Abstract
Context: Despite the traditional use of Bergia ammannioides Henye ex Roth. (Elatinaceae) for the treatment of wounds in India, there is a scarcity of scientific data supporting this use.
Objective: The objective of this study is to assess wound-healing potentiality of the plant, to study pharmacological activities that may contribute in eliminating wound complications, and to investigate the biologically active fractions.
Material and methods: The ethanolic extract (EtOH) of the aerial parts was fractionated to obtain n-hexane (HxFr), chloroform (ClFr), ethyl acetate (EtFr), and n-butanol (BuOH) fractions. EtOH and its fractions were formulated in strength of 5 and 10% w/w ointment and tested for wound-healing activity using the excision model. The topical anti-inflammatory, in vitro antioxidant, and antibacterial activities were evaluated. HxFr and EtFr were chemically investigated to isolate their constituents.
Results: Application of EtOH, HxFr, and EtFr (10% w/w ointments) leads to 71.77, 85.62, and 81.29% healing of the wounds with an increase in the collagen content. HxFr had the strongest anti-inflammatory (64.5% potency relative to Voltaren®) and antibacterial activity (MIC = 104 μg/ml against Staphylococcus aureus), while EtFr showed the strongest antioxidant activity against DPPH, ABTS•+, and super oxide radical with an IC50 value of 10.25 ± 0.01, 66.09 ± 0.76, and 167.33 ± 0.91 µg/ml, respectively. β-Sitosterol, lupeol, cyclolaudenol, and cycloartenol were isolated from HxFr. Quercetin, ellagic acid, kaempferol-3-O-α-l-rhamnoside, and quercetin-3-O-α-l-rhamnoside were isolated from EtFr.
Discussion and conclusion: Our study presents scientific evidence for the efficacy of B. ammannioides in enhancing wound healing, and the first isolation of cyclolaudenol and cycloartenol from Bergia.
Introduction
Although the concept of developing drugs from plants used in traditional medical systems is an old idea, yet it is a valuable way to produce a medication (Heinrich & Gibbons, Citation2001).
Wound healing involves three overlapping phases: inflammation, cellular proliferation, and remodeling (Clark, Citation1996; Glynn, Citation1981). It involves blood clotting which is an inflammatory response to injury and alteration in the ground substances. Reactive oxygen species (ROS) may also adversely affect wound healing due to their harmful effects on cells and tissues (Aliyeva et al., Citation2004). Inflammation, which constitutes the acute response, results in influx of neutrophils at the wound site. These cells usually produce free radicals (Baboir, Citation1978). Thus, the wound site is rich in ROS along with their derivatives. Free radical scavenging agents play an essential role in the reduction, de-activation, and removal of ROS as well as promoting wound-healing process. Consequently, topical applications of compounds with free radical scavenging properties can protect tissues from oxidative damage and also significantly improve wound healing (Thiem & Grosslinka, Citation2003). Healing is not complete until the disrupted surfaces are firmly knit by collagen (Buffoni et al., Citation1993).
Clean wound is a big issue in the medical field and medical advisors are paying great attention for wound care. Wound care is a simple, but important aspect of medicine. Without proper treatment wounds can develop infections, which can delay healing, or result in disfiguring scars. Topical antibacterial agents are commonly used to facilitate wound healing in patients (Fleischer et al., Citation1997).
Genus Bergia is one of the two genera of plants in the waterwort family, Elatinaceae; it comprises about 15 species of shrubs and sub-shrubs, of which, B. ammannioides Henye ex Roth. is the one growing in Egypt. Bergia species are important medicinal plants in India which are traditionally used for wound healing and applied on sores (Anandjiwala et al., Citation2007a,Citationb). Bergia ammannioides is an annual shrub, it is about 8–35 cm tall, erect, or somewhat decumbent, branched herb. The stem and branches are characterized by being mostly pinkish in color, glandular pubescent, rarely sub-glabrous, the flowers are small, white, and bisexual (Davis & Chase, Citation2004).
Few reports were found about the isolation of gallicin, gallic acid, lupeol, and β-sitosterol from B. suffruticosa (Delile) Fenzl (Anandjiwala et al., Citation2007b), as well as evaluating its free radical scavenging activity (Anandjiwala et al., Citation2007a). Only one report was found that evaluated the free radical scavenging activity and the total phenolics of B. ammannioides, growing in Pakistan (Ur-Rehman et al., Citation2013).
Thus, our objective is to evaluate the wound-healing activity of the aerial parts of B. ammannioides, growing in Egypt, by adopting a bioassay-guided fractionation of the ethanolic extract in order to evaluate its pharmacological activity which may contribute to wound healing or to help in eliminating wound complications such as the topical anti-inflammatory, in vitro antioxidant activities, in addition to antibacterial activity against bacterial species with relevance to wound infections and also to investigate the chemical composition of the biologically active fractions of the plant.
Materials and methods
Plant material
The aerial parts of B. ammannioides were collected in April 2011 from El-Orman Garden, Giza, Egypt. The plant was kindly authenticated by Dr. Mohamed El-Gebaly, Botany Specialist. Voucher specimen (BA-2011-31) is kept in the herbarium of the Pharmacognosy Department, Faculty of Pharmacy, Cairo University.
Plant extraction
The powdered aerial parts (500 g) of B. ammannioides were extracted with 95% ethanol on cold until exhaustion, and the combined ethanolic extract was evaporated to yield 100 g of the ethanolic residue (EtOH). EtOH was suspended in distilled water and partitioned successively using n-hexane, chloroform, ethyl acetate, and n-butanol saturated with water, and each fraction was concentrated under reduced pressure to yield HxFr (45.5 g), ClFr (0.7 g), EtFr (21.4 g), and BuFr (5.8 g), respectively. The ClFr was excluded from any further investigations because of its low yield and unpromising TLC picture.
Chemicals and equipments
Dermazine® cream (silver sulfadiazine) was used as a standard wound-healing drug. Voltaren® gel (Diclofenac sodium) was used as a standard topical anti-inflammatory drug. 2,2-Diphenyl-2-picryl hydrazyl (DPPH), ascorbic acid, nitro blue tetrazolium (NBT), 2,2′-azinobis (3-ethylbenzo-thiazoline-6-sulfonic acid) diammonium salt (ABTS•+), and gallic acid were obtained from Sigma-Aldrich Co., St. Louis, MO. Ofloxacin was obtained from Sigma Pharmaceutical Industries, Quesna, Egypt.
Silica gel H (Merck, Darmstadt, Germany) for vacuum liquid chromatography (VLC), silica gel 60 (70–230) mesh ASTM and silica Rp-18, (Fluka, Steinheim, Germany), and Sephadex LH 20 (Pharmacia, Stockholm, Sweden) for column chromatography (CC) were used. Thin-layer chromatography (TLC) was performed on silica gel GF254-precoated plates (Fluka, Steinheim, Germany). The chromatograms were visualized under UV (at 254 and 366 nm) before and after exposure to ammonia vapor and spraying with AlCl3, as well as after spraying with p-anisaldehyde/sulfuric acid spray reagent. Melting points (uncorrected) were determined on an electrothermal 9100 (Markham, Ontario, Canada). UV spectra were measured using a Shimadzu UV 240 (P/N 204-58000) spectrophotometer (Shimadzu Corporation, Kyoto, Japan). 1H-NMR (300 MHz) and 13C-NMR (75 MHz) were measured on a Varian Mercury-VX-300 instrument (Varian Medical Systems, Inc., Cary, NC). The NMR spectra were recorded in CDCl3 and DMSO-d6 and chemical shifts were given in δ (ppm) relative to TMS as an internal standard.
Ointment preparation
EtOH, HxFr, EtFr, and BuFr of the aerial parts of B. ammannioides were used for the preparation of the ointment for topical application. A 5% (w/w) and 10% (w/w) of each fraction was formulated as an ointment using soft white paraffin base (British Pharmacopeia, Citation1996).
Evaluation of the biological activity
Animals
Adult male rats of Sprague–Dawley strain (130–150 g) and adult Swiss albino mice (20–25 g) of both sexes were used. They were obtained from the laboratory animal facility of the Department of Pharmacology & Toxicology, Faculty of Pharmacy, University of Ain Shams. Animals were housed in steel cages under standard conditions and fed with standard pellets and water ad libitum. All experimental procedures were conducted in accordance with internationally accepted principles for laboratory animal use and care, and were approved by the Ethics Committee (No. 9-031) in accordance with recommendations for the proper care and use of laboratory animals (NIH publication no. 80-23; revised 1978).
Evaluation of topical wound-healing activity
The rats were anaesthetized using the open mask method by ether. The back of each rat was shaved with electric clippers and prepared for the operation. Circular wound of 1.5 cm2 area was produced in the dorsal inter-scapular region of each rat by excising the full thickness skin (Majumdar & Kamath, Citation2005). Sixty-six rats were used in this experiment, which were divided into 11 groups:
Group I: no ointment was applied and served as the control.
Group II: ointment base only was applied and this group served as the vehicle control.
Group III: 5% w/w EtOH ointment was applied.
Group IV: 10% w/w EtOH ointment was applied.
Group V: 5% w/w HxFr ointment was applied.
Group VI: 10% w/w HxFr ointment was applied.
Group VII: 5% w/w EtFr ointment was applied.
Group VIII: 10% w/w EtFr ointment was applied.
Group IX: 5% w/w BuFr ointment was applied.
Group X: 10% w/w BuFr ointment was applied.
Group XI: the standard drug Dermazine® cream was applied.
The treatment in each group was started at the day of the operation and continued till the 10th day. The wounds were covered with appropriate dressings, and the dressings were changed regularly. During changing the dressings, wounds were inspected, measured, and photographed. The wound areas were measured while animals were under anesthesia on the 2nd, 6th, and 10th day after surgery. The progressive changes in wound area were measured with the help of a plantimeter, and wound contraction was expressed as percentage reduction of original wound size [wound area at day 0–wound area at day n/wound area at day 0] × 100.
Biochemical analysis of wound tissue
The animals were anaesthetized on the 2nd, 6th, and 10th day after treatment. Then, the granulation tissue was removed from each wound and the tissue was used for the estimation of total collagen (Woessner, Citation1961).
Topical anti-inflammatory effect
The effect of the extract and fractions on topical acute edema was assessed according to the method of Okoli et al. (Citation2006) using xylene-induced ear edema in mice. The tested samples and the standard (Voltaren® gel) were applied on the anterior surface of the right ear (5 mg/ear) while xylene (0.05 ml) was applied on the posterior surface of the left and the right ears, the left ear was left untreated. Control animals received an equivalent volume of the vehicle (3% v/v Tween 85). Three hours after the application, mice were sacrificed and both ears were removed. By the aid of a cork borer, circular discs (6 mm diameter) were punched out of the ear lobes and weighed. The difference in the weight of discs from the right treated and left untreated ears was calculated and used as a measure of edema (Atta & Alkohafi, Citation1998). The percentage of inhibition of edema was calculated using the following relation:
where Wt is the average weights of edema of the treated group and Wc is the average weights of edema of the control group.
Evaluation of the in vitro antioxidant activity
DPPH assay
The method used by Takao et al. (Citation1994) and modified by Delazar et al. (Citation2004) was adopted; DPPH (4 mg) was dissolved in methanol (50 ml) to obtain a concentration of 80 µg/ml. A serial dilution of the extract and fractions were prepared in methanol (20–1000 µg/ml). Diluted solutions (1.0 ml each) were mixed with 1 ml of DPPH and allowed to stand for 30 min at room temperature. The control sample was prepared by mixing 1.0 ml of DPPH with 1.0 ml methanol. The absorbance was recorded at 517 nm. The experiment was performed in triplicate, and the average absorbance for each concentration was recorded. The same procedure was followed for ascorbic acid to be used as a positive control. The 2,2-diphenyl-1-picrylhydrazyl radicals scavenging effects of test samples were calculated using the following equation:
where AB is the absorbance of the control sample and AA is the absorbance of test sample after 30 min. The IC50 value was calculated as the concentration (µg/ml) of test sample that causes 50% quenching of the UV absorption of DPPH.
ABTS•+ assay
Here the free radical scavenging capability was measured spectrophotometrically by the loss of absorbance of the mono-cation radical 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) at λ 734 nm. ABTS•+ was prepared by mixing an ABTS stock solution (7 mM in water) with 2.45 mM potassium persulfate. This mixture was allowed to stand for 12–16 h at room temperature in the dark until reaching a stable oxidative state (Ozgen et al., Citation2006). On the day of analysis, the ABTS•+ solution was diluted with methanol to an absorbance of 0.700 ± 0.01 at 734 nm. The extract and fractions were tested at the same concentrations as for the DPPH assay. For the spectrophotometric assay, 3 ml of the ABTS•+ solution and 20 µL of standard ascorbic acid or test extract or fractions were mixed and the absorbance was determined at 734 nm. The absorbance at each time point was corrected for the absorbance of an ABTS•+ blank. The % inhibition for each dilution and the IC50 value were calculated as for DPPH assay.
Scavenging of super oxide radical by alkaline DMSO method
To the reaction mixture containing 0.1 ml of NBT (1 mg/ml solution in DMSO) and 0.3 ml of the extract, fractions, and standard ascorbic acid in DMSO, 1 ml of alkaline DMSO (1 ml DMSO containing, 5 mM NaOH in 0.1 ml water) was added to give a final volume of 1.4 ml and the absorbance was measured at 560 nm (Elizabeth & Rao, Citation1990). The % inhibition for each dilution and the IC50 value was calculated as for DPPH assay.
Evaluation of the antimicrobial activity
Following strains were used for testing the antibacterial activity of the extract and fractions: Escherichia coli ATCC10536, Proteus vulgaris NCTC4175, Pseudomonas aeruginosa CNCMA21, Staphylococcus aureus ATCC4175, Bacillus subtilis NCTC6633, Sarcina lutea, and Mycobacterium phlei (laboratory collection). The antibacterial assay was performed by the agar disc diffusion method (Parekh et al., Citation2005). The molten Muller agar was inoculated with 100 µl of the inoculum (1 × 108 CFU/ml) and poured in the Petri dish plate (Hi-media). The disc (0.7) was saturated with 20 µg/ml DMSO of the test fractions and of the standard Ofloxacin, allowed to dry and was introduced on the upper layer of the seeded agar plate. The plates inoculated with Gram (+) and (−) bacteria were incubated at 37 °C for 24–48 h. The microbial growth was determined by measuring the diameter of zone of inhibition. For each bacterial strain, controls were maintained where pure DMSO was used instead of the extracts.
Determination of the minimum inhibitory concentration (MIC)
Stationary phase cultures of S. aureus were prepared at 37 °C and used to inoculate fresh 5.0 ml culture to an OD600 value of 0.05. The 5.0 ml culture was then incubated at 37 °C until an OD600 value was achieved from which standardized bacterial suspensions were prepared to a final cell density of 6 × 105 CFU/ml. Serial dilutions from the tested extract and fractions (0–400 µg/ml) were prepared and mixed with 5.0 ml of the standardized bacterial suspension then added to the plates and incubated for 24 h at 37 °C. The colony forming units (CFU) were counted for each dilution. The MIC value corresponded to the lowest concentration inhibiting observable microbial growth. All experiments were performed in triplicate (NCCLS, Citation2000).
Statistical analysis
The results were expressed as the mean ± standard error (SE). The means were compared using the ANOVA test (MSTATC software, East Lansing, MI, USA) and Duncan’s Multiple range test. Values were determined to be significant when p value was less than 0.01 (p < 0.01).
Purification of the biologically active fractions
HxFr (20 g) was chromatographed over a VLC column (6 × 20 cm, silica gel H, 250 g). Gradient elution was carried out using n-hexane/methylene chloride mixtures and methylene chloride/ethyl acetate mixtures. Fractions of 200 ml each were collected and monitored by TLC to yield three main fractions (AH–CH). Fraction AH (55–60% methylene chloride/n-hexane) was rechromatographed over a silica gel 60 column, using n-hexane/ethyl acetate (97:3 v/v) as an eluent to give compound 1 (257 mg, white needle-shaped crystals, Rf = 0.37 in n-hexane/ethyl acetate 90:10 v/v, mp 213–215 °C). Fraction BH (65% methylene chloride/n-hexane) was similarly purified using n-hexane/ethyl acetate (95:5 v/v) as an eluent to give compound 2 (410 mg, white needle-shaped crystals, Rf = 0.25 in n-hexane–ethyl acetate 90:10 v/v, mp 140–141 °C). Fraction CH (90–100% methylene chloride/n-hexane) was purified in the same way using n-hexane/ethyl acetate (90:10 v/v) as an eluent to give compound 3 (47 mg, white microcrystalline powder, Rf = 0.62 in methylene chloride/methanol 95:5 v/v, mp 124–126 °C) and compound 4 (71 mg, white microcrystalline powder, Rf = 0.35 in methylene chloride/methanol 95:5 v/v, mp 114–116 °C).
Similarly, EtFr (10 g) was fractionated over a Sephadex LH-20 column (25 × 3 cm) using methanol and methanol–water mixtures as an eluent. Fractions were collected and purified over several Sephadex LH-20 and silica Rp-18 columns using methanol–water mixtures as an eluent to yield compound 5: (222 mg, yellow microcrystalline powder, Rf = 0.6 in ethyl acetate/methanol/water 100:16.5:13.5 v/v/v, mp 314–316 °C); compound 6: (88 mg, pale yellow microcrystalline powder, Rf = 0.51 in ethyl acetate/methanol/water 100:16.5:13.5 v/v/v, mp 350 °C); compound 7: (100 mg, yellow microcrystalline powder, Rf = 0.45 in ethyl acetate/methanol/water 100:16.5:13.5 v/v/v, mp 190–192 °C); and compound 8: (52 mg, yellow microcrystalline powder, Rf =0.37 in ethyl acetate/methanol/water 100:16.5:13.5 v/v/v, mp 182–184 °C).
Spectroscopic data of the isolated compounds
Compound 1 (lupeol)
EIMS: (70 eV rel. int.), m/z at 426 [M]+ (12%), 411 (6%), 393 (8%), 207 (43%), 203 (31%), and 189 (100%).
1H-NMR: δ (300 MHz, DMSO) 0.75 (3H, s, Me-24), 0.78 (3H, s, Me-28), 0.82 (3H, s, Me-25), 0.94 (3H, s, Me-27), 0.96 (3H, s, Me-23), 1.02 (3H, s, Me-26), 1.67 (3H, s, Me-30), 3.19 (1H, m, H-3), 4.56 (1H, br.s, H-29a), and 4.68 (1H, br. s, H-29b) ppm.
13C-NMR: δ (75 MHz, DMSO) 14.5 (C-27), 15.3 (C-24), 16.0 (C-26), 16.8 (C-25), 18.3 (C-28), 19.2 (C-30), 21.3 (C-11), 25.1 (C-12), 27.3 (C-2), 27.5 (C-15), 28.0 (C-23), 29.8 (C-21), 34.3 (C-7), 35.5 (C-16), 37.1 (C-10), 38.0 (C-13), 38.7 (C-1), 38.8 (C-4), 39.9 (C-8), 40.1 (C-22), 42.9 (C-14), 42.9 (C-17), 47.9.23 (C-18), 48.2 (C-19), 55.2 (C-5), 79.0 (C-3), 109.2 (C-29), 150.8 (C-20).
Compound 2 (β-sitosterol)
MS (EI, 70 eV): m/z (%) = 414 [M]+ (100%), 396 (51%), 329 (42%), 303 (44%), 273 (60%), and 255 (80%).
1H-NMR: δ (300 MHz, CDCl3) 0.68 (3H, s, Me-18), 0.70 (3H, d, J = 4 Hz, Me-21), 0.80 (3H, t, J = 3 Hz, Me-29), 0.85 (3H, d, J = 3 Hz, Me-26), 0.91 (3H, d, J = 3 Hz, Me-27), 1.01 (3H, s, Me-19), 3.51 (1H, m, H-3), 5.34 (1H, br.s., H-6) ppm.
Compound 3 (cyclolaudenol)
MS (EI, 70 eV): m/z (%) = 426 [M]+ (73%), 407 (49%), 379 (18%), 353 (33%), 315 (27%), 300 (100%), and 175 (70%).
1H-NMR: δ (300 MHz, CDCl3) 0.33 (1H, d, J = 4.2 Hz, 19a), 0.55 (1H, d, J = 4.2 Hz, 19b), 0.81 (3H, s, Me-29), 0.88 (3H, d, J = 6.3 Hz, Me-21), 0.90 (3H, s, C-28), 0.97 (3H, s, Me-30), 0.97 (3H, s, Me-18), 1.26 (3H, d, J = 7 Hz, Me-24), 1.73 (3H, s, Me-26), 3.25 (1H, m, H-3), 4.84 (1H, br.s, H-27a), 4.93 (1H, br.s, H-27b).
13C-NMR: δ (75 MHz, DMSO) 13.9 (C-29), 17.9 (C-18), 18.2 (C-21), 18.6 (C-26), 19.2 (C-28), 19.9 (C-9), 20.1 (24-CH3), 21.0 (C-6), 25.3 (C-30), 25.9 (C-11), 26.0 (C-10), 26.4 (C-16), 28.0 (C-7), 29.8 (C-19), 30.3 (C-2), 31.6 (C-23), 31.9 (C-15), 35.5 (C-22), 35.9 (C-20), 40.4 (C-4), 41.6 (C-24), 45.2 (C-13), 47.0 (C-5), 47.9 (C-8), 48.7 (C-14), 52.1 (C-17), 78.8 (C-3), 110.8 (C-27), 150.0 (C-25).
Compound 4 (cycloartenol)
MS (EI, 70 eV): m/z (%) = 426 [M]+ (54%), 411 (50%), 408 (70%), 393 (62%), 365 (27%), 339 (25%), 315 (9%), 286 (59%), 175 (41%), and 97 (100%).
1H-NMR: δ (300 MHz, CDCl3) 0.33 (1H, d, J = 4.2 Hz, 19a), 0.56 (1H, d, J = 4.2 Hz, 19b), 0.82 (3H, s, Me-29), 0.88 (3H, d, J = 6.3 Hz, Me-21), 0.89 (3H, s, Me-28), 0.99 (3H, s, Me-30), 0.97 (3H, s, Me-18), 1.60 (3H, s, Me-27), 1.62 (3H, s, Me-26), 3.25 (1H, m, H-3), 5.5 (1H, m, H-24).
13C-NMR: δ (75 MHz, DMSO) 13.9 (C-29), 17.5 (C-27), 18.0 (C-18), 18.4 (C-21), 19.2 (C-28), 19.9 (C-9), 21.0 (C-6), 25.3 (C-23), 25.3 (C-30), 25.9 (C-7), 26.0 (C-10), 26.4 (C-11), 28.1 (C-16), 29.6 (C-19), 30.3 (C-2), 35.5 (C-15), 35.7 (C-20), 36.7 (C-22), 40.4 (C-4), 45.2 (C-13), 47.0 (C-5), 47.9 (C-8), 48.7 (C-14), 52.1 (C-17), 78.8 (C-3), 129 (C-24), 133.9 (C-25).
Compound 5 (quercetin)
UV λmax nm (MeOH): 256, 272 (sh.), 302 (sh.), 372; NaOMe: 248 (sh.), 332; AlCl3: 272, 308 (sh.), 335, 450; AlCl3/HCl: 268, 304 (sh.), 360, 428; NaOAC: 272, 320, 406; NaOAC/H3BO3: 260, 306 (sh.), 386.
1H-NMR: δ ppm (300 MHz, DMSO), 6.18 (1H, d, J = 1.8 Hz, H-6), 6.40 (1H, d, J = 1.8 Hz, H-8), 6.87 (1H, d, J = 8.1 Hz, H-5′), 7.62 (1H, d, J = 1.2 Hz, H-2′), 7.51 (1H, d, J = 8.1, 2.1 Hz, H-6′).
13C-NMR: δ ppm (75 MHz, DMSO) 175.7 (C-4), 163.9 (C-7), 160.5 (C-5), 156.0 (C-9), 156.0 (C-2), 147.6 (C-4′), 144.9 (C-3′), 135.6 (C-3), 121.8 (C-1′), 119.9 (C-6′), 115.5 (C-5′), 114.9 (C-2′), 102.8 (C-10), 98.1 (C-6), 93.3 (C-8).
Compound 6 (ellagic acid)
1H-NMR: δ ppm (300 MHz, DMSO), 7.51 (1H, s, H-5′), 7.78 (1H, s, H-5).
13C-NMR: δ ppm (125 MHz, DMSO), 107.3 (C-1), 111.5 (C-5), 113.0 (C-1′, 6′), 113.3 (C-5′), 114.5 (C-6), 135.7 (C-2), 140.5 (C-3′), 140.7 (C-3), 141.8 (C-2′), 147.1 (C-4), 152.6 (C-4′), 158.5 (C-7′), 158.6 (C-7).
Compound 7 (kaempferol-3-O-α-l-rhamnoside)
UV λmax nm (MeOH): 268, 350; NaOMe: 274, 324sh, 400; AlCl3: 274, 302sh, 396; AlCl3/HCl: 274, 300sh, 394; NaOAC: 274, 382; NaOAC/H3BO3: 258, 350.
1H-NMR: δ ppm (300 MHz, DMSO), 0.80 (3H, d, J = 6.3 Hz, 6″-Me), 5.29 (1H, d, J = 1.2 Hz, H-1″), 6.20 (1H, d, J = 1.8 Hz, H-6), 6.40 (1H, d, J = 1.8 Hz, H-8), 6.69 (2H, d, J = 8.7 Hz, H-3′, 5′), 7.73 (2H, d, J = 8.7 Hz, H-2′, 6′).
13C-NMR: δ ppm (125 MHz, DMSO), 17.3 (C-6″), 70.1 (C-5″),70.4 (C-2″), 70.5 (C-3″), 71.5 (C-4″), 95.9 (C-8), 98.9 (C-6), 101.1 (C-1″), 103.7 (C-10), 112.5 (C-3′,5′), 120.8 (C-1′), 127.7 (C-2′,6′), 131.3 (C-3), 153.6 (C-2), 154.3 (C-9), 157.1 (C-4′), 158.4 (C-5), 161.7 (C-7), 174.8 (C-4).
Compound 8 (quercetin-3-O-α-l-rhamnoside)
UV λmax nm (MeOH): 257, 362; NaOMe: 272, 327sh, 409; AlCl3: 275, 305sh, 438; AlCl3/HCl: 270, 350sh, 395; NaOAC: 273, 324sh, 380; NaOAC/H3BO3: 262, 298sh, 377.
1H-NMR: δ ppm (300 MHz, DMSO), 0.82 (3H, d, J = 6.3 Hz, 6″-Me), 5.24 (1H, d, J = 1.2 Hz, H-1″), 6.1 (1H, d, J = 2.1 Hz, H-6), 6.38 (1H, d, J = 1.8 Hz, H-8), 6.86 (1H, d, J = 8.4 Hz, H-5′), 7.24 (1H, dd, J = 2.1 and 8.4 Hz, H-6′), 7.30 (1H, d, J = 2.1 Hz, H-2′).
13C-NMR: δ ppm (125 MHz, DMSO), 17.6 (C-6″), 70.2 (C-5″), 70.5 (C-2″), 70.7 (C-3″), 71.3 (C-4″), 93.8 (C-8), 98.9 (C-6), 101.9 (C-1″), 104.1 (C-10), 115.6 (C-2), 115.8 (C-5′), 120.9 (C-6′), 121.2 (C-1′), 134.3 (C-3), 145.3 (C-3′), 148.5 (C-4′), 156.6 (C-2), 157.4 (C-9), 161.4 (C-5), 164.0 (C-7), 177.4 (C-4).
Results
Wound-healing activity
The ointments formulated from EtOH, HxFr, and EtFr at both 5% and 10% concentration showed significant activities at p < 0.01 relative to the control and other tested fractions. The activities were dependent on the concentration. HxFr (10% ointment) showed the most pronounced activity ( and ). Meanwhile, BuFr showed weak activity.
Figure 1. The effect of the HxFr ointment (10% w/w) of B. ammannioides on the wound induced on male albino rats is shown. (A) At zero time; (B) 2nd day; (C) 6th and 10th day.
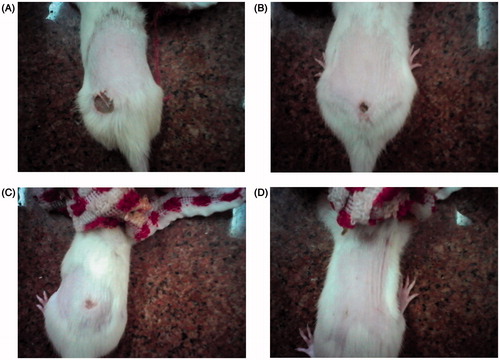
Table 1. Effect of the ethanol extract and fractions of B. ammannioides Henye ex Roth. on wound area in male albino rats.
Effect on total collagen content of the granulation tissue
The topical application of ointments formulated with EtOH, HxFr, and EtFr at both 5% and 10% concentrations significantly increased the content of collagen of the granulation tissue by the 6th and 10th day as compared with control (). BuFr showed non-significant activity.
Table 2. Effect of the ethanol extract and fractions of B. ammannioides Henye ex Roth. on total collagen content of granulation tissue on post-wounding days.
Topical anti-inflammatory activity
The EtOH and its fractions (HxFr and EtFr) inhibited topical edema induced by xylene in the mouse ear (). On one hand, HxFr showed the highest inhibitory activity (64.5% relative to voltaren®), so its major component β-sitosterol (compound 2) was also tested and showed the greatest inhibitory action (83.8% relative to voltaren®). On the other hand, BuFr showed very weak activity.
Table 3. Results of the topical anti-inflammatory activity of the extract, fractions, and the major isolated compound of B. ammannioides Henye ex Roth.
The in vitro antioxidant activity
The EtOH, HxFr, and EtFr showed free radical scavenging activities against DPPH, ABTS+•, and super oxide radicals (). EtFr was the most active fraction by showing the lowest IC50 values. BuFr almost had no free radical scavenging activity.
Table 4. In vitro antioxidant activity of the extract and fractions of B. ammannioides Henye ex Roth.
The antimicrobial activity and MIC evaluation
EtOH, HxFr, and EtFr showed the highest antibacterial activity against S. aureus (), meanwhile BuFr showed weak activity. On one hand, HxFr showed the most potent antibacterial activity on the basis of MIC values, as it had an MIC value of 104 μg/ml, on the other hand, EtOH and EtFr showed an MIC value of 178 and 152 μg/ml.
Table 5. The results of antibacterial activity of the extract and fractions of B. ammannioides Henye ex Roth.
Purification of the biologically active fractions
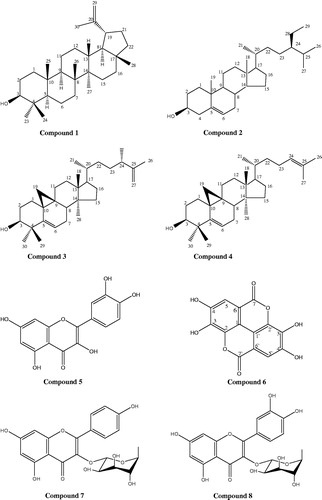
The results of the biological activities indicated that HxFr and EtFr are the most active fractions, so they were purified to isolate their major compounds. Lupeol (1), β-sitosterol (2), cyclolaudenol (3), and cycloartenol (4) were isolated from HxFr. Quercetin (5), ellagic acid (6), kaempferol-3O-α-L-rhamnoside (7), and quercetin-3-O-α-l-rhamnoside (8) were isolated from EtFr. Identification of the isolated compounds depended on comparing their spectral data with the published literature (Goad & Akihisa, Citation1997; Mabry et al., Citation1970; Markham, Citation1982; Öksüz et al., Citation2002; Yan & Guo Citation2004). Structures of the isolated compounds are shown in .
Discussion
Medicinal plants have been reported to be very beneficial in wound care, promoting the rate of wound healing with minimal scarring to the patient (Kumar et al., Citation2007). The different phases of the wound-healing process overlap and ideally a plant-based remedy should affect at least two different processes before it can be said to have some scientific support for its traditional use (Kumar et al., Citation2007).
This study is, therefore, provided a basis for the traditional use of the aerial parts of B. ammannioides as a remedy for wound healing and to control the complications threatening the wounded tissues. In order to assess the bioactivities of B. ammannioides, fractions of increasing polarities were prepared from the total ethanolic extract (EtOH) and tested for wound-healing activity using the excision model. Evaluation of topical anti-inflammatory activity, in vitro antioxidant, and anti-microbial tests was also performed.
The 5 and 10% w/w ointments of EtOH showed significant wound-healing activity when compared to that of the control (i.e., simple ointment-treated group), their effects started at the 2nd day onwards, and were comparable to that of Dermazine®. The 10% w/w EtOH ointment-treated animals achieved 71.77% wound closure by the 10th day. Wound healing is the restoration of the continuity of injured tissues. Collagen plays a crucial role in healing of wounds of skin and other tissues. There is a general agreement that synthesis of collagen is required for the restoration of physical strength of wounds. Evaluation of collagen content in wound tissues of control and treated groups obviously confirms that the application of ointments formulated with EtOH at both 5% and 10% concentrations promotes collagen synthesis and deposition. Moreover, EtOH exerted 54.8% potency in topical anti-inflammatory activity relative to Voltaren gel®. It also showed free radical scavenging activity against DPPH, ABTS•+, and super oxide anion and antimicrobial activity.
Consequently, the subfractions of increasing polarity prepared from EtOH were also tested for wound-healing activity. The wound closure time was the least in the group treated with HxFr (10% ointment), the percentage of closure by the 10th day was 85.62% which was almost close to that of Dermazine®. The 10% w/w EtFr ointment-treated animals also showed significant wound healing from the 2nd day onwards and achieved 81.29 of the wound closure by the 10th day. HxFr and EtFr at both 5% and 10% concentrations promoted collagen synthesis and deposition.
Among the tested subfractions, on one hand, HxFr showed the highest topical anti-inflammatory activity (64.5% potency relative to Voltaren gel®) and antimicrobial activity (MIC value of 104 μg/ml against S. aureus one of the bacterial species with relevance to wound infections). On the other hand, EtFr showed the highest free radical scavenging activity against DPPH, ABTS•+, and super oxide anion (an IC50 value of 86.00 ± 0.12, 219.56 ± 0.21, and 313.34 ± 0.18 µg/ml).
Purification of HxFr yielded four compounds: lupeol (1), β-sitosterol (2), cyclolaudenol (3), and cycloartenol (4). Compounds 1 and 2 were isolated before from B. suffruticosa growing in India (Anandjiwala et al., Citation2007b), but here we report the isolation of cyclolaudenol (3) and cycloartenol (4) from genus Bergia for the first time.
The spectral data of compound 3 showed the characteristic signals of a 24-methyl cycloartane triterpene (Öksüz et al., Citation2002) with a 31 carbon, seven methyls, and a side chain terminating in an isopropenyl group, which was indicated from the signals of two olefenic protons at δH 4.84 and 4.93 (br s, 2H-27) both correlated to carbon signal at δC 110.8 (C-25 and C-27), this was assigned from the HMBC. In addition to the downfield shift of the methyl signal at δH 1.73 (s, H3-26) and δC 18.6 (C-26), the C-24 methyl group appeared at δH 1.26 and δC 20.1, this was assigned from the correlations in the HSQC. Compound 3 was identified as 24-methyl-5-α-cycloartenol-25-en-3β-ol (cyclolaudenol) (Goad & Akihisa, Citation1997). Compound 4 also showed the characteristic signals of a cycloartane triterpene, with the presence of an olefinic proton appeared as a multiplete at δH 5.50 (H-24) which is directly correlated to carbon signal δC 129 (C-24) in HMQC. The position of the double bond between C-24 and C-25 was confirmed from the long-range correlation (HMBC) between the methyl protons at δH 1.60 and 1.62 (Me-27 and Me-26) and the carbon signal at δC 133.9 (C-25). From the aforementioned data and by comparison with the reported data (Goad & Akihisa, Citation1997; Öksuz et al., Citation2002), compound 4 could be identified as 5α-cycloart-24-en-3β-ol (cycloartenol). The major isolated compound β-sitosterol was subjected to evaluation of its topical anti-inflammatory activity against topical edema induced by xylene in the mouse ear and it had the highest potency (83.8% potency relative to Voltarene gel©). It was also reported that β-sitosterol possesses antibacterial activity against S. aureus (MIC 5 μg/ml) (Hoskeri et al., Citation2012). β-Sitosterol was also reported to exhibit significant wound-healing potential (Saleem, Citation2009).
Purification of EtFr yielded quercetin (5), ellagic acid (6), kaempferol-3-O-α-l-rhamnoside (7), and quercetin-3-O-α-l-rhamnoside (8), and it is reported here for the first time in genus Bergia. Phenolic compounds are known to promote the wound-healing process mainly due to their astringent and antioxidant properties (Narendhirakannan et al., Citation2012).
The wound-healing activity of the aerial parts of B. ammannioides can be possibly a synergistic effect of its constituents, for instance, β-sitosterol, which was reported to produce rapid re-epithelialization of wounds (Jewo et al., Citation2009), lupeol, which was reported to have a topical anti-inflammatory activity (Saleem, Citation2009), quercetin and its glycosides, which were reported to increase the wound contraction rate (Gomathi et al., Citation2003; Manivannan et al., Citation2014), in addition to other phenolic compounds with free radical scavenging activity. These help in improving wound healing and protect tissues from oxidative damage by reducing the levels of free radicals.
Conclusion
This study highlighted the wound-healing activity of the crude extract and fractions of B. ammannioides. The anti-inflammatory, antioxidant, and antibacterial activities of the plant may contribute in the elimination of the complications associated with the wounds. The constituents like phenolics, sterols, and triterpenes may possibly play a major role in promoting the wound-healing process through their antioxidant, astringent, and antimicrobial properties which seem to be responsible for re-epithelialization of injured tissues.
Acknowledgements
The authors are deeply thankful to Dr. Mariane Tadros, Department of Pharmacology, Faculty of Pharmacy, Ein Shams University, Egypt, for carrying out the biological experiments. Also, the authors appreciate the cooperation of the Microanalytical Center, Faculty of Science, Cairo University in evaluating the antimicrobial activities.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Aliyeva E, Umur S, Zafer E, Acigoz G. (2004). The effect of polylactide membranes on the levels of reactive oxygen species in periodontal flaps during wound healing. Biomaterials 25:4633–7
- Anandjiwala S, Srinivasa H, Kalola J, Rajani M. (2007a). Free-radical scavenging activity of Bergia suffruticosa (Delile) Fenzl. Nat Med 6:59–62
- Anandjiwala S, Srinivasa H, Rajani M. (2007b). Isolation and TLC Densitometric quantification of gallicin, gallic acid, lupeol and β-sitosterol from Bergia suffruticosa, a hitherto unexpected plant. Chromatographia 66:725–34
- Atta AH, Alkohafi A. (1998). Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plants extracts. J Ethnopharmacol 60:117–24
- Baboir BM. (1978). Oxygen dependent microbial killing by phagocytes (first of two parts). N Eng J Med 298:629–68
- British Pharmacopoiea. (1996). Simple Ointment. London: HMSO, 1360
- Buffoni F, Bancheli G, Cambi S, et al. (1993). Skin wound healing: Some biochemical parameters in Guinea pig. J Pharmaceutics Pharmacol 45:784–90
- Clark RAF. (1996). Wound repair: Overview and general consideration. In: Clark RA, Henson PM, eds. Molecular and Cellular Biology of Wound Repair. New York: The Plenum Press, 112–13
- Davis CC, Chase MW. (2004). Elatinaceae are sister to Malpighiaceae; Peridiscaceae belong to Saxifragales. Am J Bot 91:262–73
- Delazar A, Byres M, Gibbons S, et al. (2004). Iridoid glycosides from Eremostachys glabra. J Nat Prod 67:1584–7
- Elizabeth K, Rao MNA. (1990). Oxygen radical scavenging activity of curcumin. Int J Pharm 58:237–40
- Fleischer AB, Feldman SR, White RE, et al. (1997). Procedures for skin diseases performed by physicians in 1993 and 1994: Analysis of data from the national ambulatory medical care survey. J Am Acad Dermatol 37:719–24
- Glynn LE. (1981). The pathology of scar tissue formation. In: Glynn LE, ed. Handbook of Inflammation, vol. 3. Tissue Repair and Regeneration. Amsterdam: Elsevier/North Holland Biomedical Press, 34–5
- Goad J, Akihisa, T. (1997). Analysis of Sterols, 1st edn. London: Blackie Academic and Professional Press, Champan and Hall
- Gomathi K, Gopinath D, Rafiuddin AM, Jayakumar R. (2003). Quercetin incorporated collagen matrices for dermal wound healing processes in rats. Biomaterials 24:2767–72
- Heinrich M, Gibbons S. (2001). Ethnopharmacology in drug discovery: An analysis of its role and potential contribution. J Pharmacy Pharmacol 53:425–32
- Hoskeri YH, Krishna V, Jignesh S, et al. (2012). In-silico drug designing using β-sitosterol isolated from Flaveria trinervia against peptide deformylase protein to hypothesize bactericidal effect. Int J Pharmacy Pharm Sci 4:192–6
- Jewo PI, Fadeyibi IO, Babalola OS, et al. (2009). A comparative study of the wound healing properties of moist exposed burn ointment (Mebo) and silver sulphadiazine. Ann Burns Fire Disasters XXII:79–82
- Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. (2007). Ethnopharmacological approaches to wound healing – Exploring medicinal plants of India. J Ethnopharmacol 114:103–13
- Mabry TJ, Markham KR, Thomas MB. (1970). The Systematic Identication of Flavonoids. Berlin: Springer-Verlag
- Manivannan R, Prabakaran K, Ilayaraja S. (2014). Isolation, identification and antibacterial and wound healing studies of quercetin-3-o-α-l-rhamnopyranoside-2″-gallate. Int J Appl Sci Eng 12:99–106
- Markham KR. (1982). Techniques of Flavonoid Identification. London: Academic Press
- Majumdar MR, Kamath JV. (2005). Herbal concept on wound healing. J Pharmaceut Res 4:01–07
- Narendhirakannan RT, Nirmala JG, Caroline A, et al. (2012). Evaluation of antibacterial, antioxidant and wound healing properties of seven traditional medicinal plants from India in experimental animals. Asian Pac J Trop Biomed 2:S1245–53
- NCCLS (National Committee for Clinical Laboratory Standards). (2000). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically – 5th edn. Approved Standard M7-A5. Wayne (PA): NCCLS
- Ozgen M, Reese RN, Tulio AZ, et al. (2006). Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J Agric Food Chem 54:1151–7
- Okoli CO, Akah PA, Ezugworie U. (2006). Anti-inflammatory activity of extracts of root bark of Securidaca longipedunculata Fres (Polygalaceae). Afr J Trad Compl Alter Med 3:54–63
- Öksüz S, Ulubelen A, Barla A. (2002). Terpenoids and aromatic compounds from Euphorbia heteradena. Turk J Chem 26:457–63
- Parekh J, Nair R, Chanda S. (2005). Preliminary screening of some folklore medicinal plants from western India for potential antimicrobial activity. Ind J Pharmacol 37:408–9
- Saleem M. (2009). Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett 285:109–15
- Takao T, Watanabe N, Yagi I, Sakata K. (1994). A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotech Biochem 58:1780–3
- Thiem B, Grosslinka O. (2003). Antimicrobial activity of Rubus chamaemorus leaves. Fitoter 75:93–5
- Ur-Rehman A, Siddiqa A, Abbasi MA, et al. (2013). Bergia ammannioides: Phytochemical screening, antioxidant activity and radical scavenging effects of its various fractions. Asian J Chem 25:7921–6
- Yan XH, Guo YW. (2004). Two newellagic acid glycosides from leaves of Diplopanax stachyaanthus. J Asian Nat Prod Res 6:271–6
- Woessner JF. (1961). The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 93:440–7