Abstract
Context: Phytopharmacology is a complex but very promising research area. The different plant parts and extraction methods may result in opposed effects. Phlomis species have been reported for both anti-inflammatory and tonic properties.
Objective: The effect of Phlomis lanata Willd. (Lamiaceae) protein extracts on immune cell reactivity was studied in the experimental mouse model.
Materials and methods: Protein extracts from P. lanata aerial parts were fractionated by Q-sepharose ion-exchange chromatography and applied to whole spleen cells or T-cell subsets at 5 μg/ml. Cell growth and cytokine production were evaluated after 4 and 2 d of culture using 3H-thymidine-uptake and ELISA techniques, respectively.
Results: Among the protein fractions tested, column wash proteins (W1) and the fraction eluted using 600 mM NaCl (F6) reduced by 76% and increased by 78% spleen cell proliferation, respectively. W1 suppressed proliferation of effector T-cells, but stimulated the growth of suppressor/regulatory cells by 62–148%. Although W1 stimulated IL-2 and IL-10 production from total spleen cells, it significantly increased IL-10 (50%) and reduced IL-2 (30–50%) production from T-cells, while TNF-α release was enhanced in CD25+CD4+ by 92% and reduced by 50% in CD25+CD8+ cells. F6 stimulated whole spleen cell growth, reduced proliferation of CD8+ and CD25+ cells by approximately 50%, while decreasing by 60–80% TNF-α production from CD25− and CD25+CD8+ cells.
Discussion and conclusion: The suppressive activity of W1 could be attributed to IL-10 and TNF-α, while the stimulatory effect of F6 could be attributed to the inhibition of T-regulatory cells. In the same plant, coexisting protein fractions induce both immunostimulatory and immunosuppressive activities.
Introduction
The genus Phlomis s.l. (selsu lato) which is one of the largest members of the subfamily Lamioidae (Lamiaceae) comprises over 100 species with a wide distribution from China through Eurasia to the Mediterranean with two centers of diversity comprising southeast Anatolia and northwestern Iran, as well as from the old Soviet parts of Central Asian to eastern China (Azizian & Moore, Citation1982). Phlomis species are mentioned by Dioscorides as herbal drugs and used in folk medicine to treat various conditions (Amor et al., Citation2009). Some Phlomis species are also popular herbal teas (decoction or infusion) in the Mediterranean region (Couladis et al., Citation2003; López et al., Citation2010). Concerning their use in traditional medicine, herbal tea is used to treat gastric, intestinal, and abdomen pains and it is also used as a tonic, sedative, carminative, and astringent (Fernandez-Ocana et al., Citation1996; Pardo de Santayana et al., Citation2005; Rivera Nunez & Obon De Castro, Citation1993; Tardio et al., Citation2006; Vazquez et al., Citation1997). The juice of leaves is used in psoriasis, chronic skin eruptions, chronic rheumatism, and also applied to disperse painful swellings (Khanam & Abul Hassan, Citation2005). A cicatrizant plaster is prepared with the chopped leaves (Gonzalez-Tejero et al., Citation1995; Rivera Nunez & Obon De Castro, Citation1993), while dried leaves are applied directly on fresh cuts and burns (Boukef, Citation1986; Quezel & Santa, Citation1963). The juice of the plant is also used in malarial fever (Manandhar & Manandhar, Citation2002). Although the phytochemistry and biological activities of essential oils of Phomis species have been extensively studied (Amor et al., Citation2009; Li et al., Citation2010), the research on their protein extracts has widely been neglected. The use of Phlomis species as dry powder, decoction, and chopped leaves, where the protein content of the tissue should be unaffected, implies the possibility of attributing the various benefits of these plants to proteins. To date, there seems to be no research concerning the effect of Phlomis proteins in vivo or in vitro in mammals. Concentrating on Phlomis lanata Willd., which is an endemic plant of the island of Crete (Greece), the present work was designed to evaluate the role of fractionated protein extracts from aerial parts on murine immune cell proliferation and cytokine production in vitro.
The central role in the immune system (Murphy, Citation2012) is played by CD4-positive T helper (TH) cells, which upon activation will regulate humoral immunity through the stimulation of B cells as well as cellular immunity through activation of CD8-positive cytotoxic T cells (TCYT). Both humoral and cellular immunities are regulated by T regulatory/suppressor cells (Treg) that will express the CD25 surface glycoprotein. These cells will exert their effect either through antigen-specific factors or antigen non-specific cytokines. Cytokines are short-lived molecules that transduce cellular signals through binding to specific receptors and regulate cell proliferation, differentiation, migration, apoptosis, and necrosis at almost every cell type of the organism. The present report is concentrated on interleukin-2 (IL-2), interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α), which are considered to be representative members of cytokines for immune regulation. Thus, IL-2 and TNF-α are two pro-inflammatory cytokines that regulate proliferation and differentiation of other immune cells (including T cells, B cells, and NK cells). IL-2 is a very important cytokine for the proliferation of T cells and is produced by the TH1 population (Arai et al., Citation1991; Cohen & Cohen, Citation1996). Along with TNF-α which displays a cytotoxic effect, they regulate the beginning of an immune response. On the other hand, IL-10, an anti-inflammatory cytokine, is associated with the suppression of the immune response and is produced by the TH2 population (Mosmann & Moore, Citation1991). The work presented here examined the role of protein extracts of P. lanata on the proliferation and cytokine production of total spleen cells or T cell subsets. The obtained results could primarily distinguish an immunosuppressive fraction of proteins which could be proved very useful in immune regulation. To our knowledge, this is the first time that the effect of P. lanata proteins on the immune system of mammals is studied.
Materials and methods
Animals and tissues
Normal, inbred BALB/c mice were bred in the Department of Biology under standard conditions of temperature (18–25 °C), humidity (45–50%), and photoperiod of 12 h light/dark. Male mice 4–8 weeks of age were handled according to the international and national bioethical rules and conformed to the bioethics regulations of the University of Crete, approved by the Animal Facility responsible officer of the Department of Biology. No in vivo work was carried out. Mice were sacrificed by cervical dislocation and spleens were removed under antiseptic conditions. Spleen cells were isolated by pushing the tissue through a mesh wire and used either as total spleen cells or isolated myeloid and lymphoid cell populations in proliferation and enzyme-linked immunosorbent assays (ELISA).
Plant species
Phlomis lanata is a shrub that may grow up to 55 cm. The lower leaves (1.5–2.8 cm) are broadly elliptical, oblong, obovate or suborbicular, cuneate to rounded at base with petiole up to 1 cm. The floral leaves are subsessile, suborbicular, and obtuse. Verticillasters 2- to 10-flowered and bracteolates (6–10 × 3–5.5 mm2) broadly elliptical, oblanceolate or obovate, straight at apex. The calyx (10–12 mm) is stellate and the corolla (20–23 mm) is yellow.
In Crete, P. lanata shares the popular name “agkarathia” with Phlomis fruticosa L. (own data). Most of the times, in popular medicine, the two species are used for the same purposes, e.g., preparation of “sarantovotano”, a mix of 40 herbs that midwives used for baby bath therapy. The sharing of a common popular name between two different Phlomis species has also been reported by Martínez-Francés et al. (Citation2012). Phlomis crinita Cav. and Phlomis purpurea L. share the name “Salvió”, while as reported by Amor et al. (Citation2009), many Phlomis species over the world have the same mode of use.
Phlomis lanata is present mainly in the central and eastern part of Crete, growing from the sea level up to 1200 m. Plants of the species, identified by Dr. Stergios Pirintsos (Director of the Botanical Garden, University of Crete), were harvested in spring (April–May 2010 and 2011) from a phryganic ecosystem located at the northern site of the island of Crete, at an altitude of 129 m, with the following coordinates X,Y: 0 614 047, 3 908 687 (EGSA87, the Greek Projection).
Voucher specimens have been deposited at the Herbarium TAU of the Aristotle University of Thessaloniki (UOCL101-1, UOCL101-2, and UOCL101-3) and the material is available for other users. The sampling site is located in public land. This land is open to local people for herb collection in order to cover their ethnopharmacological needs. The sampling area is not a National Park or protected area and the sampling collection did not involve endangered and protected species. The studied species is not protected by National law or EU legislation. It is endemic in Crete, but not endangered, as it is widespread and common in the central and eastern part of the island.
Protein extraction and fractionation
Total proteins were extracted using a buffer containing 50 mM Tris/HCl (pH 7.5), 1 mM EDTA, 2 mM EGTA, 150 mM NaCl, 1% Triton, 10% glycerol, 1% cellulose, 1% PVP, 250 mM PMSF, and 10 μl protease inhibitors (Sigma-Aldrich Co., St. Louis, MO, P8340-1 ml). The extract was centrifuged at 10 000 rpm, at 4 °C, for 10 min. The supernatant was stored at −80 °C. The concentration of the proteins was estimated using the Lowry assay (Lowry et al., Citation1951).
Protein extracts were dialyzed using a molecular porous membrane tubing (Spectra/Por, Spectrum Labs, Row Irving, TX), in 25 mM Tris/HCl (pH 7.8) buffer at 4 °C, overnight and fractionated using an anion exchange Q-sepharose column (Sigma-Aldrich, St. Louis, MO). Different concentrations of NaCl (100 mM–2 M) in Tris 25 mM were applied to the column and the proteins were collected in 21 fractions: FT (flow through), W1 (wash 1), W2 (wash 2), and fractions 1–18 corresponding to the NaCl gradient from 100 mM to 2 M. The total extraction and fractionation products were visualized by SDS-PAGE (4% stacking gel and 10% separating gel) electrophoresis.
Isolation of T-cell subsets and macrophages
Total spleen cells were cultured in the DMEM culture medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY) for 24 h in Petri dishes at a concentration of 1 × 106 cells/ml. The non-adherent lymphoid cell population was further processed to isolate specific sub-population of cells, while the adherent macrophages were processed to proliferation assays in the presence or not of selected protein fractions. The non-adherent cells were washed 3 × with sterile PBS and were initially submitted to positive selection of B cells using a magnetic bead cell isolation protocol (DYNAL, Oslo, Norway). Thus the cells were incubated with a rat anti-mouse CD45 antibody (Immunotools, Friesoythe, Germany; 1 μg/ml, in PBS-BSA 0.1%) at room temperature (RT) for 40 min. The cells were washed with PBS and a second sheep anti-rat IgG coupled to magnetic beads antibody (DYNAL) was added. The cells were incubated at RT for 30 min under slight stirring and CD45 + cells were eliminated using a magnet. The CD45− cells (T cells) were further centrifuged at 1200 rpm for 6 min, resuspended in DMEM 10% FBS and placed in anti-mouse CD25-coated Petri dishes (10 μg/ml in PBS were incubated overnight at 4 °C; Serotec, Oxford, UK) for 1 h at RT. The cells that adhered to the plastic represented the CD25+ cell population, while the non-adherent cells represented the CD25− cells. Both cell populations were washed with sterile PBS and were further separated into CD4+ and CD4− T cells using an anti-mouse CD4 antibody (1 μg/ml in PBS-BSA 0.1%; Becton Dickinson, San Diego, CA) and the magnetic bead isolation technique as described above. Following this procedure, four T-cell sub-populations were isolated: CD25+CD4+, CD25+CD4−, CD25−CD4+, and CD25−CD4−. These cells were further used in proliferation and ELISA experiments in the presence or not of selected protein fractions. The purity of the separated population was assessed by double immunofluorescence staining using antiCD25-PE-antiCD4-FITC and antiCD25-PE-antiCD8-FITC combinations (1 μg/ml in PBS-BSA 0.1%; Immunotools) as described by Kyvelidou et al. (Citation2009). Flow cytometry analysis revealed a 90–95% purity of the isolated populations (data not shown).
Cell proliferation
Spleen cells from BALB/c mice were cultured for 4 d at the concentration of 1 × 106 cells/well in DMEM 10% FBS culture medium in 96-well plates (Sarstedt, Numbrecht, Germany) in the presence or not of 5 μg/ml of the protein. Eighteen hours prior to harvest, the cultures were pulsed with 1 μCi of 3HTdR/well (NEN-Du Pont, Les Ulis, France). After transferring the cells to cellulose filters, these were put in scintillation fluid (toluene–omnifluor, Omnifluor, Boston, MA, 1.38 g/l, NEN) and counted using an LKB beta-counter (LKB Instruments, Frederick, MD).
Detection of cytokine production
Culture supernatants were tested for their content in cytokines by ELISA. Thus, total spleen cells or isolated T cell sub-populations (1 × 106 cells/ml) were cultured in 24-well plates for 48 h in the presence or not of selected protein fractions (5 μg/ml). Upon culture termination, the supernatants were collected, centrifuged at 1200 rpm for 6 min and analyzed for the presence of IL-2, IL-10, and TNF-α by ELISA as previously described (Kyvelidou et al., Citation2009). The mouse anti-IL-2, anti-IL-10, and anti-TNF-α were purchased from Endogen (Woburn, MA) and used at the concentration of 0.1 μg/ml. The reaction was developed using a secondary anti-mouse IgG coupled to horse radish peroxidase (Santa Cruz, CA)
Statistical analysis
Statistical analysis of the relevant data was performed by Student’s t-test. Results are expressed as the means ± SD (SPSS statistics 19, SPSS Inc., Chicago, IL); p values <0.05 were considered to be significant.
Results
Many agents of plant origin have been described to activate or suppress the function of immune system (Bakuridze et al., Citation1993). Based on the essential oils and metabolites, Phlomis species have been reported for both anti-inflammatory and tonic properties (Aligiannis et al., Citation2004; Amor et al., Citation2009; Shang et al., Citation2011; Tammaro & Xepapadakis Citation1986; Ullah et al., Citation2011). Studies on P. lanata are also limited to essential oils (Couladis et al., Citation2000, Citation2003). The effect of plant proteins on mammalian immune system is a field generally neglected in the literature. The present work was designed to define the immunostimulatory and/or an immunosuppressive effect of protein extracts derived from P. lanata on murine total spleen cells and specific T cell subsets.
Isolation and fractionation of P. lanata protein extracts
Total protein extracts from aerial parts were isolated as described in the section Materials and methods. Low molecular weight proteins and salts were eliminated through dialysis against 25 mM Tris and the sample was loaded onto an anion exchange Q-sepharose column and fractionated using a NaCl gradient at a concentration varying from 0 to 2 M (). Flow through (FT) contained all material that did not react with the column, while W1 and W2 contained proteins that were not specifically bound to the column.
Figure 1. Isolation and fractionation of protein extracts from Phlomis lanata. Protein extracts from the aerial parts of the plant were dialyzed and submitted to ion exchange chromatography using a Q-Sepharose column and a 0–2 M NaCl gradient (A). The nomenclature used for the different fractions is marked at the upper part of the graphic. The isolated fractions were concentrated and submitted to SDS-PAGE electrophoresis (B) revealed either with Coomassie (upper panel) or silver staining (lower panel). Protein concentration in the different fractions was determined by the Lowry assay (C).
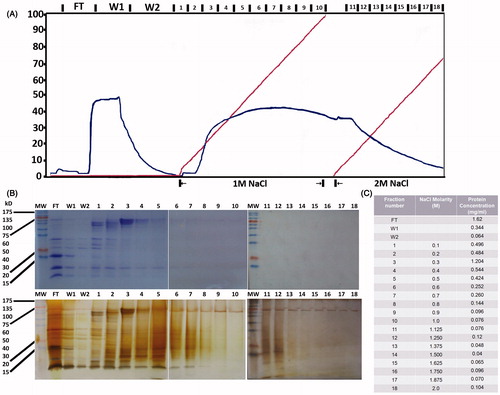
Upon concentration, the protein content of the 18 fractions in addition to FT, W1, and W2 was visualized in SDS-PAGE gels stained with either coomassie blue (, upper panel) or silver staining (, lower panel). Protein concentration was determined using the Lowry assay (). Only proteins eluted with up to 1 M NaCl were used in further experimentation at the concentration of 5 μg/ml. Proteins eluted with 1–2 M NaCl concentrations were at quite low concentrations, only detected after silver staining in the SDS-PAGE analysis. In addition, because of their high ionic force, they were excluded from the in vitro cellular assays. The protein concentration chosen to be used in the cellular assays (5 μg/ml) was quite arbitrary, but within the range of phytohemagglutinin concentrations used (Barta et al., Citation1992) avoiding toxic effects and allowing only specific events to be recorded. Yet in future work, kinetic experiments will define the optimal doses for protein members of the selected fractions.
Effect of fractionated P. lanata protein extracts on immune cell proliferation
As an initial approach, FT, W1, W2, and the fractions collected at molarities 100 mM–1 M NaCl were examined for their proliferative activity on total spleen cells (). Except from FT, W1, and W2 that significantly suppressed spleen cell proliferation (76–90% decrease, p < 0.001), only the fraction eluted with 600 mM NaCl (Fraction 6; F6) significantly increased by 78% cell proliferation as compared with untreated controls (p = 0.0055). The results with all protein fractions were consistent over six different experiments using protein fractions isolated from eight different extractions. Among these fractions, W1 and F6 were chosen for their suppressive and stimulatory activity, respectively, and used in further experimentation.
Figure 2. Effect of Phlomis lanata fractionated protein extracts on proliferation of total spleen cells or isolated T cell subpopulations. All FT, W1, W2, and fractions generated with 100 mM–1 M NaCl were added at the concentration of 5 μg/ml to total spleen cells and proliferation was assessed by 3HTdR uptake experiments (A). In these experiments concanavalin A was used as positive control for T cell proliferation and generated 7602 ± 755 cpm. The W1 and F6 (600 mM) fractions were chosen for further experimentation and added to CD4+ and CD8+ (B1) or CD25+CD4+ and CD25+CD8+ (B2) T cell populations in the context of 3HTdR uptake experiments. The results are expressed as cpm ± SEM. One out of five experiments with similar results is shown here. The asterisks (*) denote a statistically significant difference as compared with the untreated control (*p < 0.005, **p < 0.001).
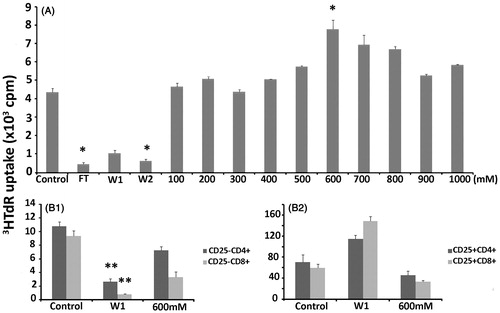
Since the initiation and the regulation of an immune response lie on T cells and their products, the selected fractions were applied on isolated T cell subsets, namely CD25+CD4+, CD25+CD8+, CD25−CD4+, and CD25−CD8+ cells. In all cases, proliferation of CD25− cells was suppressed by both W1 and F6 fractions. Yet, the decrease observed with F6 on CD25−CD4+ cells was not statistically significant (). Interestingly, the W1 fraction increased proliferation of CD25+CD4+ and CD25+CD8+ cells by 62 and 148%, respectively. In addition, F6 decreased proliferation of CD25+CD4+ and CD25+CD8+ cells by 35 and 43%, respectively, which was statistically significant ().
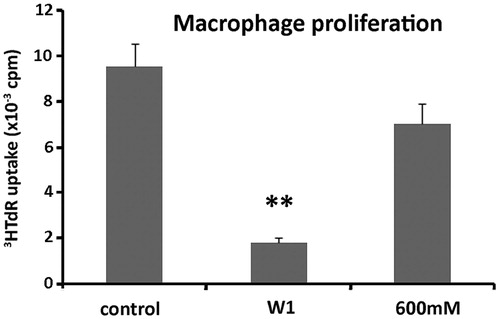
These results indicated that the suppressive effect of W1 proteins targeted all T cell populations tested. Thus, W1 proteins decreased proliferation of effector CD25−CD4+ and CD25−CD8+ cell populations that represent the TH and TCYT cells, respectively, while increasing the suppressive Tregs cell populations. Although the stimulatory effect of the F6 fraction on total spleen cells was not due to increase of proliferation of effector T cells, it can be argued that this was manifested through the suppression of regulatory/suppressive T cells. In order to evaluate whether macrophages could be the target of these proteins, the adherent cell population isolated from total spleen cultures was submitted to proliferation assays in the presence or not of W1 and F6. The results showed that both fractions reduced proliferation (). Therefore, macrophages were not the target of proteins contained in the F6 fraction, which was shown to have a stimulatory effect on total spleen cells. Although the F6 fraction did not seem to play a significant role in immune cell stimulation, W1 was constantly showing a suppressive activity for all T populations as well as macrophages.
Figure 3. Effect of protein fractions W1 and F6 on macrophage proliferation. Macrophages were cultured in the presence or not of W1 and F6 (600 mM) and assessed for proliferation following 3HTdR uptake experiments. The results are expressed as cpm ± SEM. One out of three experiments with similar results is shown here. The asterisks (*) denote a statistically significant difference as compared to the untreated control (**p < 0.001).
Effect of fractionated P. lanata protein extracts on cytokine production
T cell activity is mainly mediated by cytokines, which, depending on their inflammatory (TH1 products) or anti-inflammatory (TH2 products) activity, regulate immune responsiveness. The cytokines tested here included IL-2 (TH1 product), IL-10 (TH2 product), and TNF-α (TH1 product). In total spleen cell culture supernatants, W1 was shown to induce significant production of IL-2 (p < 0.001) and IL-10 (p < 0.001) but not TNF-α (p > 0.005), while F6 could not significantly alter productions of the cytokines tested ().
Figure 4. Effect of protein fractions W1 and F6 on cytokine production by total spleen cells (A) or T cell subpopulations (B1–B4). Total spleen cells or isolated T cell sub-populations were cultured in the presence of W1 or F6 (600 mM) and culture supernatants were tested for their content in IL-2, IL-10, or TNF-α by ELISA. The results are expressed as relative optical density (OD) increase over background ± SEM. One out of six experiments with similar results is shown here. The asterisks (*) denote a statistically significant difference as compared with the untreated control (*p < 0.005, **p < 0.001).
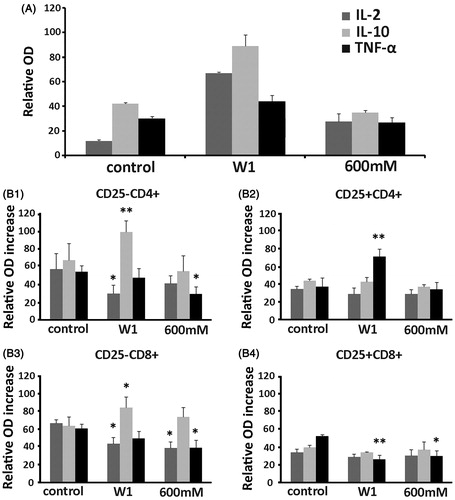
Interestingly, W1 was shown to significantly increase IL-10 and decrease IL-2 in the CD25−CD4+ and CD25−CD8+ cell populations, results that are in accordance with the suppressive effect of W1 on the proliferation assays presented above. This fraction was shown to significantly increase TNF-α production from CD25+CD4+, while decreasing its production by CD25+CD8+ cells. The F6 fraction was found to significantly decrease TNF-α production from CD25−CD4+, CD25−CD8+, and CD25+CD8+ cells, while also decreasing IL-2 production from CD25−CD8+ cells. Macrophages did not seem to be involved in IL-2, IL-10, or TNF-α production, since no statistically significant changes were observed in the presence of W1 or F6 proteins (data not shown).
It has to be noted that in all proliferation and ELISA experiments, the same numbers of cells were used in the different cell types tested. Yet, in the spleen, the relative numbers for the populations CD25−CD4+:CD25−CD8+:CD25+CD4+:CD25+CD8+ were 60:10:1:3 (data not shown), which automatically make the interpretation of the results quite difficult. In addition, the synergistic or antagonistic effects of cytokines cannot be ignored. However, it can be generally concluded that W1 exerted a suppressive effect on the immune system, which is at least mediated by increased IL-10 and decreased IL-2 production, while the F6 fraction by reducing TNF-α production could decrease cytotoxicity and therefore provide an overall stimulatory activity.
Discussion
Phlomis species have been reported for both anti-inflammatory and tonic properties (Aligiannis et al., Citation2004; Amor et al., Citation2009; Shang et al., Citation2011; Tammaro & Xepapadakis, Citation1986; Ullah et al., Citation2011). In fact, tonic properties have been reported for many other plant species. The Rasayana therapy in Ayurvedic medicine encompasses many plant preparations used as tonic drugs to restore and rejuvenate positive health by maintaining organic balance (Atal et al., Citation1986; Agarwal & Singh, Citation1999; Sharma & Khosa, Citation1994). The use of a medicinal botanical as a tonic usually indicates that the botanical has the ability to enhance certain immune responses (Borchers et al., Citation2000). In “tonic-debate”, the generally assumed immunomodulating effect is disputable, since the placebo effect was not clinically tested (Gertsch et al., Citation2011). In addition, it has been considered that the idea of a “toning agent” of plant origin determines the ability of a given group of substances to stimulate the activity of the central nervous system, which does not necessarily involve the immune system (Bakuridze et al., Citation1993). In this study, the immunosuppressive and immunostimulatory role for protein-fractions of P. lanata was examined in a mouse model showing that specific protein extracts of P. lanata exhibit 2-way immunomodulatory activity.
Phlomis lanata aerial parts were submitted to protein extraction and subsequently fractionated through anion exchange chromatography. Cationic proteins that were not retained by the column displayed an immunosuppressive/immunotoxic activity since they were shown to stimulate proliferation of CD25+ regulatory T cells, increase IL-10 and TNF-α release, while decreasing IL-2 production. In mammals, cationic proteins produced by polymorphonuclear cells have been shown to display anti-microbial activity (Zeya & Spitznagel, Citation1966). Similar proteins with enzymatic activity have been reported in plants (Wenzela et al., Citation2014). The results presented here indicate that the effect of cationic proteins could also be mediated indirectly through the immune modulators (i.e., TNF-α). Although the cationic W1 fraction was shown to display an immunosuppressive activity, the anionic F6 fraction was shown to stimulate spleen cell proliferation probably by exerting negative proliferative effect of suppressor T regulatory cells, while decreasing TNF-α production. Noteworthy, in the case of Pelargonium sidoides DC (Geraniaceae), an important species used in traditional medicine in Southern Africa, where moderate direct antibacterial capabilities against a spectrum of Gram-positive and Gram-negative bacteria were accompanied by significant immunostimulatory properties (Kolodziej & Kiderlen, Citation2007), where the results in clinical trials showed significant effectiveness as compared with placebo (Lizogub et al., Citation2007).
It is well known that agents derived from different plant species activate or suppress the function of the immune system. The wide effective spectrum on the immune system spreading from immunostimulation to immunosuppression has already been extensively reported in the literature for agents derived from different plant species (Bakuridze et al., Citation1993). Based on the results presented here, it is essential to note that both functions can be promoted by protein fractions of the same plant species which exhibit two-way immunomodulatory activity. It is worth noticing that the W1 and F6 protein fractions used in the functional studies presented here include several protein bodies and, therefore, the observed effect corresponds to a synergistic/antagonistic effect of included proteins. Although identification of the different protein members in the fractions studied is an important task to pursuit, single purified proteins are not expected to display the same biological effects.
Conclusion
In conclusion, the results presented in the present study showed that the cationic protein fraction of the aerial parts of P. lanata exerts an immunosuppressive activity in vitro through an increase of suppressor T cell populations, IL-10 and TNF-α, while the anionic F6 protein fraction displays an immunostimulatory activity by inhibiting the T suppressor cell growth and decreasing the levels of TNF-α. The same plant can display both immunostimulatory and immunosuppressive activities, so the two-way immunomodulatory activity should not be considered paradoxical anymore.
Acknowledgements
The authors would like to thank Mrs Vlata Chara FORTH (Crete, Greece) for technical assistance in cell harvesting.
Declaration of interest
The authors report that they have no conflicts of interest. The authors declare that there is no financial conflict of interest that might be construed to influence the results or interpretation of this manuscript. This work was supported by the Special Account for Research Resources of the University of Crete through services to the private sector (KA1226). The authors also thank the Special Account for Research Resources of the University of Crete for covering publication costs.
References
- Agarwal SS, Singh VK. (1999). Immunomodulators: A review of studies on Indian medicinal plants and synthetic peptides. Part 1: Medicinal plants. Proc Indian Natl Sci Acad Part B: Biol Sci B65:179–204
- Aligiannis N, Kalpoutzakis E, Kyriakopoulou I, et al. (2004). Essential oils of Phlomis species growing in Greece: Chemical composition and antimicrobial activity. Flavour Fragr J 19:320–4
- Amor ILB, Boubaker J, Sgaier MB, et al. (2009). Phytochemistry and biological activities of Phlomis species. J Ethnopharmacol 125:183–202
- Arai KI, Lee F, Miyajima A, et al. (1991). Cytokines: Coordinators of immune and inflammatory responses. Annu Rev Biochem 59:783–836
- Atal CK, Sharma ML, Kaul A, Khajuria A. (1986). Immunomodulating agents of plant origin. I: Preliminary screening. J Ethnopharmacol 18:133–41
- Azizian D, Moore DM. (1982). Morphological and palynological studies in Phlomis L. Eremostachys Bunge and Paraphlomis Prain (Labiatae). Bot J Linn Soc 85:225–48
- Bakuridze AD, Kurtsikidze MS, Pisarev VM, et al. (1993). Immunomodulators of plant origin (review). Pharm Chem J 27:589–95
- Barta O, Barta V, Pierson FW. (1992). Optimum conditions for the chicken lymphocyte transformation test. Avian Dis 36:945–55
- Borchers AT, Keen CL, Stern JS, Gershwin ME. (2000). Inflammation and Native American medicine: The role of botanicals. Am J Clin Nutr 72:339–47
- Boukef MK. (1986). Les plantes dans la medecine traditionnelle Tunisienne. Paris: Agence de Cooperation Culturelle et Technique
- Cohen MC, Cohen S. (1996). Cytokine function. A study in biologic diversity. Am J Clin Pathol 105:589–98
- Couladis M, Tanimanidis A, Tzakou O, et al. (2000). Essential oil of Phlomis lanata growing in Greece: Chemical composition and antimicrobial activity. Planta Med 66:670–2
- Couladis M, Tzakou O, Verykokidou E, Harvala C. (2003). Screening of some Greek aromatic plants for antioxidant activity. Phytother Res 17:194–5
- Fernandez-Ocana AM, Ortuno-Moya I, Martos-Gilabert AI, Fernandez-Lopez C. (1996). Saber y utilizacion de plantas en la provicia de Jaen. Campana de 1993. Boletin del Instituto de Estudios Giennenses 161:199--318
- Gertsch J, Viveros-Paredes JM, Taylor P. (2011). Plant immunostimulants – Scientific paradigm or myth? J Ethnopharmacol 136:385–91
- Gonzalez-Tejero MR, Molero-Mesa J, Casares-Porcel M, Lirola MJM. (1995). New contributions to the ethnopharmacology of Spain. J Ethnopharmacol 45:157–65
- Khanam M, Abul Hassan MD. (2005). A critical study of the genus Leucas R.Br. (Lamiacea) from Bangladesh. Bangl J Plant Taxon 12:1–10
- Kolodziej H, Kiderlen AF. (2007). In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs 7630. Phytomedicine 14:18–26
- Kyvelidou C, Chatzi K, Semitekolou M, et al. (2009). Characterization of CD25-positive T cells during syngeneic pregnancy: Production of stimulatory class II MHC molecules. Scand J Immunol 70:584–95
- Li M-X, Shang X-F, Jia Z-P, Zhang R-X. (2010). Phytochemical and biological studies of plants from the genus Phlomis. Chem Biodivers 7:283–301
- Lizogub VG, Riley DS, Heger M. (2007). Efficacy of a Pelargonium sidoides preparation in patients with the common cold: A randomized, double blind, placebo-controlled clinical trial. Explore (NY) 3:573–84
- López V, Jäger AK, Akerreta S, et al. (2010). Antioxidant activity and phenylpropanoids of Phlomis lychnitis L.: A traditional herbal tea. Plant Foods Hum Nutr 65:179–85
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–75
- Manandhar NP, Manandhar S. (2002). Plants and People of Nepal. Portland, OR: Timber Press. ISBN: 0-88192-527-6
- Martínez-Francés V, Hahn E, Juan-Vicedo J, et al. (2012). Ethnobotanical study of the sages used in traditional Valencian medicine and as essential oil: Characterization of anendemic Salvia and its contribution to local development. Contrib Sci 8:77–84
- Mosmann TR, Moore KW. (1991). The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today 12:A49–53
- Murphy KM. (2012). Janeway’s Immunobiology, 8th edition, Kindle Edition. Taylor & Francis Group, Informa UK Ltd., ISBN 13: 978-0815342434
- Pardo de Santayana M, Blanco E, Morales R. (2005). Plants known as te in Spain: An ethno-pharmaco-botanical review. J Ethnopharmacol 98:1–19
- Quezel P, Santa S. (1963). Nouvelle flore de l’Algerie et des regions desertiques et meridionales. Paris: Tome II, Editions CNRS
- Rivera Nunez D, Obon De Castro C. (1993). Ethnopharmacology of Murcia (SE Spain), Medicaments et aliments: L’approche ethnopharmacologique. Actes du 2e Colloque Europeen d‘Ethnopharmacologie et de la Ile Conference internationale d‘Ethnomedecine, Heidelberg, 2.4–27 mars, 215–39
- Shang X, Wang J, Li M, et al. (2011). Antinociceptive and anti-inflammatory activities of Phlomis umbrosa Turcz extract. Fitoterapia 82:716–21
- Sharma DN, Khosa RL. (1994). Immunomodulators of plant origin: A review. Anc Sci Life 13:326–9
- Tammaro F, Xepapadakis G. (1986). Plants used in phytotherapy, cosmetics and dyeing in the Pramanda district (Epirus, North-West Greece). J Ethnopharmacol 16:167–74
- Tardio J, Padro-De-Santayana M, Morales R. (2006). Ethnobotanical review of wild edible plants in Spain. Bot J Linn Soc 152:27–71
- Ullah R, Al-Zeghayer YS, Haider S. (2011). Immunomodulatory potential of Phlomis bracteosa. Afr J Pharm Pharmacol 5:1811–12
- Vazquez FM, Suarez MA, Perez A. (1997). Medicinal plants used in the Barros Area, Badajoz Province (Spain). J Ethnopharmacol 55:81–5
- Wenzela M, Chiriacb AJ, Ottoc A, et al. (2014). Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc Natl Acad Sci 111:E1409–18
- Zeya HI, Spitznagel JK. (1966). Cationic proteins of polymorphonuclear leukocyte lysosomes I. Resolution of antibacterial and enzymatic activities. J Bacteriol 91:750–4
