285–90
- NCCLS. (2008). Performance standards for antimicrobial susceptibility testing; ninth informational supplement, NCCLS documents. M100-S9. Wayne: National committee for Clinical Laboratory Standard, 120–6
- Nirmal NP, Benjakul S. (2011). Use of tea extract for inhibition of polyphenoloxidase and retardation of quality loss of Pacific white shrimp during iced storage. LWT – Food Sci Technol 44:924–32
- Nirmal NP, Panichayupakaranant P. (2014). Anti-Propionibacterium acnes assay-guided purification of brazilin and preparation of brazilin rich extract from Caesalpinia sappan heartwood. Pharm Biol 52:1204–7
- Panichayupakaranant P, Issuriya A, Sirikatitham A, Wang W. (2010). Antioxidant assay-guided purification and LC determination of ellagic acid in pomegranate peel. J Chromatogr Sci 48:456–9
- Saraya S, Temsiririrkkul R, Manamuti C, et al. (2009). Sappan wood extract used as preservative in chili paste. Mahidol Univ J Pharm Sci 36:13–21
- Sasaki Y, Hosokawa T, Nagai M, Nagumo S. (2007). In vitro study for inhibition of NO production about constituents of Sappan Lignum. Biol Pharm Bull 30:193–6
- Wijeratne SSK, Amarowicz R, Shahidi F. (2006). Antioxidant activity of almonds and their by-product in food model systems. J Am Oil Chem Soc 83:223–30
- Williams LA, O'Connar A, Latore L, et al. (2008). The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med J 57:327–31
- Xu HX, Lee SF. (2004). The antibacterial principle of Caesalpinia sappan. Phytother Res 18:647–51
- Zhao H, Bai H, Wang Y, et al. (2008). A new homoisoflavan from Caesalpinia sappan. J Nat Med 62:325–7