Abstract
Context: The biotransformation of lilial results in an acid that is used in the dairy industry, in perfumery, as an intermediate in the manufacture of pharmaceuticals and cosmetics, and as a food additive for enhancing taste.
Objective: This study investigates the biotransformation of lilial by Staphylococcus aureus and Staphylococcus epidermidis, two bacterial species isolated from human skin.
Materials and methods: Both species of Staphylococcus were isolated in samples taken from the skin of individuals living in a rural area of Iran. The pH of the culture medium was optimized, and after culturing the microorganisms, the bacteria were added to a flask containing a nutrient broth and incubated for several hours. The flasks of bacteria were combined with lilial, and various biochemical tests and diagnostics were performed, including Fourier transform infrared spectroscopy (FT-IR), ultraviolet-visible spectrophotometry (UV–Vis), and gas chromatography-mass spectroscopy (GC-MS).
Results: The S. aureus produced isobutyric acid (2-methylpropanoic acid) after 72 h (71% of the total products yielded during biotransformation), whereas the S. epidermidis produced terpenoid alcoholic media after 24 h (90% of total products obtained).
Discussion and conclusion: The results obtained indicate that biotransformation of lilial by S. aureus is more desirable than by S. epidermidis due to the highly efficient production of a single product. Bourgeonal and liliol were two toxic compounds produced during biotransformation, which indicates that the use of lilial in cosmetics can be harmful to the skin.
Introduction
Monocyclic compounds are widely distributed in nature and have found extensive applications in the flavor, fragrance, and cosmetics industries. Their simple structures make them ideal targets for microbial biotransformation, during which they have been demonstrated to yield several commercially important products (Esmaeili et al., Citation2012). The use of microorganisms for transformation of complex chemicals resulting in single and specific products has been effectively exploited in recent decades. The biological, medical, and food industries have found a variety of microorganisms useful in the production of organic and food compounds, either in improving the yield of already known substances or in the production of novel substances. Bacteria and fungi have been widely employed in steroid biotransformation studies as early as 1937 (Schlosser et al., Citation1995). The changes produced in natural-product compounds by biotransformation can be important in the search for new uses of these compounds as medicinal drugs and in food processing. For example, investigators studying biotransformation of geraniol, nerol, and citral have reported that a combination of these alcohols extracted from the lavender plant can help prevent liver and lung cancer (Demyttenaere et al., Citation2000; Reddy, Citation2004).
In a previous study carried out on the biotransformation of terpenoids by Bacillus stearothermophilus, the materials produced were perillyl alcohol, perillaldehyde, and α-terpineol (Duetz et al., Citation2003). Biotransformation of plasmid-controlled mercury by the bacteria Clostridium cochlearium (Pan-Hou et al., Citation1980) and of 2,4-dinitrotoluene and 2,6-dinitrotoluene by C. acetobutylicum (Hughes et al., Citation1999) has also been studied. In 1969, a geraniol culture was converted into dihydrolinalool and oxidized to citral using the sporulated surface culture method (Wood, Citation1969). The biotransformation of geraniol and nerol as performed by five species of Aspergillus niger and three species of Penicillium has also been reported on (Carnesecchi et al., Citation2001).
An important factor in medication therapy is the biotransformation of drugs on the skin, which can have significant consequences. The skin’s ability to perform metabolic reactions such as the biotransformation of drugs has been documented (Esmaeili et al., Citation2009). In this study, we used the bacteria Staphylococcus epidermidis and S. aureus isolated from the skin surface to effect biotransformation of lilial, a compound employed in cosmetology and a common ingredient in preparations applied to the skin. The biotransformation products were extracted and investigated by Fourier transform infrared spectroscopy (FT-IR), ultraviolet-visible spectrophotometry (UV–Vis), and gas chromatography-mass spectroscopy (GC-MS). One of the materials obtained from this biotransformation was isobutyric acid, which is used in perfumery ().
Methods
Nutrient agar and mannitol salt agar were purchased from Merck & Co. (Kenilworth, NJ). Ethylene acetate (90%), sodium sulfate anhydride (99%), and chloroform (99%) were purchased from Merck (Darmstadt, Germany). Müller Hinton broth, nutrient agar, and agar–agar were purchased from Sigma-Aldrich (St. Louis, MO). Lilial was donated by oil companies from Amol (Mazandaran Province, Iran).
Microorganisms and cultural conditions
Staphylococcus aureus and S. epidermidis, two symbiotic species of bacteria found on human skin, were sampled from the skin of students between the ages of 25 and 35 at Islamic Azad University North Tehran Branch, Iran, on 19 November 2013. The two strains were isolated from the samples and identified using various biochemical and diagnostic tests. All experiments were conducted under laboratory conditions.
Batch operation of fermentation procedure
Initially, both species were cultured for 24 h in mannitol salt agar; following this, a test pipe containing a liquid nutrient broth was prepared, and the bacteria were transferred to the pipe and then placed in a shaking incubator for 4 h at 36 ± 2 °C and 135 rpm. The contents of the tube were then transferred to a 250 ml flask containing 50 ml of nutrient broth. Finally, the contents were returned to the shaking incubator for an additional 4 h at 36 ± 2 °C and 135 rpm.
Biotransformation of lilial by S. aureus and S. epidermidis
Two 250 ml flasks were prepared, one containing S. epidermidis and the other containing S. aureus. Following this, 50 µL of lilial were transferred to each container. The container with S. epidermidis was put in an incubator and analyzed after 8, 24, and 48 h. The container with S. aureus was incubated and analyzed after 24, 48, and 72 h. In both cases, the temperature was set to 36 ± 2 °C and the circulation was set to 135 rpm. For each microorganism, the same conditions were used for bioconversion. The biotransformation using S. epidermidis obtained a high conversion percentage in this experiment.
Extraction of products of lilial by S. aureus and S. epidermidis
Following incubation, the biotransformation product was then extracted, and the bacteria were destroyed by 10 min of centrifugation. The supernatant was extracted with ethyl acetate and the products were analyzed by GC-MS, FT-IR, and UV–Vis at least four times.
Analysis of S. aureus and S. epidermidis biotransformation with GC-MS
For the GC-MS spectroscopy a Hewlett-Packard 5973 (Agilent Technologies Inc., Santa Clara, CA) equipped with an HP 5 MS column (30 mm × 0.25 mm) was used. The temperature of the oven was set at 60 ± 2 °C for 3 min. Identification of the constituents of the oil was made by comparing their mass spectra and retention indices with those given in the literature and the authentic samples (Aligiannis et al., Citation2001).
Results
The pH of the culture medium was optimized before the bioconversion. To find the best pH, we tested five ranges for the pH, from 5 to 9. The results showed that the best range for microbial conversion for both microorganisms was pH 7. We therefore conducted all conversions in the range of pH 7.
Biotransformation of lilial by S. aureus
After the pH of the culture medium nutrient broth was optimized, the S. aureus bacteria were added to it. When the microorganisms had achieved sufficient growth, the lilial extract was added and the solution put in an incubator for varying lengths of time. The extraction operations were then conducted four times using ethyl acetate, and the remaining solution was identified by GC-MS. Isobutyric acid (2-methylpropanoic acid) was the primary product obtained, representing 69% of the total products yielded after 24 h, 46% after 48 h, and 71% after 72 h. The results demonstrated that the optimum length of time for the conversion by S. aureus was 72 h, which produced the highest percentage of material. The longer time allowed for biotransformation by S. aureus raised the percentage of alcoholic terpenoid obtained ().
Table 1. Percentages of products yielded in biotransformation of lilial by microorganisms isolated from human skin.
Biotransformation of lilial by S. epidermidis
After the pH of the culture medium nutrient broth was optimized, the S. epidermidis bacteria were added to it. When the microorganisms had achieved sufficient growth, the extract was added and the solution put in the incubator for varying lengths of time. The extraction operations were then conducted four times using ethyl acetate, and the remaining solution was identified by GC-MS. The main product formed initially was liliol, the maximum amount of this product (90% of total products yielded) being obtained after 24 h. After 8 h, a smaller percentage of liliol (82%) had been produced. After 48 h, 2-methylpropanoic acid (40.2%), benzoic acid (13.7%), bourgeonal (11.8%), and 2-methyl-3-phenylpropanal (24.2%) were produced (). The best efficiency was achieved for S. epidermidis, which yielded a 90% conversion after 24 h, as compared with a 71% conversion by S. aureus after 72 h. Of course, the advantage of S. aureus over S. epidermidis is the production of a single product.
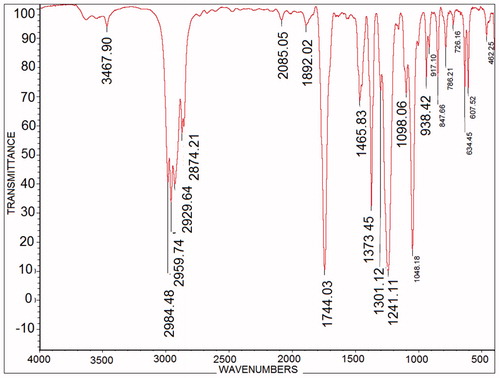
FT-IR spectroscopy
FT-IR spectroscopy was used to identify compounds that emerged from the 24 h conversion of lilial by S. epidermidis (). For compounds of lily alcohol, the signal at 3467 cm−1 is related to the alcohol group −OH (Beneventi et al., Citation1994), which confirms that the combination of alcohol and signal in region 2874 cm−1 is related to the –C–H stretching and is asymmetric. The signal in the region 1465 cm−1 is related to the –CH2 bending, and the signal in the area of 2984 cm–1 is related to the tert-butylbenzene compound. Comparison with previous studies on the biotransformation of homologous compounds and the compounds obtained in this study showed that the FT-IR spectral data for the functional groups are in accordance with our functional groups (Esmaeili et al., Citation2011). In an earlier study, the signal obtained in the region of 3400 cm−1 was attributed to O–H monoterpene alcohol, and signals for the compounds –CH3 and –CH2 corresponded with the signals obtained from our compounds (Esmaeili et al., Citation2011).
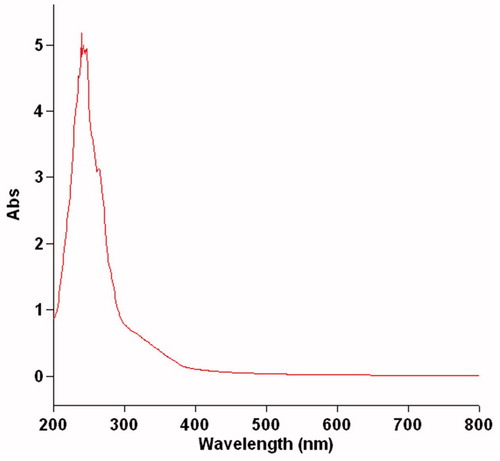
UV–Vis spectroscopy
UV–Vis spectra for biotransformation of lilial by S. epidermidis after 24 h are shown in . The spectra show the absorption compound for alcohol lilial. In the diagram, the wavelength for tert-butylbenzene is seen in the region of 200–300 nm. The spectra for –C=C– as well as the spectra for –C–O can also be observed. The strong signal confirms the conversion of lilial from aldehyde to form the alcohol. In the same tasks, the UV–Vis spectra were compared with the obtained spectra in this article, and this comparison confirmed our products in this study.
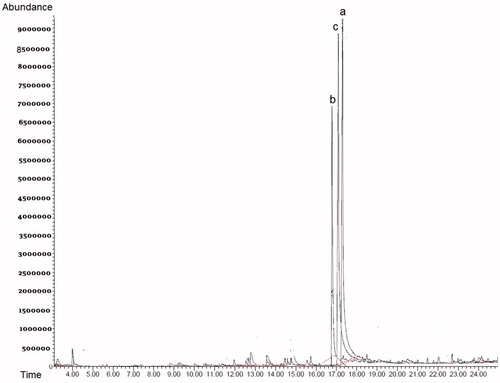
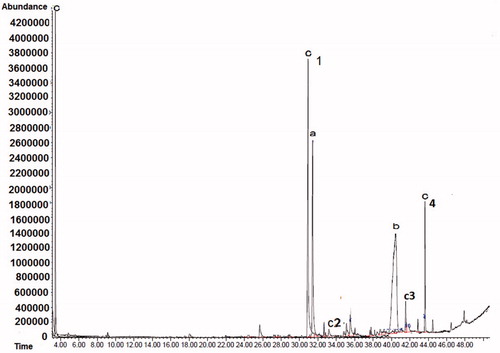
GC–MS spectra
and show the GC–MS spectra for the biotransformation of lilial by S. aureus and S. epidermidis. The diagram in shows the GC–MS spectra of the biotransformation by S. aureus. Regions (a), (b), and (c) show the amount of 2-methyl propionic acid compound produced (69, 46, and 71%, respectively). The GC–MS diagram in shows the biotransformation by S. epidermidis in region (a). The spectrum of (b) is related to lily alcohol, obtained after 31 min. The compound of spectrum (b) was 2-methyl propionic acid, obtained after 33 min, and the spectrum of (c) was related to 2-methyl-3-phenylpropanal. After 42 min bourgeonal was observed, and the spectrum after 44 min was related to benzoic acid, corresponding to the composition. A closer look at the eight peaks reveals that at least 60% of the peaks are similar to the peaks of our compounds (Adams, Citation2007; Ardrey et al., Citation1983). The main products obtained in the bioconversion by S. epidermidis and S. aureus of lilial were bourgeonal (82% at 8 h and 90% at 24 h) and 2-methylpropanoic acid (46% at 8 h, 69% at 24 h, and 71% at 72 h), respectively.
Mass spectra of the eight main peaks are given below:
Bourgeonal: 190 [M+]: 175(100), 131(20), 190(10), 176(10), 57(9), 91(7), 60(4), 30(5).
2-Methylpropanoic acid: 88[M+]: 43(100), 41(39), 73(25), 27(27), 42(9), 98(5), 56(4), 81(40):
Discussion
The purpose of this study was to investigate the microbial conversion of lilial by S. aureus and S. epidermidis. Lilial was added to bacterial growth in a culture medium and incubated for different lengths of time. Two of the primary products obtained from biotransformation of lilial by S. aureus and S. epidermidis were bourgeonal and liliol.
Bourgeonal, or 3-(4-tert-butylphenyl)propanal, an aromatic aldehyde used in fragrances, is toxic if ingested, and can cause skin irritation and sensitization on contact. It is the only known odor substance to which males have a higher average sensitivity than females, and acts as a chemo-attractant for human spermatozoa, apparently playing a role in attracting sperm to the ovum. This is thought to be because the nonolfactory tissue in sperm cells possesses the same olfactory receptor as the olfactory tissue of the nose.
Liliol, or 3-(4-tert-butylphenyl)-2-methylpropan-1-ol, is a member of the 2-methylpropan-1-ol family. Liliol is harmful if absorbed through skin, resulting in irritation, burning, or tingling. Its vapors have a narcotic effect and may cause headache, fatigue, dizziness, and nausea.
In a previous study of biotransformation of an organic compound similar to lilial, Penicillium chrysogenum and P. rugulosum were found to have low efficiency (Esmaeili & Tavassoli, Citation2010). Biotransformation of menthol (also similar to lilial) by A. niger and Penicillium spp. produced more monocyclic compounds (Esmaeili et al., Citation2009). Comparison with these studies showed that S. epidermidis was more selective for biotransformation of monocyclic compounds.
Earlier studies involving the conversion of β-pinene by S. epidermidis produced myrtenol (19.8%), sabinol (32%), myrtenal (25.3%), and pinocarvone (18.3%). In the biotransformation by S. aureus, these compounds were produced at a much lower level (0.1%). In the bioconversion of α-pinene, the materials produced were α-pinene oxide, trans-verbenol, and verbenone. In our conversion by S. epidermidis, a high product percentage was achieved, as was the percentage of products converted by S. aureus. In contrast, in the biotransformation of β-pinene and α-pinene, the percentage of products was very low, especially for the biotransformation by S. aureus. The biotransformation of p-cymene by S. epidermidis produced the alcoholic terpenoid p-cymene-8-ol (40.2%), and S. aureus did not yield a product (Javidnia et al., Citation2009). However, in our work, S. aureus demonstrated the ability to produce products in high percentages. Biotransformation of lilial by S. epidermidis for 8 h produced liliol (82% of total products obtained), and after 24 h, the highest percentage of liliol (90%) was obtained. The main product of biotransformation after 48 h was 2-methyl propanoic acid (40.2%). In the biotransformation of lilial by S. aureus, the resulting product was isobutyric acid (71%), the highest percentage being achieved after 72 h.
In the biotransformation of α-pinene in the earlier study, an oxidation operation was performed. In contrast, in the biotransformation of lilial by both bacteria, a revival reaction was observed. In a previous study of biotransformation of shikonin (a Hindi spice extract) by human intestinal bacteria Bacteroides fragilis subsp., after 72 h of anaerobic incubation, the products obtained included anhydro alkannin, deoxyshikonin, cycloshikonin, and metaboshikonin (Meselhy et al., Citation1994). That biotransformation was performed in anaerobic conditions, whereas the biotransformation of lilial in the current study was carried out in aerobic conditions. Our product is almost derivative of shikonin, as the products obtained are not very different from the initial material, yet in our work, the product is slightly different from the primary products.
Conclusion
In this study, the biotransformation of lilial by S. aureus and S. epidermidis, two species of bacteria isolated from human skin, was investigated. In this work, we conducted an experimental study using bacteria isolated from the skin of people residing in a rural area of Iran. The novelty of this research is the use of microorganisms isolated from the skin; the test results have interesting applications in the cosmetics industry. In this experiment, biotransformation by S. aureus produced 2-methylpropanoic acid (71%) after 72 h, whereas S. epidermidis showed the highest turnover, with 90% conversion to liliol after 24 h. The research results show that the biotransformation activity of S. epidermidis has a high selectivity with a high conversion percentage. The biotransformation of lilial by bacteria commonly found on human skin resulted in compounds known to be unhealthful. Therefore, the use of lilial in formulation of perfumes or cosmetics or other preparations that come in contact with human skin is not advisable.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Adams RP. (2007). Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Carol Stream (IL): Allured Publishing Corporation
- Aligiannis N, Kalpoutzakis E, Mitaku S, Chinou IB. (2001). Composition and antimicrobial activity of the essential oils of two Origanum species. J Agric Food Chem 49:4168–70
- Ardrey RE, Brown C, Allan A, et al. (1983). An Eight Peak Index of Mass Spectra of Compounds of Forensic Interest. Edinburgh, Scotland, and London: Scottish Academic Press
- Beneventi P, Capelletti R, Kovács L, et al. (1994). FTIR spectroscopy of OH stretching modes in BSO, BGO and BTO sillenites. J Phys: Condens Matter 6:6329
- Carnesecchi S, Schneider Y, Ceraline J, et al. (2001). Geraniol, a component of plant essential oils, inhibits growth and polyamine biosynthesis in human colon cancer cells. J Pharmacol Exp Ther 298:197–200
- Demyttenaere J, del Carmen Herrera M, de Kimpe N. (2000). Biotransformation of geraniol, nerol and citral by sporulated surface cultures of Aspergillus niger and Penicillium sp. Phytochemistry 55:363–73
- Duetz W, Bouwmeester H, Van Beilen J, Witholt B. (2003). Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl Microbiol Biotechnol 61:269–77
- Esmaeili A, Hashemi E, Safaiyan S, Rustaiyan A. (2011). Biotransformation of myrcene by Pseudomonas putida PTCC 1694. Herba Pol 57:72–82
- Esmaeili A, Hoseiny Zarea A, Sharafian S, et al. (2009). Biotransformation of menthol by sporulated surface cultures of Penicillium sp. and study of the pathways involved. Herba Pol 55:78–83
- Esmaeili A, Khodadadi A, Safaiyan S. (2012). Biotransformation of thymol by Aspergillus niger. Chem Nat Compd 47:966–8
- Esmaeili A, Tavassoli A. (2010). Microbial transformation of citral by Penicillium sp. Acta Biochim Pol 57:265
- Hughes JB, Wang CY, Zhang C. (1999). Anaerobic biotransformation of 2,4-dinitrotoluene and 2,6-dinitrotoluene by Clostridium acetobutylicum: A pathway through dihydroxylamino intermediates. Environ Sci Technol 33:1065–70
- Javidnia K, Aram F, Solouki M, et al. (2009). Microbial biotransformation of some monoterpene hydrocarbons. Annals Microbiol 59:349–51
- Meselhy MR, Kadota S, Tsubono K, et al. (1994). Biotransformation of shikonin by human intestinal bacteria. Tetrahedron 50:3081–98
- Pan-Hou H, Hosono M, Imura N. (1980). Plasmid-controlled mercury biotransformation by Clostridium cochlearium T-2. Appl Environ Microbiol 40:1007–11
- Reddy BS. (2004). Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen 44:26–35
- Schlosser D, Irrgang S, Schmauder HP. (1995). Fermentation kinetics of free and immobilized Penicillium raistrickii able perform the 15α-hydroxylation of 13-ethyl-gon-4-ene-3, 17-dione. Acta Biotechnol 15:161–71
- Wood J. (1969). Microbial fermentation of lower terpenoids. Process Biochem 2:50–2