Abstract
Context: Salvianolic acids are the most abundant water-soluble compounds extracted from the herb Salvia miltiorrhiza L. (Lamiaceae) with antioxidant and protective effects.
Objective: This study evaluates the antidiabetic effect of salvianolic acid B (Sal B) in multiple low-dose streptozotocin (MLDS)-induced diabetes in rat.
Materials and methods: Rats were divided into control, Sal B40-treated control, diabetic, Sal B20-, and Sal B40-treated diabetic groups. Sal B was daily administered at doses of 20 or 40 mg/kg (i.p.), started on third day post-STZ injection for 3 weeks. Serum glucose and insulin level and some oxidative stress markers in pancreas were measured in addition to the oral glucose tolerance test (OGTT), histological assessment, and apoptosis determination.
Results: After 3 weeks, treatment of diabetic rats with Sal B20 and Sal B40 caused a significant decrease of the serum glucose (p < 0.05–0.01) and improvement of OGTT. Meanwhile, serum insulin was significantly higher in Sal B20- and Sal B40-treated diabetics (p < 0.01) and treatment of diabetics with Sal B40 significantly lowered malondialdehyde (MDA) (p < 0.05), raised glutathione (GSH) (p < 0.05), and activity of catalase (p < 0.01) with no significant change of nitrite. Furthermore, the number of pancreatic islets (p < 0.05) and their area (p < 0.01) was significantly higher and apoptosis reactivity was significantly lower (p < 0.05) in the Sal B40-treated diabetic group versus diabetics.
Discussion and conclusion: Three-week treatment of diabetic rats with Sal B exhibited antidiabetic activity which is partly exerted via attenuation of oxidative stress and apoptosis and augmentation of antioxidant system.
Introduction
Diabetes mellitus (DM) comprises a heterogeneous group of metabolic disorders characterized by defect in glucose regulation and ensuing hyperglycemia (Maraschin Jde, Citation2012). Type 1 DM is a serious condition due to an autoimmune process and characterized by the infiltration of T-cells, macrophages, and natural killer cells into and around islets of Langerhans that is followed by selective destruction of insulin-secreting beta cells (Bending et al., Citation2012). In addition to enhanced oxidative stress burden, antioxidant defense systems are markedly disturbed in diabetic pancreas, leading to eventual apoptotic cell death (Cernea & Dobreanu, Citation2013; Zhu et al., Citation2014). The diabetogenic agent streptozotocin (STZ) is widely used to induce diabetes due to its specific toxic effect on pancreatic beta cells in rodents like rat (Nasri et al., Citation2011). Multiple low-dose streptozotocin (MLDS) induces diabetes via autoimmunity-mediated destruction of pancreatic β-cells (Xiang et al., Citation2010), which resembles its pathologic process in patients with type 1 DM (Chang et al., Citation2013).
Salvianolic acids, especially salvianolic acid B (Sal B), are the most abundant water-soluble compounds extracted mainly from the medicinal herb Salvia miltiorrhiza L. (Lamiaceae), also known as danshen, with potent antioxidative effect due to their polyphenolic structure (Ho & Hong, Citation2011). Sal B could protect pancreatic beta-cells against cytotoxicity (Cheng et al., Citation2013) and prevent high glucose-induced apoptosis (Sun et al., Citation2012). Sal B is capable to protect vascular endothelial cell against oxidative injury (Yang et al., Citation2011). Hepatoprotective effect of Sal B (Gao et al., Citation2012) and its protective effect in chronic pancreatitis (Lu et al., Citation2009) have also been reported. This study was undertaken to evaluate the antidiabetic effect of Sal B in multiple low-dose (MLD) model of type 1 diabetes in rat and to assess some underlying mechanisms.
Materials and methods
Animals
Male albino Wistar rats (Pasteur’s Institute, Tehran, Iran) weighing 210–260 g were housed in an air-conditioned colony room on a light/dark cycle (21–23 °C and a humidity of 30–40%) and supplied with free access to standard pelleted diet and tap water. The procedures involving animals use and care were approved by ethics committee of Iran University of Medical Sciences (Tehran, Iran) and conducted in conformity with the National Institutes of Health guidelines.
Experimental procedure
The rats (n = 60) were randomly allocated and similarly grouped into five groups, i.e., control, Sal B40-treated control (40 mg/kg), diabetic, and Sal B20- and Sal B40-treated diabetics (20 or 40 mg/kg, respectively). For induction of autoimmune type 1 DM, streptozotocin (STZ) was administered daily (i.p.) for 5 consecutive days at a dose of 20 mg/kg according to a previous study (Hall et al., Citation2013). STZ (Sigma-Aldrich, St. Louis, MO) were freshly dissolved in cold normal saline. Sal B (Sigma-Aldrich, St. Louis, MO) at doses of 20 (Sal B20) or 40 (Sal B40) mg/kg (p.o. using a gavage needle) was daily administered, started on third day post-STZ injection for 3 weeks. The used doses were selected according to a previous report (Wang et al., Citation2011) and our earlier pilot study. Overnight fasting serum glucose concentration was measured using the glucose oxidation method (Zistshimi, Tehran) and blood samples taken from the tail vein before the study (week 0) and at the end of weeks 1, 2, and 3 after STZ injection. Body weight was also measured weekly. Serum insulin concentration was determined by an ELISA technique (EIA Kit, Cayman Chemical, Ann Arbor, MI) according to the instructions of the manufacturer.
Oral glucose tolerance test (OGTT)
After overnight fasting (n = 7–9 for each group), on the day of animal sacrifice, blood samples were obtained from snipped tails by tail milking at 0, 30, 60, 90, and 120 min after administration of glucose (a 20% solution; 2 g/kg b.w.) using a gavage needle and the blood glucose measurement was made using a research-grade glucometer.
Oxidative stress assessment
Pancreas tissues from rats (n = 4–5 from each group) were dissected, washed in cold normal saline, blotted dry, weighed, then made into 5% tissue homogenate in ice-cold normal saline, centrifuged at 4 °C, the obtained supernatant was aliquotted, then stored at −70 °C until being assayed.
Determination of pancreas malondialdehyde content
The malondialdehyde (MDA) concentration was measured as described by Kiasalari et al. (Citation2013). The results were expressed as MDA equivalents using tetraethoxypropane as a standard.
Pancreas nitrite assay
Supernatant nitrite content was measured by the Griess method according to a previous study (Mirshekar et al., Citation2010).
Assay of catalase activity
Claiborne's method (Citation1985) was used for this purpose. Briefly, H2O2 was added to a mixture of potassium phosphate buffer and supernatant and the rate of H2O2 decomposition was assessed by measuring the absorbance changes at 240 nm.
Reduced glutathione measurement (GSH)
Glutathione measurement (GSH) was measured as described before (Ellman, Citation1959; Sedlak & Lindsay, Citation1968). Briefly, the supernatant was centrifuged with trichloroacetic acid. Then, phosphate buffer and 5′5-dithiobis-(2-nitrobenzoic acid) (DTNB) was added. The mixture was vortexed and the absorbance was read at 412 nm.
Protein assay
The protein content was measured with the Bradford (Citation1976) method using bovine serum albumin as the standard.
Pancreas histology
Hematoxylin and eosin staining
Pancreas of animals (n = 3 from each group) was fixed in 10% buffered formalin for 2–3 d and then embedded in paraffin. Tissue sections (5 µm thick) were deparaffinized and rehydrated sequentially in xylene, xylene/ethanol, and ethanol (100, 95, and 70), and then the sections were placed in distilled water for 10 min. Sections were stained with hematoxylin and eosin (H&E) using the standard protocol, where cytoplasm stained pink, nuclei stained deep purple, and pancreatic islets appeared pale in color. Finally, the average number of islets/mm2 and their area were calculated.
Apoptosis assay
In order to detect DNA fragmentation and apoptotic cell death, a transferase dUTP nick-end labeling (TUNEL) assay was performed using the in situ cell death detection kit (Roche, Penzberg, Germany). In this assay, four rats from each group was used and euthanized on day 5 after diabetic induction by STZ. Briefly, the pancreatic sections were incubated with proteinase K (100 µg/ml), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using a converter-POD and subsequent incubation with DAB (3–3′-diamonobenzidine tetrachloride) and H2O2, coverslipped and evaluated. A dark brown color indicating DNA breaks developed. TUNEL reactivity density was assessed in at least 10 islets for each rat and its average was taken as the final value.
Data and statistical analysis
All values were given as means ± SEM. For morphometric and densitometric analysis, UTHSCSA image tool software (version 3, 2002, UTHSCSA, San Antonio, TX) was used. Statistical analysis of data was carried out using repeated measure and one-way ANOVA followed by the Tukey post hoc test. A statistical p value less than 0.05 considered significant.
Results
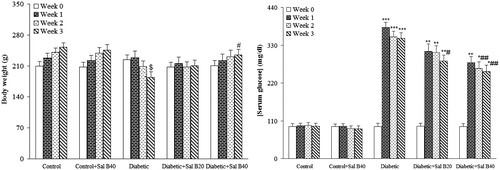
One rat in the diabetic group was excluded from the study due to its morbid state and final death. Body weight and serum glucose data () indicated that before induction of MLD-STZ diabetes, there were no significant differences among the experimental groups. At the end of week 3, the weight of the vehicle-treated diabetic rats was found to be significantly lower versus control rats (p < 0.05) and SAL B40-treated diabetics showed no reduction of body weight as compared with vehicle-treated diabetics. Untreated diabetic rats also had an elevated serum glucose level over those of control rats (p < 0.0005) and treatment of diabetic rats with Sal B20 or Sal B40 caused a significant decrease in the serum glucose (p < 0.05–0.01) at week 3 versus vehicle-treated diabetics. In addition, Sal B treatment of control rats at none of the doses did not produce any significant change regarding serum glucose level relative to the control group.
Figure 1. Body weight and serum glucose level at different weeks. Sal B20 and Sal B40 indicate salvianolic acid B at doses of 20 and 40 mg/kg, respectively (n = 10–11 for each group). $p < 0.05 (versus week 0), *p < 0.005, **p < 0.001, ***p < 0.0005 (versus week 0), #p < 0.05, ##p < 0.01 (versus diabetic in the same week).
Oral glucose tolerance test (OGTT)
The OGTT procedure was performed at the end of week 3 after induction of diabetes (). The values are percent change in blood glucose level at time t, relative to initial blood glucose level at time 0. In control and control + Sal B40 groups, blood glucose almost returned to its initial value at min 120, while in the diabetic group, it was still higher than its initial level. However, in both the treated diabetic groups, blood glucose level finally resettled to the initial level by 120 min. Meanwhile, changes of blood glucose in all diabetic groups, especially in treated diabetics, were lower than that of the control groups.
Figure 2. Changes of blood glucose as percentage of initial value (min 0) at different time points in OGTT and serum insulin level. Sal B20 and Sal B40 indicate salvianolic acid B at doses of 20 and 40 mg/kg, respectively (n = 7–9 for each group). *p < 0.05, **p < 0.01, ***p < 0.001 (versus control), #p < 0.01 (versus diabetic).
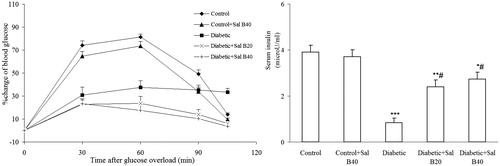
Serum insulin measurement
The effect of Sal B20 and Sal B40 administration on serum insulin level is shown in . In diabetic rats, serum insulin showed a significant reduction (p < 0.001) as compared with the control group and treatment of diabetics with Sal B20 and Sal B40 significantly improved it (p < 0.01). However, the insulin level in the latter groups was still significantly lower as compared with the control group (p < 0.05–0.01).
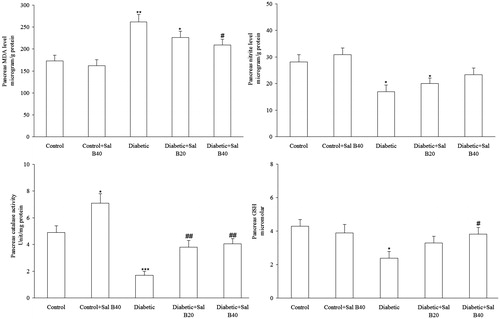
Oxidative stress markers
With respect to pancreatic biochemical markers (), MLD-STZ diabetes resulted in significant elevation of MDA (p < 0.01), decreased nitrite (p < 0.05), and GSH (p < 0.05), and reduced activity of the defensive enzyme catalase (p < 0.005) versus the control group and treatment of the diabetic group with Sal B40 significantly lowered MDA content (p < 0.05), raised GSH content (p < 0.05), and activity of catalase (p < 0.01) with no significant effect on nitrite. Meanwhile, there was no significant changes in the Sal B40-treated control group relative to control animals regarding these parameters.
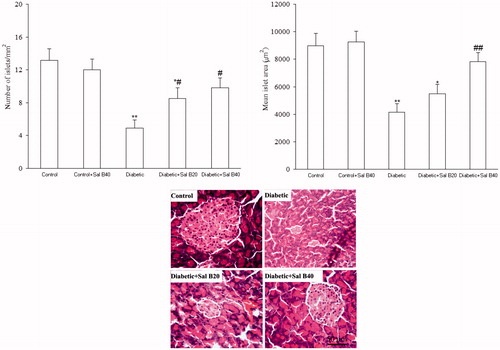
Histological evaluation of the pancreas
Histology of pancreas in control rats in H&E staining was normal in all the specimens. In the diabetic group, a significant decrease of islet numbers/mm2 (p < 0.01) and their mean area (p < 0.01) (), an atrophy and vacuolation and subtle invasion of connective tissues in parenchyma of pancreatic islets were observed relative to the control group. These abnormal histological changes dramatically decreased in the diabetic + Sal B40 group as compared with diabetic one. In this respect, the number of islets/mm2 significantly increased in diabetic + Sal B20 (p < 0.05) and diabetic + Sal B40 (p < 0.05) groups as compared with diabetic one. Regarding the islets mean area, it significantly decreased in the diabetic group as compared with the control group (p < 0.01) and it showed significant increase in the diabetic + Sal B40 group in comparison with the diabetic group (p < 0.01).
Figure 4. The number of pancreatic islets/mm2 and their mean area (top panel) and photomicrographs of pancreatic sections stained with H&E (bottom panel) after 3 weeks of Sal B administration. Sal B20 and Sal B40 indicate salvianolic acid B at doses of 20 and 40 mg/kg, respectively (n = 3 for each group). *p < 0.05, **p < 0.01 (versus control), #p < 0.05, ##p < 0.01 (versus diabetic).
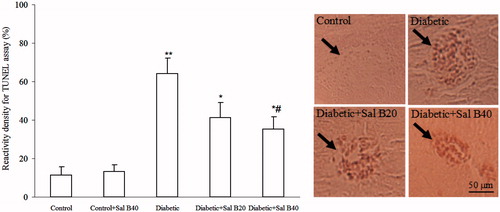
Apoptosis assay
With respect to islet cell apoptosis (), islet cells undergoing apoptosis were determined by TUNEL assay. Using a densitometric method, it was found out that reactivity density was significantly greater in the diabetic group versus control one (p < 0.005) and this parameter was significantly lower in diabetic + Sal B20 (p < 0.05) and diabetic + Sal B40 (p < 0.05) groups as compared with diabetic one.
Figure 5. Immunoreactivity density for TUNEL assay of pancreatic islets and their photomicrographs on day 5 after diabetic induction by STZ. Sal B20 and Sal B40 indicate salvianolic acid B at doses of 20 and 40 mg/kg, respectively (n = 4 for each group). Each arrow shows a pancreatic islet. *p < 0.05, **p < 0.005 (versus control), #p < 0.05 (versus diabetic).
Discussion
In this study, Sal B administration at a dose of 40 mg/kg for 3 weeks to MLD-STZ diabetic rats exerted a hypoglycemic effect and prevented body weight reduction, improved OGTT, and increased serum insulin level, attenuated oxidative stress, and augmented antioxidant defense system, and prevented loss and degeneration of pancreatic islets due to its anti-apoptotic effect.
In our research, we decided to start Sal B administration at early stages of diabetes process, i.e., several days post-STZ injection (on third day), not to have a mere protective or restorative intervention. In this respect, some previous works have administered their beneficial agents before the first STZ injection in the MLD-STZ model (Amirshahrokhi & Ghazi-Khansari, Citation2012) that is a protective strategy and some works have administered their agents after the last STZ injection in this model (Jiang et al., Citation2014) that is more resembled to a restorative protocol. Even, some researchers have begun first administration of their protective agent at day 1 post-STZ injection (Fukudome et al., Citation2008). In addition, since mononuclear infiltration into pancreatic islets is an initial event inducing insulitis in the development of type 1 diabetes in the MLD-STZ model and occurs within several days post-STZ injection (Takamura et al., Citation1999), therefore, it is better to begin the treatment at the first days of diabetes induction.
Although glucose-lowering effect of Sal B was not observed for the control group in this study, but its administration for 3 weeks exerted a marked hypoglycemic effect in diabetic rats, indicating hypoglycemic mechanism of Sal B to be different in diabetic and non-diabetics. A previous study has shown that Sal B could protect and even restore function of pancreatic beta cells (Cheng et al., Citation2013), in this way possibly exerting its anti-hyperglycemic and antidiabetic effect. In addition, it has been shown that Sal B has protective effect in mammalian cells against toxicity induced by high glucose condition through inhibition of apoptotic pathway (Sun et al., Citation2012) that was also observed in our study. Until now, there has not been any report on insulinomimetic and/or insulin releasing ability of SAL B, thus it is more possible that such compounds act via their anti-oxidant and/or anti-inflammatory effect, which itself needs further investigation. Histological analysis of the pancreas using H&E and TUNEL staining showed the degeneration of islets, as evidenced by their lower area and being less scattered. In support of this idea, part of deleterious effect of multiple low-dose STZ is exerted through activation of an apoptotic pathway (Mensah-Brown et al., Citation2002) and it has been reported that Sal B exhibits anti-apoptotic effect via reducing the expression of tumor necrosis factor alpha receptor type 1, balancing the expression of Bcl-2 family members, reducing the release of cytochrome C from the mitochondria into the cytosol, and inhibiting activated caspase-3 (Yan et al., Citation2010). Thus, these mechanism may be of significance in such studies and warrant more researches in future.
Sal B treatment could also attenuate the inflammation induced by some toxic agents (Gao et al., Citation2012), therefore, part of its beneficial effect in our study could be attributed to its anti-inflammatory property (Chen et al., Citation2011). In this respect, Sal B could suppress the expression of pro-inflammatory cytokines TNF-α and IL-1β, whereas enhance the expression of anti-inflammatory cytokines IL-10 and TGF-β1 (Chen et al., Citation2011). The same mechanism may have occurred in our study to produce the protective role of Sal B in the MLD-STZ model of diabetes and suggest that its protective effect might be associated with its anti-inflammatory activity. However, determination of exact mode of action of Sal B in this model is strongly recommended.
Reactive oxygen species (ROS) and NO also have crucial roles in pathogenesis of diabetes complications (Baluchnejadmojarad et al., Citation2013). Possible sources of oxidative stress in diabetes include an increased production of ROS, especially from enhanced glycation and lipoxidation processes (Fiorentino et al., Citation2013). Substantial evidence suggest that Sal B could elicit anti-oxidant properties by attenuating the lipid peroxidation caused by various forms of free radicals and to boost antioxidant defensive system (Yang et al., Citation2011), in this way may have exerted its antidiabetic effect in our study. This fact was verified by a lower MDA content, raised GSH, and an increased activity of catalase in pancreatic tissue of treated diabetics in this study. Finally, one of the limitations of our study was that we did not have a positive control group and, for this reason, we could not directly compare the magnitude of the effect of Sal B in this study with other available drugs.
The data obtained from the present study indicate antidiabetic effect of Sal B in the MLD-STZ model of diabetes. We provide evidence for the first time that Sal B may exert antidiabetic effect by protecting pancreatic beta cell against chemical insult and may also improve insulin secretory function of beta cells. Since the loss of functional beta cell mass is the key for deterioration of glycemic control in diabetic patients, more researches are warranted to further characterize and unravel the molecular mechanisms underlying the antidiabetic effect of Sal B.
Declaration of interest
The authors report that they have no conflicts of interest. This study was part of a Ph.D. thesis project that was financially supported by a research grant (No. 90-04-30-15967) from Iran University of Medical Sciences in 2011 (Tehran, Iran).
References
- Amirshahrokhi K, Ghazi-Khansari M. (2012). Thalidomide attenuates multiple low-dose streptozotocin-induced diabetes in mice by inhibition of proinflammatory cytokines. Cytokine 60:522–7
- Baluchnejadmojarad T, Roghani M, Jalali Nadoushan MR, et al. (2013). The sesame lignan sesamin attenuates vascular dysfunction in streptozotocin diabetic rats: Involvement of nitric oxide and oxidative stress. Eur J Pharmacol 698:316–21
- Bending D, Zaccone P, Cooke A. (2012). Inflammation and type one diabetes. Int Immunol 24:339–46
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–54
- Cernea S, Dobreanu M. (2013). Diabetes and beta cell function: From mechanisms to evaluation and clinical implications. Biochem Med (Zagreb) 23:266–80
- Chang CL, Chen YC, Chen HM, et al. (2013). Natural cures for type 1 diabetes: A review of phytochemicals, biological actions, and clinical potential. Curr Med Chem 20:899–907
- Chen T, Liu W, Chao X, et al. (2011). Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res Bull 84:163–8
- Cheng B, Gong H, Li X, et al. (2013). Salvianolic acid B inhibits the amyloid formation of human islet amyloid polypeptide and protects pancreatic beta-cells against cytotoxicity. Proteins 81:613–21
- Claiborne A. (1985). Catalase activity. In: Greenwald RA, ed. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press, 283–4
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–7
- Fiorentino TV, Prioletta A, Zuo P, Folli F. (2013). Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des 19:5695–703
- Fukudome D, Matsuda M, Kawasaki T, Ago Y, Matsuda T. (2008). The radical scavenger edaravone counteracts diabetes in multiple low-dose streptozotocin-treated mice. Eur J Pharmacol 583:164–9
- Gao HY, Li GY, Lou MM, et al. (2012). Hepatoprotective effect of matrine salvianolic acid B salt on carbon tetrachloride-induced hepatic fibrosis. J Inflamm (Lond) 9:16
- Hall KE, McDonald MW, Grise KN, et al. (2013). The role of resistance and aerobic exercise training on insulin sensitivity measures in STZ-induced type 1 diabetic rodents. Metabolism 62:1485–94
- Ho JH, Hong CY. (2011). Salvianolic acids: Small compounds with multiple mechanisms for cardiovascular protection. J Biomed Sci 18:30
- Jiang X, Bai Y, Zhang Z, et al. (2014). Protection by sulforaphane from type 1 diabetes-induced testicular apoptosis is associated with the up-regulation of Nrf2 expression and function. Toxicol Appl Pharmacol 279:198–210
- Kiasalari Z, Roghani M, Khalili M, et al. (2013). Antiepileptogenic effect of curcumin on kainate-induced model of temporal lobe epilepsy. Pharm Biol 51:1572–8
- Lu XL, Dong XY, Fu YB, et al. (2009). Protective effect of salvianolic acid B on chronic pancreatitis induced by trinitrobenzene sulfonic acid solution in rats. Pancreas 38:71–7
- Maraschin Jde F. (2012). Classification of diabetes. Adv Exp Med Biol 771:12–19
- Mensah-Brown EP, Stosic Grujicic S, Maksimovic D, et al. (2002). Downregulation of apoptosis in the target tissue prevents low-dose streptozotocin-induced autoimmune diabetes. Mol Immunol 38:941–6
- Mirshekar M, Roghani M, Khalili M, et al. (2010). Chronic oral pelargonidin alleviates streptozotocin-induced diabetic neuropathic hyperalgesia in rat: Involvement of oxidative stress. Iran Biomed J 14:33–9
- Nasri S, Roghani M, Baluchnejadmojarad T, et al. (2011). Vascular mechanisms of cyanidin-3-glucoside response in streptozotocin-diabetic rats. Pathophysiology 18:273–8
- Sedlak J, Lindsay RH. (1968). Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25:192–205
- Sun LQ, Zhao J, Zhang TT, et al. (2012). Protective effects of salvianolic acid B on Schwann cells apoptosis induced by high glucose. Neurochem Res 37:996–1010
- Takamura T, Ando H, Nagai Y, et al. (1999). Pioglitazone prevents mice from multiple low-dose streptozotocin-induced insulitis and diabetes. Diabetes Res Clin Pract 44:107–14
- Wang T, Shan SY, Han B, et al. (2011). Salvianolic acid A exerts antiamnesic effect on diazepam-induced anterograde amnesia in mice. Neurochem Res 36:103–8
- Xiang FL, Lu X, Strutt B, et al. (2010). NOX2 deficiency protects against streptozotocin-induced beta-cell destruction and development of diabetes in mice. Diabetes 59:2603–11
- Yan X, Zhou T, Tao Y, et al. (2010). Salvianolic acid B attenuates hepatocyte apoptosis by regulating mediators in death receptor and mitochondrial pathways. Exp Biol Med (Maywood) 235:623–32
- Yang TL, Lin FY, Chen YH, et al. (2011). Salvianolic acid B inhibits low-density lipoprotein oxidation and neointimal hyperplasia in endothelium-denuded hypercholesterolaemic rabbits. J Sci Food Agric 91:134–41
- Zhu M, Guo M, Fei L, et al. (2014). 4-Phenylbutyric acid attenuates endoplasmic reticulum stress-mediated pancreatic beta-cell apoptosis in rats with streptozotocin-induced diabetes. Endocrine 47:129–37