Abstract
Context: Cardiomyocyte apoptosis plays a critical role in the progress of heart diseases. Fucoidan, a complex-sulfated polysaccharide, has been reported to possess potential cardioprotective efficacy in vivo.
Objective: The present study determines whether fucoidan could provide cardioprotection on hypoxia-induced cardiomyocyte apoptosis.
Materials and methods: H9c2 cardiomyoblast cells were incubated with various concentrations (15, 30, and 60 μg/ml) of fucoidan in a humidified incubator at 37 °C with 95% O2 and 5% CO2. After 6 h, hypoxia was processed and the cardioprotective effects of fucoidan were evaluated by applying MTT, ELISA, Hoechst 33258 nucleus staining, and western blot.
Results: Following a 6 h exposure of H9c2 to hypoxic condition, significant reduction was found in cell survival (0.57-fold) and superoxide dismutase (SOD) activity (0.56-fold), which were associated with the increase of malondialdehyde (MDA) level (2.58-fold), creatine phosphokinase (CK, 3.57-fold), and lactate dehydrogenase (LDH) activities (2.39-fold). Moreover, hypoxia-induced apoptosis was confirmed by Hoechst 33258 nuclear staining, and these changes were accompanied by the increase of Bcl-2 (1.27-fold) and Bax expression (2.6-fold). However, preincubation of the cells with fucoidan prior to hypoxia exposure elevated the cell viability (30 μg/ml, 1.18-fold; 60 μg/ml, 1.32-fold) and SOD activity (30 μg/ml, 1.12-fold; 60 μg/ml, 1.25-fold), but decreased the MDA level (30 μg/ml, 0.70-fold; 60 μg/ml, 0.80-fold), CK (30 μg/ml, 0.69-fold; 60 μg/ml, 0.76-fold), and LDH (30 μg/ml, 0.67-fold; 60 μg/ml, 0.86-fold) leakages. Hoechst 33258 nuclear staining observations demonstrated the same protective effect of fucoidan on hypoxia-induced myocardial injury. Also, cardioprotective effects of fucoidan were reflected by increasing Bcl-2 (60 μg/ml, 1.84-fold), as well as decreasing Bax (60 μg/ml, 0.6-fold).
Conclusion: Fucoidan had protective effect against hypoxia-induced cardiomyocytes apoptosis, and the mechanism might involve protections of the cell from oxidative injury.
Introduction
As we all know, cardiovascular disease is one of the world's major killer diseases, such as cardiac infarction and myocardial hypertrophy, accounting for approximately 20% of deaths every year in the world (Ferrero et al., Citation2014). Of note, a large number of animal experiments and clinical trials have shown that myocyte apoptosis is an important event after acute myocardial infarction and may be responsible for a significant portion of myocyte death during the acute ischemic stage, as well as progressive loss of surviving myocytes during the subacute and chronic stages (Bathgate-Siryk et al., Citation2014; Takemura & Fujiwara, Citation2004). Thus, therapeutic strategies focusing on inhibiting myocardial cell apoptosis may be beneficial to impede the occurrence and progression of these cardiovascular diseases. It has been proposed that cardiac myocyte cell loss is the result of apoptosis and is of great importance to various cardiovascular diseases (Gao et al., Citation2013), and hypoxia is regarded as a key initiator to arouse the loss of cardiac myocyte cells (Kakinuma et al., Citation2005). Therefore, suppression of hypoxia-induced apoptosis might be beneficial to block the occurrence and progression of many heart diseases.
The brown seaweed, Laminaria japonica Aresch (Laminariales), is one of the thousands of types of seaweed found around the world, and is consumed as a marine vegetable. Fucoidan is a complex-sulfated polysaccharide, derived from brown seaweed. Fucoidan has been reported to possess a wide spectrum of biological activities, such as antiviral, anticoagulant, antioxidant, antitumor, and anti-inflammatory activities (Gao et al., Citation2012). Moreover, Li et al. (2011) also have reported the protective anti-inflammatory effect of fucoidan on myocardial ischemia–reperfusion injury in rats.
It is well known, however, that several multi-target interference approaches have been used in traditional Chinese medicine (TCM) for a very long time (Li et al., Citation2007). Considering the complexity of ischemic heart disease and the biological activity of fucoidan, we hypothesized that antioxidation and anti-apoptosis of fucoidan cannot be the exclusive mechanism responsible for their cardioprotection. In the current study, we investigated the cardioprotective effects of fucoidan on hypoxia-induced apoptosis in H9c2 cardiomyoblast cells and the possible mechanisms involved.
Materials and methods
Chemicals
Fucoidan was provided by Ci Yuan Biotechnology Co., Ltd., Xi’an, China. The purity of test substance was more than 98.0%.
Cell culture
H9c2 cardiomyoblast cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). The cells were routinely cultured in Dulbecco's modified Eagle's medium (DMEM) as previously described with minor modifications (Fang et al., Citation2012). The medium was changed every 2 d and when confluence was reached, the cells were subcultured by detaching with 0.25% trypsin–EDTA solution and re-seeding into new plates at a ratio of 1:5. Then, cells were incubated in DMEM containing 2% fetal bovine serum (FBS). All tests were performed 6 h after incubation.
In vitro hypoxia model
First, we cultured the cardiomyocyte with various concentrations (15, 30, and 60 μg/ml) of fucoidan in a humidified incubator at 37 °C with 95% O2 and 5% CO2. After 6 h, hypoxia was processed. Briefly, the hypoxia solution contained 0.9 mM NaH2PO4, 6.0 mM NaHCO3, 1.0 mM CaCl2, 1.2 mM MgSO4, 40 mM Natrium lacticum (C3H5O3Na), 20 mM HEPES, 98.5 mM NaCl, and 10.0 mM KCl. Hypoxic condition was produced by placing the plates of cultured cells in an anaerobic chamber and O2 was adjusted to 1.0% and CO2 was adjusted to 5.0%.
Analysis of H9c2 viability
Next, H9c2 viability was detected as previously described methods (Santos et al., Citation2011). MTT (Amresco, Solon, OH) was added to the medium at a final concentration of 0.5 mg/ml for the last 12 h of the experiment. The resulting formazan crystals were dissolved in dimethyl sulfoxide and measured at an optical absorbance of 490 nm on a microtitre plate reader (Bio-Rad 550, BioRad, Hercules, CA). The cell viability was presented as a percentage of the control absorbance (untreated cells) after subtracting the background absorbance of the culture medium without cells.
Determination of LDH and CK activities
LDH and CK have been regarded as two reliable markers of primary cardiac myocytes death (Liu et al., Citation2013). Thus, we next detected these two parameters according to the previous study (Sadoshima, Citation2006). The levels of LDH and CK in supernatant were measured using a colorimetric assay to measure the conversion of pyruvic acid to lactic acid by LDH and the conversion of triphosphare and creatine to phosphagen by CK, respectively.
Measurement of MDA and SOD levels
MDA, the degradation product of the oxygen-derived free radicals and lipid oxidation, reflects the damage caused by reactive oxygen species (ROS) (Qin et al., Citation2009). SOD is a secreted enzyme that regulates levels of extracellular superoxide and protects the extracellular matrix from degradation by reactive species. MDA level and SOD activity were measured using commercial kits (Jian Cheng Bioengineering Institute, Jiangsu, China).
Hoechst 33258 nucleus staining
Chromosomal condensation and morphological changes in the nucleus of H9c2 cardiomyoblast cells were identified as in the previous study with some medications (Calvert & Vohra, Citation2013). In brief, H9c2 cardiomyoblast cells received similar treatment as described in the previous section. Then, cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 25 min at room temperature. After rinsing with PBS two times, cells were exposed to Hoechst 33258 (5 μg/ml) at room temperature for 20 min. Cells were examined under a fluorescence microscope. Samples were analyzed in triplicate. Viable cells displayed normal nuclear size and uniform fluorescence, whereas apoptotic cells showed condensed, fractured, or distorted nuclei.
Detecting of proteins by western blot
After rinsing twice with cold PBS, H9c2 cardiomyoblast cells were homogenized in lysis buffer containing proteinase inhibitors. Western blot was then carried out to detect protein expression using polyclonal antibodies Bcl-2, Bax, and β-actin followed by incubation with the corresponding secondary antibodies at room temperature.
Statistical analyses
All data are expressed as means ± SD. Differences among groups were determined by ANOVA and the differences between groups were determined by Student's t-test. A value of p < 0.05 was considered statistically significant.
Results
Analysis of H9c2 viability
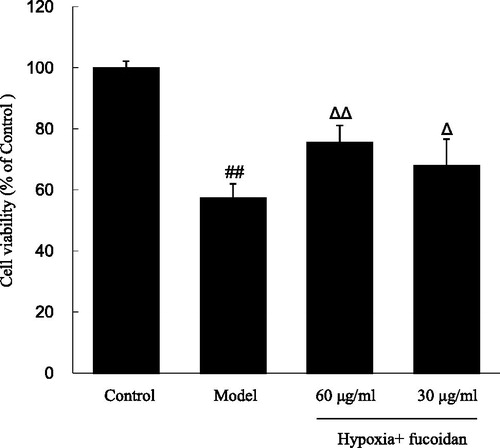
The cell survival was obviously decreased when exposed to hypoxia from 1 to 8 h in a time-dependent manner (data not shown). Notably, H9c2 viability was only 57.4 ± 4.5% when compared with the control after exposed to hypoxia for 6 h, and then this was chosen as a standard apoptosis induction in the subsequent experiments. showed H9c2 viability using the MTT assay, and the results indicated that fucoidan (30 and 60 µg/ml) prevented cells from hypoxia-induced damage in a dose–response relationship, restoring cell survival to 68.0 ± 8.6% and 75.8 ± 5.3%, respectively (p < 0.05 and p < 0.01).
Determination of SOD and MDA levels
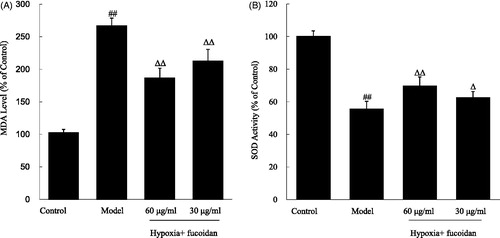
As shown in , exposure of H9c2 cardiomyoblast cells to hypoxia for 6 h caused significant elevation in the level MDA (2.58-fold increase relative to control value), while reduction in the activity of SOD (1.79-fold decrease relative to control value), when compared with the vehicle control group. Meanwhile, pretreatment of H9c2 cardiomyoblast cells with fucoidan at concentrations of 30 and 60 µg/ml significantly reduced the MDA level (, all p < 0.01), and markedly increased SOD activity (, p < 0.05 and p < 0.01, respectively), when compared with the cells treated with hypoxia only.
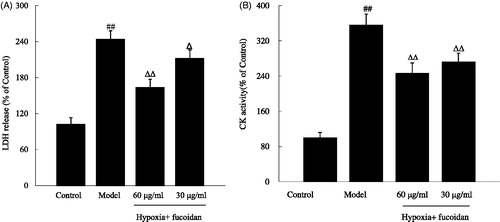
Determination of LDH and CK activities
To further investigate how fucoidan influenced hypoxia-induced H9c2 cardiomyoblast cells' damage, we measured the activities of LDH and CK in the medium. When primary cardiac myocytes exposed to hypoxia for 6 h, LDH and CK activities were obviously increased (243.4 ± 24.5% and 355.8 ± 25.0%, respectively, p < 0.01). On the contrary, these changes could be blocked by pretreating with fuciodan at doses of 30 and 60 µg/ml ().
Figure 3. Effect of fucoidan on lactate dehydrogenase (LDH) and creatine phosphokinase (CK) activities in the culture supernatant of cardiomyocytes subjected to hypoxia. All data were shown as mean ± SD of three experiments. N = 6. ##p < 0.01 versus control and ΔΔ, Δp < 0.01 and p < 0.05 versus hypoxia alone.
Hoechst 33258 nucleus staining
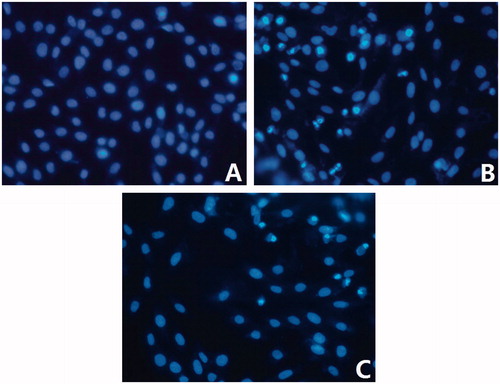
In order to clarify whether the observed cell death was due to apoptosis or necrosis, nuclear morphological change was observed by Hoechst 33258 staining. shows the nuclear staining of H9c2 cardiomyoblast cells pretreated with hypoxia or hypoxia plus 60 µg/ml fuciodan. The results indicated that H9c2 cardiomyoblast cells, exposed to hypoxia, featured typical characteristics of apoptosis, showing the shrinkage of nuclei, the condensation of chromatin, and the appearance of a few apoptotic bodies. Fuciodan pretreatment inhibited hypoxia-induced apoptosis as indicated by the normal nuclear size and uniform fluorescence.
Detecting of proteins by western blot
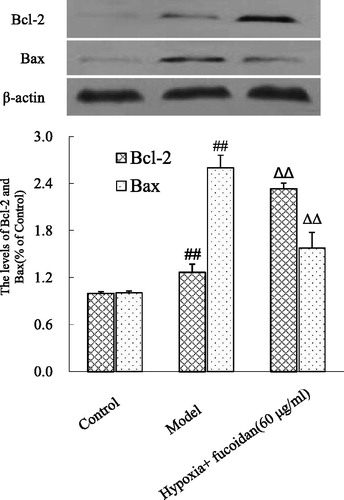
To study the molecular mechanisms that fuciodan protected H9c2 cardiomyoblast cells from apoptosis induced by hypoxia, we also measured the levels of Bcl-2 and Bax. After 6 h exposure to hypoxia, the expression of Bcl-2 and Bax levels was increased when compared with control cells (). However, fuciodan significantly decreased Bax expression and strikingly elevated Bcl-2 expression.
Discussion
CK and LDH, cardiac enzymes found primarily in the myocardium, are used to evaluate the existence and the extent of cardiac myocytes injury (Singh et al., Citation2008). An increased leakage of them from mitochondria as an indicator of hypoxia induced cell toxicity. Our previous results were similar to this report. The present study confirmed that hypoxia for 6 h significantly increased LDH and CK activities in the culture medium. However, pretreatment with different concentrations (30 and 60 μg/ml) of fucoidan greatly ameliorated these cardiac enzyme activities. These results showed that fucoidan suppressed high hypoxia-induced cardiomyoblast cells oxidative stress which is indicated by inhibiting LDH and CK activities.
Although the exact etiology is still unknown, extensive studies for the last several decades have shown that hypoxia-induced cardiac myocyte loss plays a major part in patients with many kinds of heart diseases (Maeda et al., Citation2013). In recent years, more and more attention has been focused on researching the chemical substance and nature compounds with advantages of anti-apoptotic activity and low toxicity for cardioprotective agents. In the present study, we focused on demonstrating the effect of fucoidan on hypoxia-induced cardiomyocytes apoptosis. The results showed that exposure of H9c2 cardiomyoblast cells to hypoxia was followed by decreased cell viability and SOD activity, but increased the MDA level. On the contrary, these changes were sharply blocked by treatment with fucoidan (30 and 60 µg/ml). In view of the obtained results, fucoidan prevented H9c2 from oxidative damage; further investigations were focused on the underlying mechanisms.
The balance between ROS production and removal of excess ROS is essential in maintaining the redox state and homeostasis balance in the cell (Wang et al., Citation2012). At the subcellular level, increased ROS level can cause damage to nucleic acids and proteins leading to apoptosis (Lee et al., Citation2013; Qin et al., Citation2014). Since H9c2 cardiomyocyte cells respond in a manner similar to myocytes in primary cultures or isolated heart preparations under oxidative stress conditions (Liao et al., Citation2013), it was chosen as an in vitro model to study how fucoidan exerts a protective effect against hypoxia-induced cell apoptosis. In the experiment, we made use of Hoechst 33258 nucleus staining to determine H9c2 cardiomyoblast cells' apoptosis. The results strongly suggested that fucoidan might exert a protective effect against the hypoxia-induced cytotoxicity and apoptosis in response to hypoxia. Moreover, these anti-apoptosis effects were further confirmed by the western blot analysis, as shown in .
Bcl-2 and Bax expressions are of great importance in deciding susceptibility to cell apoptosis (Rong et al., Citation2010). When Bax is overexpressed in cells, programmed cell death will occur (Hou & Hsu, Citation2005). While Bcl-2 is up-regulated, it will heterodimerize with Bax, and cells will survive. That is to say, overexpression of Bcl-2 might lead to the heterodimerization with Bax, and then there might be less free Bax protein to form homodimerization. In the current study, the effects of fuciodan on Bcl-2 and Bax were tested by western blot, and the results indicated that fuciodan remarkably inhibited the expression of Bax, indicating that it relieved the degree of apoptosis after exposed to hypoxia, and increased Bcl-2 expression so as to enhance the rate of cell survival, these results were supported by Hoechst 33258 nucleus staining.
Taking all the current in vitro findings together, ROS-induced change of apoptosis-related proteins was regulated by treatment with fuciodan, which strongly indicated that antioxidant power and anti-apoptosis play an important role in the cardioprotective effects of test substance. It is thus logical to postulate that fuciodan may have a protective effect on heart diseases related to ROS defense. However, there is still a long way to go from fundamental research of fuciodan to its clinical development. The results of the present study only pave the way for further understanding of the cardioprotective effect of fuciodan. Continued attempts to clarify the active molecules and identify the mechanism of test substance in detail are needed in the future.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bathgate-Siryk A, Dabul S, Pandya K, et al. (2014). Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension 63:404–12
- Calvert RJ, Vohra S. (2013). Doxorubicin-treated H9c2 cells: Caution with luminescent ATP and Hoechst 33258 assays. In Vitro Cell Dev Biol Anim 49:95–6
- Fang J, Song XW, Tian J, et al. (2012). Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis 17:410–23
- Ferrero JM, Trenor B, Romero L. (2014). Multiscale computational analysis of the bioelectric consequences of myocardial ischaemia and infarction. Europace 16:405–15
- Gao G, Xie A, Zhang J, et al. (2013). Unfolded protein response regulates cardiac sodium current in systolic human heart failure. Circ Arrhythm Electrophysiol 6:1018–24
- Gao Y, Li C, Yin J, et al. (2012). Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ Toxicol Pharmacol 33:304–11
- Hou Q, Hsu YT. (2005). Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis: Development of a GFP-Bax-stable H9c2 cell line for apoptosis analysis. Am J Physiol Heart Circ Physiol 289:H477–87
- Kakinuma Y, Ando M, Kuwabara M, et al. (2005). Acetylcholine from vagal stimulation protects cardiomyocytes against ischemia and hypoxia involving additive non-hypoxic induction of HIF-1alpha. FEBS Lett 579:2111–18
- Lee WL, Huang JY, Shyur LF. (2013). Phytoagents for cancer management: Regulation of nucleic acid oxidation, ROS, and related mechanisms. Oxid Med Cell Longev 2013:925804
- Liao LZ, Chen YL, Lu LH, et al. (2013). Polysaccharide from Fuzi likely protects against starvation-induced cytotoxicity in H9c2 cells by increasing autophagy through activation of the AMPK/mTOR pathway. Am J Chin Med 41:353–67
- Li C, Gao Y, Xing Y, et al. (2011). Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia-reperfusion injury in rats via regulating the inflammation response. Food Chem Toxicol 49:2090–5
- Li S, Zhang Z, Wu L, et al. (2007). Understanding ZHENG in traditional Chinese medicine in the context of neuro-endocrine immune network. IET Syst Biol 1:51–60
- Liu C, Guo W, Maerz S, et al. (2013). 3,5-Dimethoxy-4-(3-(2-carbonyl-ethyldisulfanyl)-propionyl)-benzoic acid 4-guanidino-butyl ester: A novel twin drug that prevents primary cardiac myocytes from hypoxia-induced apoptosis. Eur J Pharmacol 700:118–26
- Maeda H, Nagai H, Takemura G, et al. (2013). Intermittent-hypoxia induced autophagy attenuates contractile dysfunction and myocardial injury in rat heart. Biochim Biophys Acta 1832:1159–66
- Qin F, Liu YX, Zhao HW, et al. (2009). Chinese medicinal formula Guan-Xin-Er-Hao protects the heart against oxidative stress induced by acute ischemic myocardial injury in rats. Phytomedicine 16:215–21
- Qin J, Kang Y, Xu Z, et al. (2014). Dioscin prevents the mitochondrial apoptosis and attenuates oxidative stress in cardiac H9c2 cells. Drug Res (Stuttg) 64:47–52
- Rong SL, Wang YJ, Wang XL, et al. (2010). Recombinant proteins secreted from tissue-engineered bioartificial muscle improve cardiac dysfunction and suppress cardiomyocyte apoptosis in rats with heart failure. Chin Med J (Engl) 123:3626–33
- Sadoshima J. (2006). Redox regulation of growth and death in cardiac myocytes. Antioxid Redox Signal 8:1621–4
- Santos CX, Anilkumar N, Zhang M, et al. (2011). Redox signaling in cardiac myocytes. Free Radic Biol Med 50:777–93
- Singh G, Singh AT, Abraham A, et al. (2008). Protective effects of Terminalia arjuna against doxorubicin-induced cardiotoxicity. J Ethnopharmacol 117:123–9
- Takemura G, Fujiwara H. (2004). Role of apoptosis in remodeling after myocardial infarction. Pharmacol Ther 104:1–16
- Wang X, Jian C, Zhang X, et al. (2012). Superoxide flashes: Elemental events of mitochondrial ROS signaling in the heart. J Mol Cell Cardiol 52:940–8