Abstract
Context: Asclepias subulata Decne. (Apocynaceae) is a shrub used in the Mexican traditional medicine for the treatment of cancer.
Objective: The objective of this study was to evaluate the antiproliferative activity of methanol extract of aerial parts of A. subulata and its fractions against different cancer cell lines. Additionally, we analyzed the mechanism of action of the active fractions.
Materials and methods: Methanol extract fractions were prepared by serial extraction with n-hexane, ethyl acetate, and ethanol. The antiproliferative activity of methanol extract and its fractions was evaluated, against several murine (M12.C3.F6, RAW 264.7, and L929) and human (HeLa, A549, PC-3, LS 180, and ARPE-19) cell lines by the MTT assay, using concentrations of 0.4–400 µg/mL for 48 h. Ethanol and residual fractions were separated using silica gel column. Apoptosis induction of cancer cells was evaluated by Annexin and JC-1 staining using flow cytometry.
Results: Methanol extract and its fractions showed antiproliferative activity against all human cancer cell lines tested. Methanol extract had the highest antiproliferative activity on A549 and HeLa cells (IC50 values < 0.4 and 8.7 µg/mL, respectively). Ethanol and residual fractions exerted significant antiproliferative effect on A549 (IC50 < 0.4 µg/mL) and PC3 cells (IC50 1.4 and 5.1 µg/mL). Apoptotic assays showed that CEF7, CEF9, CRF6, and CRF5 fractions induced mitochondrial depolarization in A549 cells, 70, 73, 77, and 80%, respectively. Those fractions triggered the apoptosis mitochondrial pathway.
Conclusion: Our data show that A. subulata extracts have potent antiproliferative properties on human cancer cell lines. This plant should be considered an important source of potent anticancer compounds.
Introduction
Asclepias L. (Apocynaceae), a genus of herbaceous perennial, dicotyledonous plants, is widely spread with 164 species in nine subgenera in North America (Fernández et al., Citation2008). Several species of the genus Asclepias have been part of traditional medicine since ancient times for the treatment of snakebites, skin problems, warts, headaches, angina, colds, pain, and cancer (Fernández et al., Citation2008; Mena et al., Citation2009; Navarro et al., Citation2003).
Phytochemical studies have revealed the presence of pregnane glycosides, secopregnane glycosides, triterpenoids, steroidal glycosides, and, mainly, cardenolide glycosides in Asclepias verticillata L., Asclepias tuberosa L., Asclepias curassavica L., Asclepias incarnata L., and Asclepias linaria Cav. (Araya et al., Citation2012b; Li et al., Citation2008, Citation2009; Rodriguez & Fonseca, Citation1991; Warashina & Noro, Citation1999, Citation2009a,Citationb).
A few studies have shown the pharmacological properties of constituents of Asclepias species. Shivaprasad et al. (Citation2009) have demonstrated that latex of A. curassavica possesses pro-coagulant activity and several studies have shown the cytotoxic activity of the cardiac glycosides isolated of A. curassavica against cancer cell lines (Li et al., Citation2009; Roy et al., Citation2005).
In Mexico, there are 68 species of Asclepias and 14 of them are used as medicinal plants. Asclepias subulata Decne. is a shrub occurring in west of Arizona, in the southwest United States of America, and northern Baja California and Sonora (northwest Mexico). Different parts of this plant are used by several ethnic groups (Seris and Pimas) of Sonora, Mexico, to treat several health problems, such as gastrointestinal disorders, cancer, eye disease, among others.
Asclepias subulata was investigated as a source of rubber during World War II and it was also studied as a producer of biocrude (Beckett, Citation1935). However, few phytochemical and pharmacological studies about A. subulata have been published.
A previous chemical study about A. subulata, harvested in the University of Arizona’s overpass farm, showed the presence of some cardenolide glycosides, mainly derivatives of coroglaucigenin and calactin, and a lignan (Jolad et al., Citation1986). In vitro evaluations of the antimicrobial and cytotoxic activity of A. subulata showed that ethanol extract of the plant inhibited the growth of Mycobacterium tuberculosis by 45%, at a concentration of 100 µg/mL, and it also has antiproliferative activity against a colon adenocarcinoma cell line (HCT-116 cells) (Murillo et al., Citation2001).
Considering the popular consumption of A. subulata by Sonoran ethnic groups (Seris and Pimas) as a cancer therapy, and the previous phytochemical reports, in the present study, we evaluated the antiproliferative activity of the methanol extract of A. subulata and its solvent fractions against different cancer cell lines. Additionally, we also analyzed the molecular mechanism involved in the anticancer properties of this plant.
Materials and methods
Plant material
The stems and flowers of A. subulata were collected around the city of Hermosillo, Sonora, Mexico (29°8′43.25″N, 110°57′10.15″O) in September 2011. Botanical specimens were identified by Professor Jesus Sánchez Escalante (Herbarium of the University of Sonora) (voucher specimen no. 17403). All plant materials were air dried in the shade at room temperature. The dried samples were powdered and stored at 4 °C.
Chemicals
All chemicals used in the present study, such as methanol (PubChem CID: 887), ethanol (PubChem CID: 702), ethyl acetate (PubChem CID: 8857), and n-hexane (PubChem CID: 8058), were purchased from Fermont Chemicals (Monterrey, NL, Mexico). Annexin V-FITC, propidium iodide, JC-1 (5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetra-ethylbenzimidazol-carbocyanine iodide) (PubChem CID: 5492929), Dulbecco’s Modified Eagle’s Medium (DMEM) high glucose, dimethyl sulfoxide (DMSO) (PubChem CID: 679), trypsin–EDTA solution 0.25%, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] (PubChem CID: 64965), were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was obtained from Gibco Life Technologies (Grand Island, NY).
Preparation of methanol extract and solvent fractions
The methanol extract was obtained by maceration of dried powder of A. subulata (1.8 kg) in methanol (18 L) for 10 d (Jiménez-Estrada et al., Citation2013). The solvent was removed by evaporation under reduced pressure at 40 °C on a rotary evaporator, affording 232 g (yield 12.9%) of the methanol extract. The crude extract was fractionated with each of the following solvents: n-hexane, ethyl acetate, and ethanol. A sample of the methanol extract and solvent fractions was diluted in dimethyl sulfoxide (DMSO) prior to testing (stock solution of 80 mg/mL). The final dilutions were made in DMEM containing fetal bovine serum (5% FBS).
Phytochemical screening
In order to identify the major classes of phytochemical present in the methanol extract and solvents fractions of A. subulata, a qualitative phytochemical screening was conducted. We used the next specific test: phenolic compounds (Shinoda test), tannins (ferric chloride test) steroids–triterpenoids (Libermann–Burchard test), saponins (Rosenthaler test), quinones (Borntraguer test), cumarines (KOH reaction), alkaloids (Wagner test), and glycosides (Keller–Killiani test) (Brain &Turner, Citation1975; Harborne, Citation1984; Samejo et al., Citation2013).
Cell lines
Cell lines L929 (murine subcutaneous connective tissue), HeLa (human cervical carcinoma), A549 (human alveolar adenocarcinoma), PC-3 (human prostatic adenocarcinoma), LS 180 (human colorectal adenocarcinoma), and ARPE-19 (human retinal pigmented epithelium) were purchased from the American Type Culture Collection (ATCC, Rockville, MD). The cell lines M12.C3.F6 (murine B cell lymphoma) and RAW 264.7 (murine macrophages transformed by virus Abelson leukemia) were provided by Dr. Emil A. Unanue (Department of Pathology and Immunology, Washington University in St. Louis, MO). Cells were cultured in DMEM supplemented with 5% FBS (Sigma, St. Louis, MO). Experiments were conducted when the cell cultures were approximately 70% confluent.
Cell viability assays
Cell viability was evaluated by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium] reduction assay with some modifications (Mosmann, Citation1983; Valencia et al., Citation2012). Briefly, cells (1 × 104 per well, 50 μL) were placed in each well of a 96-well plate. After 24 h incubation at 37 °C in an atmosphere of 5% CO2 to allow cell attachment, aliquots (50 μL) of medium containing different concentrations of extracts were added and the cell cultures were incubated for 48 h. Preliminary experiments established that the use of DMSO concentrations ranging from 0.06 to 2.0% in the cell cultures caused no cell damage. Previously, extracts were dissolved in DMSO and subsequently diluted in the culture medium. In the last 4 h of incubation time, the cells were washed with PBS. Fresh culture medium and 10 μL of a MTT solution (5 mg/mL) were added to each well. The cell viability was assessed by the ability of metabolically active cells to reduce tetrazolium salt to colored formazan. The formed formazan crystals were dissolved in acidic isopropyl alcohol. The absorbance of the samples was measured with an ELISA plate reader (Multiskan EX, Thermo Scientific, Waltham, MA), using a test wavelength of 570 nm and a reference wavelength of 650 nm. Proliferative cells were expressed in terms of percentage, where the optical density measured from vehicle-treated cells was considered 100% of proliferation. Antiproliferative activity of extracts and fractions was reported as IC50 values (the IC50 value was defined as the concentration of extract required to inhibit cell proliferation by 50%) using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). Values of proliferation of at least three experiments, with six doses, in triplicate, were log transformed and normalized; and non-linear regression analysis was used to generate a dose–response curve to calculate IC50 values. The differences in means were analyzed using one-way analysis of variance (one-way ANOVA) followed by Tukey’s test (Sigma Stat 3; Systat Software Inc., San Jose, CA).
Purification of the ethanol and residual fractions
In order to identify active fractions, 3 g of ethanol and residual fractions were subjected to gravity silica-gel (200–400 mesh) column chromatography using a glass column of 70 cm and 2.5 cm internal diameter and eluting with stepwise gradient mixtures of n-hexane–ethyl acetate (20, 40, 60, 80, and 100% of ethyl acetate) and ethyl acetate–methanol (20, 40, 60, 80, and 100% of methanol). The solvent gradient was at a flow rate of 2.5 mL/min. Fractions were collected each 250 mL. The purification was monitored through TLC in silica gel plates GF 254 (Merck, Darmstadt, Germany), which were revealed with a solution of ceric sulfate.
Apoptosis detection
Annexin V-propidium iodide staining
To evaluate the apoptotic activity of chromatographic fractions that possess antiproliferative activity, annexin V and propidium iodide staining was carried out as previously described by Chan et al. (Citation2010) with minor modifications. Briefly, A549 cells (4.5 × 105 cells) were treated with 12.5 μg/mL of the active chromatographic fractions for 24 h. Caffeic acid phenethyl ester (CAPE) (120 μM) was used as the apoptosis positive control. After treatment period, cells were washed three times with cold PBS (centrifugation 200 × g, 7 min, 4 °C), and cell pellet was resuspended with a solution of Annexin V-FITC (1 μg/mL) in binding buffer and incubated for 10 min at room temperature in the dark, then a solution of propidium iodide (0.5 μg/mL) was added, allowed for a 10 min of incubation at room temperature in the dark. Finally, the cells were washed twice and resuspended in PBS. Cells were analyzed by flow cytometry as soon as possible (a maximum time of 1 h) using the FACS Canto II cytometer (BD Systems, San Jose, CA).
Mitochondrial membrane potential measurement
The breakdown of mitochondrial membrane potential was measured by flow cytometry using the cationic lipophilic fluorocrome JC-1 (5,5′,6,6′-tetra-chloro-1,1′,3,3′-tetra-ethylbenzimidazol-carbocyanine iodide). A549 cells (4.5 × 104/mL) were treated for 24 h with chromatographic fractions at a final concentration of 12.5 μg/mL. After trypsination and PBS washing (twice), cells were incubated for 15 min in freshly prepared JC-1 solution (5 μg/mL in culture medium) at 37 °C in the dark. Then, cells were washed twice in PBS, resuspended in culture medium, and immediately analyzed by flow cytometry using a FACS Canto II cytometer (BD Systems, San Jose, CA). CAPE (120 μM) was used as a positive control of mitochondrial membrane potential breakdown (Chen et al., Citation2011).
Results
Phytochemical screening
The qualitative phytochemical analyses of solvent fractions of methanol extract of A. subulata revealed the presence of the major phytochemical groups. Saponins and steroids/triterpenes were detected in the n-hexane and ethyl acetate fractions. Additionally, saponins, alkaloids, quinones, phenolic compounds, and glycosides were detected in the ethanol and residual fractions. Tannins were detected in the residual fraction.
Antiproliferative activity
In order to evaluate the anticancer activity of A. subulata, the crude methanol extract and its solvent fractions were assayed against six different cancer cell lines and two non-cancer cell lines.
Antiproliferative activity of methanol extract and solvent fractions of A. subulata on murine cell lines
In the MTT reduction assay, methanol extract was tested over a concentration range of 12.5–400 μg/mL on murine cancer cell lines M12.C3.F6 and RAW 264.7, and on L929 non-cancer cells. The methanol extract was shown to be more active on M12.C3.F6 cells (IC50 value = 80.9 ± 1.2 μg/mL) than on RAW 264.7 cells (IC50 value = 188.4 ± 1.1 μg/mL). L929 cells were the least affected from this extract (IC50 value = 245.4 ± 1.1 μg/mL) ().
Table 1. Antiproliferative activity on murine cells of fractions obtained from Asclepias subulata methanol extract.
The methanol extract was fractioned with solvents of increasing polarity, n-hexane, ethyl acetate, and ethanol, resulting in the respective extract and the residual fraction. These solvent fractions were tested for antiproliferative activity under same conditions previously described. It can be seen that non-polar fractions showed higher antiproliferative activity on the cancer cell lines M12.C3.F6 and RAW 264.7 than polar fractions (n-hexane (IC50 values 86.3 ± 1.0 and 70.4 ± 1.1 μg/mL, respectively) and ethyl acetate (IC50 values 72.3 ± 1.1 and 100.3 ± 1.1 μg/mL, respectively)); while the polar fractions, ethanol (IC50 values 85.1 ± 1.1 and 180.3 ± 1.1 μg/mL, respectively) and residual (IC50 values 212.5 ± 1.2 and >400 μg/mL, respectively), were the least active fractions. The L929 cells were least affected from the treatments with the solvent fractions ().
Antiproliferative activity of methanol extract and solvent fractions of A. subulata on human cancer cell lines
To evaluate the antiproliferative activity of methanol extract and solvent fractions of A. subulata on human cancer cells, methanol extract of A. subulata and solvent fractions were assayed on HeLa, A549, PC-3, and LS 180 human cancer cell lines, and on ARPE-19 human non-cancer cells. A range of extract concentration of 0–400 μg/mL was tested.
Treatment with methanol extract of A. subulata inhibited the cell proliferation by 50% at low concentrations on all cancer cells tested, <0.4 μg/mL for A549, 8.7 ± 1.3 μg/mL for HeLa, 15.6 ± 1.3 μg/mL for LS 180, and 16.3 ± 1.2 μg/mL for PC-3. ARPE-19 cells were the least affected from the methanol extract of A. subulata with IC50 values of 141.0 ± 1.2 μg/mL ().
Table 2. Antiproliferative activity on human cells of fractions obtained from the Asclepias subulata methanol extract.
The four solvent fractions tested exhibited potent inhibitory activity on the growth of all human cancer cell lines tested with IC50 values significantly lower than the normal cell line ARPE-19. The polar fractions, ethanol and residual, showed the highest antiproliferative activity, and they also showed selectivity against cancer cells. These fractions presented the lowest growth inhibitory activity on non-cancer cells. The ethanol fraction showed the highest antiproliferative activity on HeLa, A549, PC-3, and LS180 cells with IC50 values of 4.8 ± 1.4, <0.4, 1.4 ± 1.2, and 18 ± 1.2 μg/mL, respectively; for ARPE-19 cells, the IC50 value was higher, 198.6 ± 1.3 μg/mL. The residual fraction showed IC50 values of 7.7 ± 1.1, <0.4, 5.1 ± 1.1, 34.2 ± 1.3, and >400 μg/mL for HeLa, A549, PC-3, LS 180, and ARPE-19, respectively.
Hexane and ethyl acetate fractions showed lower antiproliferative activity than ethanol and residual fractions on human cell lines. The n-hexane fraction showed IC50 values of 14.2 ± 1.1, 12 ± 1.2, 48 ± 1.2, 174.2 ± 1.3, and 99.6 ± 1.1 μg/mL, while ethyl acetate fraction exhibited values of 9.9 ± 1.1, <0.4, 16.1 ± 1.2, 28.2 ± 1.3, and 92.9 ± 1.1 μg/mL, on HeLa, A549, PC-3, LS 180, and ARPE-19, respectively.
Chromatographic fractions of ethanol and residual fractions with antiproliferative activity
Ethanol and residual fractions showed the highest antiproliferative activity and selectivity against human cancer cells, therefore, they were submitted to further purification processes. Ethanol and residual fractions were chromatographed on a silica gel column and the fractions were eluted by a gradient of increasing the polarity. The ethanol fraction yielded 14 subfractions and the residual fraction yielded 10 subfractions. All chromatographic fractions were tested for antiproliferative activity on A549 cells, which were more sensitive to the ethanol and residual fraction treatments.
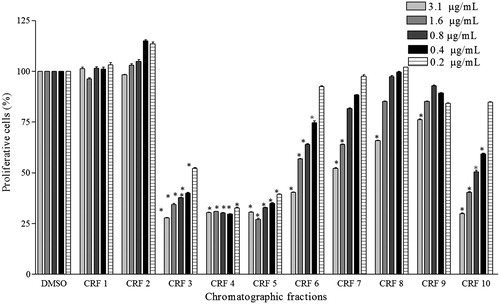
Chromatographic fractions of ethanol fraction CEF6, CEF7, CEF8, CEF9, CEF10, CEF11, and CEF14 presented the highest growth-inhibitory activity on A549 cells (). At an extract concentration of 0.2 μg/mL, CEF7 and CEF8 had proliferation of 29.3 ± 0.8% and 32.99 ± 0.4%, respectively.
Figure 1. Effect the treatment with chromatographic fractions of ethanol fraction on cellular growth of A549 cells. Values are the mean ± SD (n > 3). *Statistical significance is based on the comparison with the control cells (DMSO), p < 0.05.
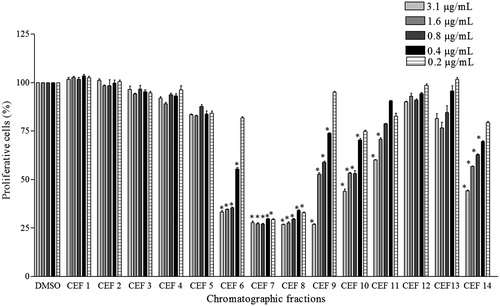
The subfractions CRF3, CRF4, CRF5, CRF6, and CRF10, from the residual fraction, showed a potent antiproliferative activity (proliferation values: 52.3 ± 0.8%, 32.8 ± 0.7%, and 39.5 ± 0.4%, respectively, at a concentration of 0.2 μg/mL) ().
Apoptosis detection
Once antiproliferative activity of the mentioned chromatographic fractions was determined, we evaluated whether cell death mediated by apoptosis is playing a role in the antiproliferative action of the chromatographic fractions.
Asclepias subulata chromatographic fractions induce the phosphatidylserine externalization on cell surface of cancer cells
The externalization of phosphatidylserine to the outer leaflet of the plasma membrane is associated with apoptosis. Phosphatidylserine, which is usually located on the inner leaflet of cytoplasmatic membrane, is translocated to the outer leaflet in the early phase of apoptosis, in vivo, exposure of phosphatidylserine is essential for the recognition of apoptotic cell by macrophages (Huigsloot et al., Citation2001). Thus, to evaluate the apoptosis induction on cancer cells due to treatment with chromatographic fractions, the externalization of phosphatidylserine was determined through staining A549 cells with Annexin V-FITC and propidium iodide (PI), followed by flow cytometric analysis.
Chromatographic fractions of ethanol fraction, CEF6, CEF7, CEF8, CEF9, CEF10, CEF11, and CEF14, increased the percentage of early apoptotic cells in A549 cells from 3.5% in control-untreated cells to 10, 19.32, 17.77, 5.85, 20.17, 41.80, and 45.82%, respectively (). These results showed that the cells treated with those ethanol fractions died by apoptosis, since it was possible to detect early events of this process (). The ratio of late apoptosis cells was higher in the treated cells with respect to control-untreated cells, which can be seen in as a shift of cell population toward quadrant Annexin V positive–PI positive (top right).
Figure 3. Percentages of cells in apoptosis induced for the treatment with active chromatographic fractions of ethanol and residual fractions. The values represent the mean ± SD of at least three independent experiments. * and ♦ statistical significance based on the comparison with the control cells (DMSO) for each condition, p < 0.05 (Tukey test).
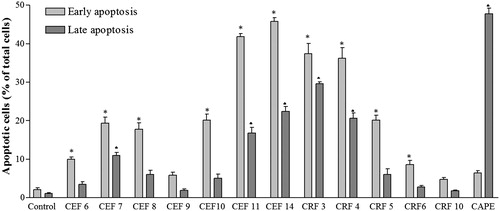
Figure 4. Effect of active chromatographic fractions on externalization of phosphatidylserine in A549 cells. Flow cytometric fluorescence patterns of Annexin V/PI double staining. The data are representative of least three independent experiments.
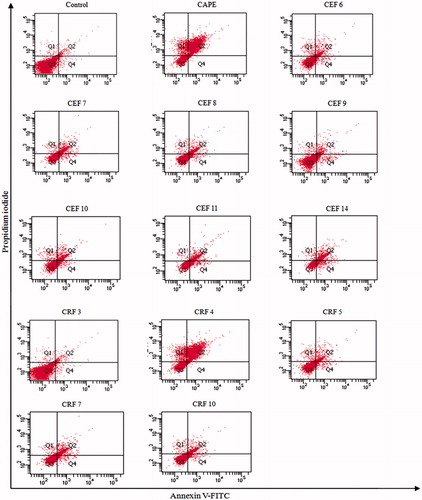
Table 3. Induced apoptosis in A549 cells for the treatment with active chromatographic fractions of ethanol and residual fractions.
Chromatographic fractions of residual fraction also induced an increase in the percentage of early apoptotic cells from 3.5% on untreated cells to 37.4, 36.3, 20.2, 8.6, and 4.8% in cells treated with CRF3, CRF4, CRF5, CRF6, and CRF10, respectively. Thus, CRF3, CRF4, and CRF5 showed significant percentages of cells in early apoptosis, while CRF6 and CRF10 did not show significant differences with respect to control-untreated cells at 24 h of treatment. CAPE, an apoptosis inducer, showed higher percentage of late apoptosis cells ().
Depolarization of mitochondrial membrane potential
Mitochondria plays a major role during apoptosis induction, leading to organized degradation of cellular structure, which results in the formation of apoptotic bodies, characteristic of this process (Bustamante et al., Citation2004). To further confirm that chromatographic ethanol and residual fractions induce cell death by apoptosis, changes in mitochondrial membrane potential were monitored in A549 cells in 24 h after treatment. In living cells, JC-1 exists either as a green fluorescent monomer at depolarized membrane potentials (positive to −100 mV) (detected in FL-1 channel) or as an orange-red fluorescent J-aggregate at hyperpolarized membrane potentials (negative to −140 mV) (detected in FL-2 channel). JC-1 undergoes a reversible shift in emission, from 527 nm to 590 nm as more J-aggregates form with increasingly negative mitochondrial potentials (Dispersyn et al., Citation1999). Thus, in apoptotic cells, when the mitochondrial potential collapses, the JC-1, which cannot accumulate within the mitochondria, remains in the cytoplasm as a green fluorescent monomeric form. Therefore, mitochondrial depolarization is indicated by a decrease in red/green fluorescence ratio.
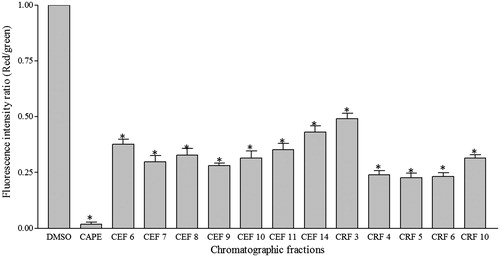
shows the red/green fluorescence intensity ratio for A549 cells treated with active chromatographic fractions. Mitochondrial depolarization is indicated by a decrease in the red/green fluorescence intensity ratio. All treatments with the active chromatographic fractions produced a significant mitochondrial depolarization as compared with control. As can be seen in , the change of FL2/FL1 ratio was more pronounced in the treatment with CEF7, CEF 9, CRF4, CRF5, CRF6, and CRF10 with approximate 70, 73, 76, 80, 77, and 70%, respectively, less than the control. These results indicate that the chromatographic fractions of ethanol and residual fractions induced depolarization of mitochondrial membrane potential.
Figure 5. Loss of mitochondrial transmembrane potential in A549 cells. The cells were treated with 12.5 μg/mL of chromatographic fractions of ethanol and residual fractions for 24 h. The results are expressed as the fluorescence intensity ratio (red/green) for JC-1 ± SD of triplicate and are representative of three separate experiments. ANOVA *significant differences with respect to control DMSO, p < 0.01 (Tukey test). CAPE was used as positive control of apoptosis.
Discussion
In Sonora, Mexico some indigenous groups use the species A. subulata in common practice for the empirical cancer treatment (López & Hinojosa, Citation1988). Based on this popular knowledge and a few scientific studies demonstrating its anticancer property, we decided to evaluate the antiproliferative activity of the methanol extract of A. subulata and its solvent fractions in different cancer cell lines.
In murine cancer cells, n-hexane and ethyl acetate fractions showed higher antiproliferative activity than ethanol and residual (polar) fractions. IC50 values ranged between 70.4 and 188.4 μg/mL for n-hexane and ethyl acetate fractions; while for ethanol and residual fractions, the IC50 values were higher (). Contrary, in human cancer cells, ethanol and residual fractions showed more effect than n-hexane and ethyl acetate fractions. The IC50 values of ethanol and residual fractions in human cells were lower than that of murine cells, <0.4 μg/mL. Ethanol and residual fractions also showed selectivity against human cancer cells; non-cancer cells, ARPE-19, were less affected for the treatments ().
According to the National Cancer Institute (NCI), plant extracts with IC50 values ≤30 μg/mL are considered active (Suffness & Pezzuto, Citation1990). Due to that the crude methanol extract displays IC50 values of 8.7, <0.4, 16.3, and 15.6 μg/mL for HeLa, A549, PC-3, and LS 180 cell lines, respectively, this can be considered as the active extract (). The ethanol and residual fractions showed IC50 values even lower than that of methanol extract, in human cell lines; therefore, these fractions could be a rich source of bioactive metabolites with antiproliferative activity. Other species of Asclepias have shown antiproliferative activity in vitro such as A. subulata. Bio-guided phytochemical studies have revealed that cardiac glycosides and triterpenoids present in A. curassavica possess antiproliferative effect in cancer cell lines (Roy et al., Citation2005). Moreover, A. verticillata contain cardenolide glycosides and polioxygenated pregnane glycosides, which are responsible of anticancer effect of the plant (Araya et al., Citation2012a,Citationb). Thus, according to the chemotaxonomy of the plants, it is possible that A. subulata contain cardiac glycosides and terpenoids with antiproliferative activity.
The current work also demonstrates differences in sensitivity of the murine and human cells to methanol extract of A. subulata and its solvent fractions. Human cancer cells showed more sensitivity for all treatments with respect to murine cells. These differences can be due to that human and murine cells possess changes in expression or activity of transporters involved in the metabolism of compounds present in the extract (Jemnitz et al., Citation2008). The genetic pattern of human and mouse presents differences, which generate a different response to the same metabolite.
Most chemotherapies used as cancer treatment cause many side effects such as vomiting, diarrhea, alopecy, myelosuppression, and, neurological, cardiac, pulmonary, and renal toxicities, which reduce the patient’s quality of life. These side effects are due to that most conventional anticancer agents have sufficient membrane permeability to penetrate both normal and cancer cells to a similar extent. Thus, a fundamental goal in cancer research is the development of drugs that selectively target cancer cells, but have limited effects against non-cancer cells. Methanol extract of A. subulata and solvent fractions showed selectivity to cancer cells, this characteristic was more pronounced in ethanol and residual fractions. This pattern is desirable because non-cancer cells are less affected. Bio-guided phytochemical studies of A. subulata could find compounds with high selectivity to cancer cells, which could be used in cancer therapy. Nowadays, two plant-derived compounds, flavopiridol and mesoindigo, have been shown to exhibit anticancer effects with less toxicity than conventional drugs (Alonso et al., Citation2011).
The phytochemical screening indicated the presence of multiple groups of bioactive metabolites in the solvent fractions generated from methanol extract of A. subulata. Lower antiproliferative activity shown for n-hexane and ethyl acetate fractions, on human cancer cell lines, can be due to the content of saponines and steroids, which do not usually present remarkable antiproliferative properties. While the high antiproliferative potential and selectivity of ethanol and residual fractions can be associated to the presence of alkaloids and glycosides, mainly.
Apocynaceae family-derived alkaloids are known to exhibit antiproliferative activity, such as vinblastine and vincristine, vinca alkaloids isolated from Catharanthus roseus (L.) G. Don. Other alkaloids in clinical use include camptothecin derivatives, topotecan, irinotecan, and etoposide, and paclitaxel (Cragg & Newman, Citation2003; Facchini, Citation2001). Also, mitraphylline, isolated from Uncaria tomentosa DC. (Rubiaceae) and berberine from Coptis chinensis Franch. (Ranunculaceae), have shown strong antiproliferative potential in cancer cell lines (García et al., Citation2007; Tang et al., Citation2009).
Cardiac glycosides are compounds used for the treatment of cardiac failure, which also display strong anticancer activity inducing impairment of cell proliferation or activation of cell death (Cerella et al., Citation2012). Several plants are recognized to contain large amounts of cardiac glycosides including members of the Ranunculaceae, Scrophulariacea, and Apocynaceae families (Heneidak et al., Citation2006; Mijatovic et al., Citation2007; Rashan et al., Citation2011). The genus Asclepias is a great source of cardiac glycosides with antiproliferative activity. Studies have revealed that Asclepias syriaca L., A. curassavica, and A. verticillata contain cardiac glycosides and related compounds, which have strong antiproliferative potential in cancer cell lines (Araya et al., Citation2012a,Citationb; Roy et al., Citation2005).
Based on the chemotaxonomic information, the glycosides contained in A. subulata can be responsible for antiproliferative activity found in this study. This hypothesis would also explain the selectivity present for methanol extract of A. subulata and solvent fractions to cancer cells. It has been postulated that cardiac glycosides affect cell metabolism by altering the glycolytic flux (Lopez, Citation2007) and the cancer cells derive most of their energy from glycolysis even under aerobic conditions, thus, cardiac glycosides affect, mainly, to aerobic glycolysis-addicted cancer cells and have lower effect in healthy cells with normal metabolism (Mijatovic et al., Citation2007).
However, the participation of others compounds detected in the polar solvent fractions cannot be excluded as possible responsible metabolites of antiproliferative activity. Phenolic compounds, particularly, flavonols, flavones, and proanthocyanidins contained in Crataegus monogyna Jacq. (Rosaceae) showed high antiproliferative activity in MCF-7, NCI-H460, HeLa, and HepG2 cell lines (Rodrigues et al., Citation2012). It has also been reported that anthocyanins present in jaboticaba peel (Myrciaria jaboticaba (Vell.) Berg.) have antiproliferative effects against K562 leukemia cell line (Leite-Legatti et al., Citation2012). Flavonols are present in A. syriaca; however, the antiproliferative potential of these flavonols has not yet been investigated (Goonnet et al., Citation1973).
In a natural products’ drug discovery, a bioassay will be used to guide fractionation of crude extracts towards isolation of pure bioactive compounds. Following this scheme, the most active fractions, ethanol and residual fractions, were separated by chromatographic methods and antiproliferative activity of obtained chromatographic fractions was evaluated through MTT assay in A549 cells. The more active chromatographic fractions were CEF6, CEF7, CEF8, CEF9, CEF10, CEF11, CEF14, CRF3, CRF4, CRF5, CRF6, and CRF10, the antiproliferative activity of those fractions was increased with respect to ethanol and residual fractions, since chromatographic fractions obtained the IC50 value even lower of 0.19 μg/mL ( and ). Active chromatographic fractions of A. subulata have been selected for future fractionation and isolation of compounds with antiproliferative activity.
Since cytotoxicity is related with antiproliferative activity, the cytotoxicity-based assays that have the advantage of all potential mechanisms involved with the cellular proliferation are monitored simultaneously. However, in many cases, the cytotoxic compounds are simply toxic; therefore, mechanism-based assays are needed to confirm antiproliferative activity. These assays due to their selectivity and sensitivity can be designed by analogy with the types of molecular responses of known antiproliferative agents and the major response of them is the apoptosis induction (Suffness & Pezzuto, Citation1990).
Apoptosis is one of the main types of programmed cell death and involves a series of biochemical events leading to cellular morphological changes and cell death. Inhibition of apoptosis constitutes an essential event in cancer process allowing the survival of cells that accumulate mutations; therefore, the search for apoptotic stimuli is an important issue in the development of anticancer drugs (Pore et al., Citation2013). Thus, successful treatment with chemotherapeutic agents is largely dependent on its ability to trigger cell apoptosis in tumor cells (Vansteenkiste et al., Citation2007).
In the present study, data on active fractions-mediated cell death were obtained by demonstrating two early characteristics connected with apoptosis that are phosphatidylserine externalization and changes in the mitochondrial membrane potential in A549 cells. One of the first events that occur in apoptosis is the exposure of phosphatidylserine in the outer layers of the cell membrane; this allows early recognition of dead cells by macrophages, leading phagocytosis without the release of proinflammatory cellular components (Wong, Citation2011). Here this event was detected by Annexin V-Propidium iodide staining. CEF 6, CEF 7, CEF 8, CEF 10, CEF 11, CEF 14, CRF 3, CRF 4, and CRF 5 were potent inducers of phosphatidylserine externalization. A549 cells treated with some active fractions as CEF 11, CEF 14, CRF3, and CRF 4 showed an increase even higher of 10-fold in early apoptotic cells with respect to untreated controls (). These data clearly show that cells treated with active fractions were dead by apoptosis.
Apoptosis pathways can generally be divided into caspase dependent and caspase independent. The former includes signaling through the death receptor (extrinsic) or the mitochondria (intrinsic) pathway; while the latter involves translocation of apoptosis-inducing factor from the mitochondria to the nucleus. Thus, mitochondria are keys to apoptotic process in mammalian cells. Loss of mitochondrial potential is considered as an early stage of apoptosis, preceding efflux of molecules from mitochondria as cytochrome c and apoptosis-inducing factor and followed by caspase cascade activation, which ultimately leads to cell death (Mou et al., Citation2011). Active chromatographic fractions treatment showed the ability to collapse mitochondrial membrane potential in A549 cells. All active chromatographic fractions tested decreased significantly the red/green fluorescence intensity compared with untreated controls and, therefore, decreased the mitochondrial membrane potential, which further suggested that these fractions induced the cell death through the mitochondrial pathway (). The analysis performed showed good agreement between the antiproliferative activity and the induction of cell death as reflected by the evaluation of early apoptosis events (phosphatidilserine externalization and loss of mitochondria membrane potential).
A few studies about the mechanism of action of compounds isolated from the species of Asclepias have been published. A previous study demonstrated that calotropin, a cardenolide isolated from A. curassavica, possesses cytotoxicity against human chronic myeloid leukemia K562 cells by the G2/M phase arrest due to upregulation of expression of p27 and downregulation of antiapoptotic signaling, leading to caspase 3 activation, which resulted in the induction of apoptosis (Wang et al., Citation2009). However, scientific reports of other species of Asclepias only described phytochemical constituents. Thus, the current study is the first evidence of antiproliferative activity of A. subulata on several cancer cell lines, and it is also the first report that describes molecular events of apoptosis induced by A. subulata.
Conclusions
The results of this study revealed that methanol extract of A. subulata and its polar fractions displayed potent antiproliferative activity against human cancer cell lines. Active chromatographic fractions induced cell death by apoptosis which is mediated by mitochondria-dependent apoptotic pathway. Thus, these data provide a rational basis for the use of A. subulata in traditional medicine of ethnic groups of northwest of Mexico, and suggest that active fractions studied deserve further investigations in order to isolate bioactive metabolites with antiproliferative activity. Currently, experiments are in process in order to determinate the chemical structure of compounds present in active fractions and to perform biological evaluations to define the molecular mechanism by which they induce the cell death.
Acknowledgements
The authors thank Ing. Jesús Sánchez Escalante, who kindly agreed to identify the specimens of A. subulata.
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported by the Consejo Nacional de Ciencia y Tecnología (CONACYT, Grant 83462).
References
- Alonso A, Villarreal M, Salazar L, et al. (2011). Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J Ethnopharmacol 133:945–72
- Araya J, Kindscher K, Timmermann B. (2012a). Cytotoxic cardiac glycosides and other compounds from Asclepias syriaca. J Nat Prod 75:400–7
- Araya J, Kindscher K, Timmermann B. (2012b). Verticillosides A-M: Polyoxygenated pregnane glycosides from Asclepias verticillata L. Phytochemistry 78:178–89
- Beckett R. (1935). The Desert Milkweed (Asclepias subulata) as a Possible Source of Rubber. Washington DC: USDA
- Brain K, Turner T. (1975). The Practical Evaluation of Phytopharmaceuticals. Bristol: Scientectica Publishers
- Bustamante J, Caldas E, Garcia M, et al. (2004). Disruption of mitochondrial membrane potential during apoptosis induced by PSC833 and CsA in multidrug-resistant lymphoid leukemia. Toxicol Appl Pharmacol 199:44–51
- Cerella C, Dicato M, Diederich M. (2012). Assembling the puzzle of anti-cancer mechanisms triggered by cardiac glycosides. Mitochondrion 13:225–34
- Chan Y, Chang C, Chien L, Wu T. (2010). Apoptotic effects of a high performance liquid chromatography (HPLC) fraction of Antrodia camphorate mycelia are mediated by down-regulation of the expressions of four tumor-related genes in human non-small cell lung carcinoma A549 cell. J Ethnopharmacol 127:652–61
- Chen Z, Huang X, Yang H, et al. (2011). Anti-tumor effects of B-2, a novel 2,3-disubstituted 8-arylamino-3H-imidazol [4,5-g] quinazoline derivative, on the human lung adenocarcinoma A549 cell line in vitro and in vivo. Chem Biol Interact 189:90–9
- Cragg G, Newman D. (2003). Plants as a source of anti-cancer and anti-HIV agents. Ann Appl Biol 143:127–33
- Dispersyn G, Nuydens R, Connors R, et al. (1999). BCl-2 protects against FCCP-induced apoptosis and mitochondrial membrane potential depolarization in PCL2 cells. Biochim Biophys Acta 1428:357–71
- Facchini P. (2001). Alkaloids biosynthesis in plants: Biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Physiol 52:29–66
- Fernández A, Juárez V, Zaragoza L. (2008). Usos de las especies del género Asclepias L. (Apocynaceae, Asclepiadoideae), información del Herbario Nacional de México, MEXU. Polibotánica 25:155–71
- García E, García M, De la Puerta R, et al. (2007). Antiproliferative effects of mitraphylline, a pentacyclic oxindole alkaloid of Uncaria tomentosa on human glioma and neuroblastoma cell lines. Phytomedicine 14:280–4
- Goonnet J, Kozjek F, Favrebonvin J. (1973). Flavonols of Asclepias syriaca. Phytochemistry 12:2773–5
- Harborne J. (1984). Phytochemical Methods: A Guide to Nodern Techniques of Plant Analysis. USA: Chapman and Hall
- Heneidak S, Grayer R, Kite G, Simmonds M. (2006). Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae). Biochem Syst Ecol 34:575–84
- Huigsloot M, Tijdens I, Mulder G, Water B. (2001). Differential regulation of phosphatidylserine externalization and DNA fragmentation by caspases in anticancer drug-induced apoptosis of rat mammary adenocarcinoma MTLn3 cells. Biochem Pharm 62:1087–97
- Jemnitz K, Veres Z, Monostory K, et al. (2008). Interspecies differences in acetaminophen sensitivity of human, rat and mouse primary hepatocytes. Toxicol In Vitro 22:961–7
- Jiménez-Estrada M, Velázquez-Contreras C, Garibay-Escobar A, et al. (2013). In vitro antioxidant and antiproliferative activities of plants of the ethnopharmacopeia from northwest of Mexico. BMC Complem Altern Med 13:1–8
- Jolad S, Bates R, Cole J, et al. (1986). Cardenolides and a lignin from Asclepias subulata. Phytochemistry 25:2581–90
- Leite-Legatti A, Batista A, Vicente N, et al. (2012). Jaboticaba peel: Antioxidant compounds, antiproliferative and antimutagenic activities. Food Res Int 49:596–603
- Li J, Liu H, Lin Y, et al. (2008). Six new C21 steroidal glycosides from Asclepias curassavica L. Steroids 73:594–600
- Li J, Qing C, Chen C, et al. (2009). Cytotoxicity of cardenolides and cardenolide glycosides from Asclepias curassavica. Bioorg Med Chem Lett 19:1956–9
- Lopez M. (2007). Digitoxin as an anticancer agent with selectivity for cancer cells: Possible mechanisms involved. Expert Opin Ther Targets 11:1043–53
- López R, Hinojosa A. (1988). Catálogo de Plantas Medicinales Sonorenses. Hermosillo, Sonora, México: Universidad de Sonora Press
- Mena G, Caamal E, Cantillo Z, et al. (2009). In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J Ethnopharmacol 121:462–5
- Mijatovic T, Quaquebeke E, Delest B, et al. (2007). Cardiotonic steroids on the road to anti-cancer therapy. Biochim Biophys Acta 1776:32–57
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application of proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
- Mou H, Zheng Y, Zhao P, et al. (2011). Celastrol induces apoptosis in non-small-cell lung cancer A549 cells through activation of mitochondria- and Fas/FasL-mediated pathways. Toxicol In Vitro 25:1027–32
- Murillo J, Encarnación D, Franzblau S. (2001). Antimicrobial and cytotoxic activity of some medicinal plants from Baja California Sur (Mexico). Pharm Biol 39:445–9
- Navarro V, Gonzalez A, Fuentes M, et al. (2003). Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol 87:85–8
- Pore M, Hiltermann T, Kruyt F. (2013). Targeting apoptosis pathways in lung cancer. Cancer Lett 332:359–68
- Rashan L, Franke K, Khine M, et al. (2011). Characterization of anticancer properties of monoglycosidic cardenolides isolated from Nerium oleander and Streptocaulon tomentosum. J Ethnopharmacol 134:781–8
- Rodrigues S, Calhehla R, Barreira J, et al. (2012). Crataegus monogyna buds and fruits phenolic extracts. Growth inhibitory activity on human tumor cell lines and chemical characterization by HPLC-DAD-ESI/MS. Food Res Int 49:516–23
- Rodriguez L, Fonseca G. (1991). The cardenolide content of Asclepias linaria. Phytochemistry 30:3941–2
- Roy M, Chang F, Huang H, et al. (2005). Cytotoxic principles from Formosan milkweed, Asclepias curassavica. J Nat Prod 68:1494–9
- Samejo M, Sumbul A, Shah S, et al. (2013). Phytochemical screening of Tamarix dioca Roxb. Ex Roch. JPR 7:181–3
- Shivaprasad H, Rajesh R, Nanda B, et al. (2009). Thrombin like activity of Asclepias curassavica L. latex: Action of cysteine proteases. J Ethnopharmacol 123:106–9
- Suffness M, Pezzuto J. (1990). Assays related to cancer drug discovery. In: Hostettmann K, ed. Methods in Plant Biochemistry: Assays for Bioactivity, vol. 6. London: Academic Press, 71–133
- Tang J, Feng Y, Tsao S, et al. (2009). Berberine and Coptidis rhizome as novel antineoplastic agents: A review of traditional use and biomedical investigations. J Ethnopharmacol 126:5–17
- Valencia D, Alday E, Robles R, et al. (2012). Seasonal effect on chemical composition and biological activities of Sonoran propolis. Food Chem 131:645–51
- Vansteenkiste J, Lara P, Le Chevalier T, et al. (2007). Phase II clinical trial of epothilone B analog, ixabepilone, in patients with non-small-cell lung cancer whose tumors have failed first-line platinium-based chemotherapy. J Clin Oncol 25:3448–55
- Wang S, Lu M, Chen H, et al. (2009). Cytotoxicity of calotropin is through caspase activation and downregulation of anti-apoptotic proteins in K562 cells. Cell Biol Int 33:1230–6
- Warashina T, Noro T. (1999). Steroidal glycosides from the aerial part of Asclepias incarnata. Phytochemistry 53:485–9
- Warashina T, Noro T. (2009a). 8,12;8,20-Diepoxy-8,14-secopregnane glicosides from aerial parts of Asclepias tuberosa. Chem Pharm Bull 58:172–9
- Warashina T, Noro T. (2009b). 8,14-Secopregnane glycosides from aerial parts of Asclepias tuberosa. Phytochemistry 70:1294–304
- Wong R. (2011). Apoptosis in cancer: From pathogenesis to treatment. J Exp Clin Canc Res 30:1–14