Abstract
Context: Trichomonosis, caused by the flagellate protozoan Trichomonas vaginalis, is the most common non-viral sexually transmitted disease (STD) and 5-nitroimidazole drugs are used for the treatment. However, a growing number of T. vaginalis isolates are resistant to these drugs, which make it becomes an urgent issue.
Objective: The current study was designed to evaluate the anti-T. vaginalis activity of the essential oil from A. tsao-ko used in traditional Chinese medicine and as a spice and its main component, geraniol.
Materials and methods: The anti-T. vaginalis activities of A. tsao-ko essential oil and geraniol were evaluated by the minimum lethal concentration (MLC) and 50% inhibitory concentration (IC50) in vitro. The morphological changes of T. vaginalis were observed by transmission electron microscopy (TEM). Additionally, sub-MLC concentration treatment with sub-MLC A. tsao-ko essential oil and geraniol was also performed.
Results: This study shows that MLC/IC50 of A. tsao-ko essential oil was 44.97 µg/ml/22.49 µg/ml for T. vaginalis isolate Tv1, and 89.93 µg/ml/44.97 µg/ml for T. vaginalis isolate Tv2. Those of geraniol were 342.96 µg/ml/171.48 µg/ml, respectively. After A. tsao-ko essential oil or geraniol treatment, obvious similar morphological changes of T. vaginalis were observed by TEM: the nuclear membrane was damaged, nuclei were dissolved, and the chromatin was accumulated; in the cytoplasm, numerous vacuoles appeared, rough endoplasmic reticulum dilated, the number of ribosomes were reduced, organelles disintegrated, the cell membrane was partially damaged, with cytoplasmic leakage, and cell disintegration was observed. The action time did not increase the effect of A. tsao-ko essential oil or geraniol against T. vaginalis, as no significant difference was observed after sub-MLC concentration treatment for 1, 3, and 5 h with A. tsao-ko essential oil and geraniol.
Discussion and conclusion: The study describes the first report on the activity and morphological changes of A. tsao-ko essential oil and geraniol against T. vaginalis. The results obtained herein presented new opportunities for antitrichomonal drugs.
Introduction
Trichomonosis, caused by the parasitic protozoan Trichomonas vaginalis, is the most prevalent non-viral sexually transmitted disease (STD) worldwide with 248 million new cases annually [World Health Organization (WHO), Citation2010)]. The infection has been associated with serious health consequences including adverse pregnancy outcomes (Cotch et al., Citation1997), acute infections associated with pelvic inflammatory disease (Cherpes et al., Citation2006), increased risks of infertility (Goldstein et al., Citation1993), and facilitation of HIV infection acquisition (Van Der Pol et al., Citation2008). So far, 5-nitroimidazole drugs have been used in the treatment of trichomonosis. Metronidazole and tinidazole were the two drugs of choice recommended by the United States Food and Drug Administration for the treatment of this STD (Helms et al., Citation2008). Although the cure rate was high, these drugs demonstrated treatment failure which was normally related to non-compliance because of side effects such as headache, dry mouth, metallic taste, glossitis, and urticaria caused by lengthy treatment or high doses (Garduño-Espinosa et al., Citation1992; Kapoor et al., Citation1999; Oxberry et al., Citation1994), or reinfection (Rocha et al., Citation2012). Moreover, an increasing number of metronidazole-resistant T. vaginalis isolates have been reported since metronidazole was introduced for the treatment of trichomoniasis in 1959 (Blaha et al., Citation2006; Butler et al., Citation2010; Durel et al., Citation1967; Narcisi & Secor, Citation1996; Schmid et al., Citation2001; Upcroft et al., Citation2006). In addition, the metronidazole-resistant isolates were cross-resistant to other members of the 5-nitrothiazole compounds including ornidazole, tinidazole, nitazoxanide, and furazolidone (Wright et al., Citation2010). Therefore, to improve the current chemotherapy against T. vaginalis infection, the natural products with high effectiveness and low toxicity may be an alternative therapeutic source.
Amomum tsao-ko Crevost et Lemaire (tsao-ko), called “Caoguo” or “tsao-ko”, which belongs to the family Zingiberaceae, was used as spice in Chinese cuisine and Chinese Traditional Medicine for the treatment of stomach disorders and throat infection (Yu et al., Citation2010). A. tsao-ko exhibited a wide range of biological activities, such as antimicrobial, antioxidant, and cytotoxic activities, as well as plasma and liver triacylglycerol decreasing activity (Martin et al., Citation2000; Moon et al., Citation2004, Citation2005; Yang et al., Citation2008, Citation2009; Yu et al., Citation2010). In a previous study, we found that A. tsao-ko essential oil from the dried fruit of tsao-ko cultured in Yunnan, China, had antibacterial effects (Peng et al., Citation2010). Geraniol shown to be one of the main components of A. tsao-ko essential oil as determined by GC-MS with a content of 13.69%. Geraniol is a widely used fragrance ingredient (Lapczynski et al., Citation2008). It also demonstrated pharmaceutical activities such as antimicrobial activity (Mendonça et al., Citation2009), restoring antibiotic activity against multidrug-resistant isolates (Lorenzi et al., Citation2009), insecticidal (Barros et al., Citation2009; Khallaayoune et al., Citation2009), and antitumor activity (Kim et al., Citation2011). In this study, the anti-T. vaginalis activities of A. tsao-ko essential oil and geraniol were assessed. The morphological changes of T. vaginalis after pretreatment with A. tsao-ko essential oil or geraniol were also studied by transmission electron microscopy (TEM).
Materials and methods
Drugs, media, and reagents
Geraniol was obtained from Sigma (St. Louis, MO) (Lot: MKBH7931V). Metronidazole was purchased from Sichuan Kelun Pharmaceutical Co., Ltd (Chengdu, China) (Lot: M11120602). Jieeryin was purchased from Sichuan Enwei Pharmaceutical Co., Ltd (Chengdu, China) (Lot: 1107102). Trichomonas medium No. 2 (each liter nutrient solution containing 13.6 g pancreatic digest of casein, 2.4 g papaic digest of soybean meal, 4.0 g sodium chloride, 2.0 g di-basic potassium phosphate, 2.0 g glucose, 14.4 g liver peptone, 16.0 g dextrose, 0.05 g calcium d-pantothenic acid, 0.125 g chloramphenicol, 200 ml horse serum) was purchased from OXOID Co., Ltd (Adelaide, South Australia). Polysorbate 80 and phosphate buffer were purchased from Chengdu Changzheng Huabo Co., Ltd (Chengdu, China). Other reagents were purchased from Sigma, Shanghai, China.
Origin and preparation of T. vaginalis
Two isolates of T. vaginalis (Tv1, Tv2) were obtained from vaginal secretions of out-patients at Sichuan Provincial Maternal and Child Health Hospital, Chengdu, China in 2012, and identified by optical microscopy and TEM.
Trichomonas vaginalis suspensions were prepared as described by Moon et al. (Citation2006) with modifications. T. vaginalis was axenically cultured at 37 °C in trichomonas medium No. 2. The growth of T. vaginalis was monitored using the trypan blue exclusion method (Mishell & Shiigi, Citation1980). Trichomonas vaginalis was harvested at the mid-logarithmic phase with more than 95% viable cells by centrifugation at 500 rpm for 10 min, and was adjusted to 2 × 105 cells/ml by pre-warmed fresh medium at 37 °C. The survival rate was more than 95% as observed by microscopy.
Preparation of essential oil from A. tsao-ko
Amomum tsao-ko cultivated in Yunnan province, China, was purchased from Beijing Tongren Drug Store (Beijing, China) in 2012 and identified by Prof. Min Li at Chengdu University of Traditional Chinese Medicine. The dried fruits were ground and distilled for 4 h using a Clevenger-type apparatus (Yang et al., Citation2008). The essential oil was dried by anhydrous sodium sulfate and then stored at 4 °C. The density of the oil was 920.90 mg/ml.
Determination of minimum lethal concentration (MLC) and 50% inhibitory concentration (IC50)
Amomum tsao-ko essential oil (or geraniol) mixed with polysorbate 80 (v/v, 10:1) was emulsified by adding physiological saline while stirring. The emulsion containing 10% essential oil (or geraniol) was diluted with trichomonas medium No. 2. This assay was performed according to Wright et al. (Citation2010) and Delmas et al. (Citation2002) with modifications. Amomum tsao-ko essential oil and geraniol were diluted by geometric dilutions in 96-well plates, ranging from 22.48 µg/ml to 46.05 mg/ml of A. tsao-ko essential oil, and from 21.43 µg/ml to 43.90 mg/ml of geraniol. In addition, three positive drug controls (trichomonas medium + metronidazole, or ornidazole, or Jieeryin + T. vaginalis), one solvent control (trichomonas medium + polysorbate 80 + T. vaginalis), and one growth control (trichomonas medium + T. vaginalis) were set up and investigated at the same time. After 48 h of incubation at 37 °C, viable T. vaginalis cells were identified and counted microscopically on the basis of the morphology and motility. The minimum lethal concentration (MLC) was defined as the lowest drug concentration in which no motile organism was observed. The 50% inhibitory concentration (IC50) value was determined as the drug concentration in which 50% T. vaginalis cells were alive and 50% died (Delmas et al., Citation2002). All experiments were carried out in triplicate.
Inspectation of ultrastructural changes of A. tsao-ko essential oil-treated or geraniol-treated T. vaginalis by TEM
The ultrastructural changes of T. vaginalis isolate (Tv2) treated with A. tsao-ko essential oil (or geraniol) were observed by TEM. Tv2 were incubated at 37 °C, and 2.0 × 105 T. vaginalis/ml were treated in trichomonas medium No. 2 containing sub-MLC A. tsao-ko essential oil or geraniol for 1, 3, and 5 h. The cells were centrifuged at 1200g at 4 °C for 10 min, and were added to PBS (pH 7.4) containing 3% glutaraldehyde at 4 °C for 1 h. The cells were then postfixed in 1% buffered osmium tetroxide for 1 h, stained with 1% uranyl acetate and dehydrated in graded series of ethanol. The samples were cryofixed with a pressure of about 2100 bars and a reduction in temperature of 8 °C/s. The cryofixed cells were kept in liquid nitrogen and cryosubstituted in pure acetone containing 2% of osmium tetroxide and 0.1% of uranyl acetate for 72 h at −90 °C. The temperature was gradually increased to 4 °C (5 °C/h). Samples were kept at this temperature for 2 h, followed by 2 h at room temperature, and were then washed twice with acetone. The fixed cells were then embedded in Epon812. Ultra-thin sections were prepared and stained with uranyl acetate and lead citrate. Microscopy was performed with a HITACHI, H-600IV TEM at the West China Medical School, Sichuan University, China. The control samples were treated with physiological saline instead of A. tsao-ko essential oil or geraniol.
Results
In vitro activity of A. tsao-ko essential oil and geraniol against T. vaginalis
As shown in , A. tsao-ko essential oil and geraniol showed obvious anti-T. vaginalis activity in vitro. The activity of A. tsao-ko essential oil was stronger than that of geraniol. MLCs and IC50 values of A. tsao-ko essential oil were 44.97 µg/ml/22.49 µg/ml for T. vaginalis isolate Tv1, and 89.93 µg/ml/44.97 µg/ml for T. vaginalis isolate Tv2. Those of geraniol were 342.96 µg/ml/171.48 µg/ml. When the activity of A. tsao-ko essential oil was compared with two standard drugs (metronidazole and ornidazole) and geraniol, the essential oil was about 10-fold less active than metronidazole, 20-fold less than ornidazole, and 4-fold to 8-fold more active than geraniol. However, in contrast to the Traditional Chinese Medicine Jieeryin, a well-known therapeutic agent for killing Trichomonas vaginitis in China, A. tsao-ko essential oil and geraniol were 80-fold and 20-fold to 40-fold more active, respectively.
Table 1. In vitro anti-T. vaginalis activity of essential oil and geraniol from A. tsao-ko.
Morphological changes of T. vaginalis after A. tsao-ko essential oil treatment
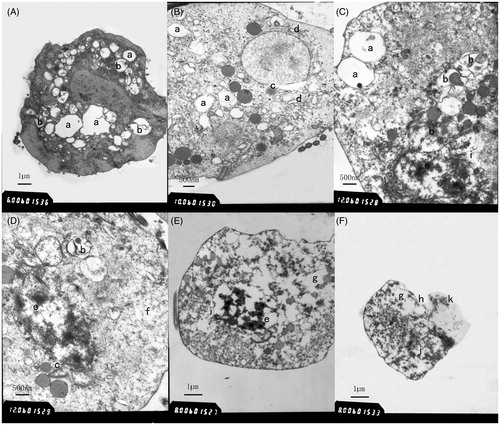
shows the internal structure of untreated T. vaginalis Tv2 cell. The cell in normal condition without environment disturbance shows an integrated membrane (6 in ) and nucleus (1 in ), hydrogenosomes (3 in ), and the base of the flagellum (5 in ). Significant ultrastructural changes in A. tsao-ko essential oil-treated Tv2 cell were observed by TEM. The morphological changes of Tv2 with sub-MLC essential oil (44.97 μg/ml) for 1 h are shown in . In the nucleus, chromatin accumulation (e in ), a damaged nuclear membrane or disappearing nuclear envelope (i in ), and dissolving nuclei (j in ) were observed. In the cytoplasm, numerous vacuoles (a in ), autophagic vacuoles (b in ), dilation of the rough endoplasmic reticulum (d in ), reduction or disappearance of ribosomes (f in ), organelles disintegration (g in ), partial cell membrane damaging (h in ), cytoplasmic leakage (k in ), cell disintegration, and necrosis () were observed. There was no obvious difference among 1, 3, and 5 h treatments. These results indicate that the treatment time is not the main factor on the effect of A. tsao-ko essential oil against T. vaginalis, and the A. tsao-ko essential oil takes action in a relatively short period of time.
Figure 1. Transmission electron micrographs of untreated T. vaginalis. In untreated-T. vaginalis cell, nucleus (1), rough surfaced endoplasmic reticulum (2), free hydrogenosomes (3), ribosome (4), flagella basal body, (5) and cytoplasmic membrane (6) were observed by TEM in this figure.
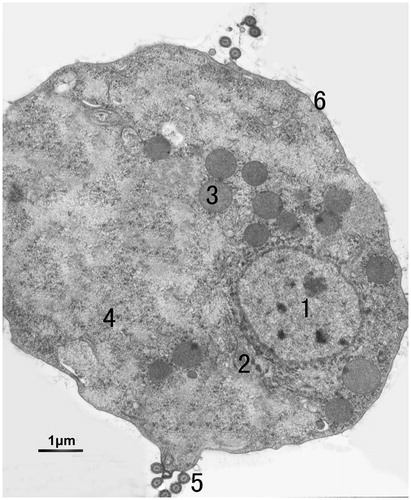
Figure 2. Transmission electron micrographs of A. tsao-ko essential oil-treated T. vaginalis. Transmission electron micrographs of T. vaginalis cell treated with sub-MLC A. tsao-ko essential oil (44.97 µg/ml) for 1 h were shown in (A–F). Vacuoles (a), autophagic vacuoles (b), perinuclear space widening (c), swollen rough surfaced endoplasmic reticulum (d), chromatin accumulation (e), ribosomes disappearing (f), organelles disintegration (g), damaged cytoplasmic membrane (h), nuclear envelope disappearing (i), nuclei dissolving (j), and cytoplasmic leakage (k) were observed.
Morphological changes of T. vaginalis after geraniol treatment
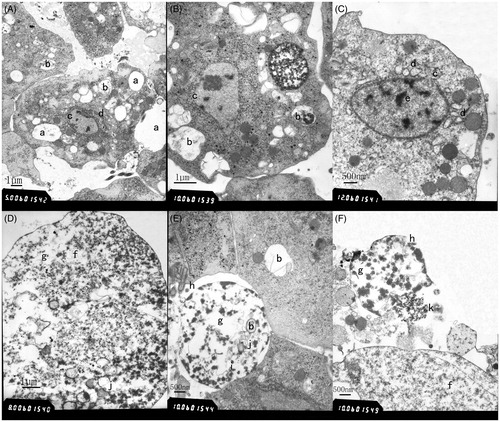
Similar morphological changes were observed in Tv2 cells treated with geraniol at sub-MLC (171.48 µg/ml) for 1, 3, and 5 h, with the observation in 1 h represented in (A–F). Compared with the changes in A. tsao-ko essential oil-treated Tv2 cells, similar morphological changes were observed, such as numerous vacuoles (a in ), autophagic vacuoles (b in , B, and E), rough endoplasmic reticulum dilation (d in ), perinuclear space widening (c in ), chromatin accumulation (e in ), disappearing nuclear envelope (i in ), dissolving nuclei (j in ), ribosome reduction or disappearing (f in ), organelles disintegration (g in D, E, and F), partial cell membrane damaging (h in ), and cytoplasmic leakage (k in ). Disintegration and necrosis of Tv2 cells were observed in . These results indicated that the anti-T. vaginalis mechanism of geraniol might be similar to that of A. tsao-ko essential oil.
Figure 3. Transmission electron micrographs of gernaiol-treated T. vaginalis. Transmission electron micrographs of T. vaginalis cell treated with sub-MLC gernaiol (171.48 µg/ml) for 1 h were observed in (A–F). Vacuoles (a), autophagic vacuoles (b), perinuclear space widening (c), swollen rough surfaced endoplasmic reticulum (d), chromatin accumulation (e), ribosomes disappearing (f), organelles disintegration (g), damaged cytoplasmic membrane (h), nuclear envelope disappearing (i), nuclei dissolving (j), and cytoplasmic leakage (k) were observed.
Discussion
Anti-T. vaginalis activity of A. tsao-ko essential oil and its main component, geraniol
Interest has increased in recent decades in natural products with medicinal activity from plants which are still the major sources of innovative therapeutic agents for infectious diseases, cancer, lipid disorders, and immunomodulation (Altmann, Citation2001). Plant extracts (Frasson et al., Citation2012), essential oils (Moon et al., Citation2006), and isolated substances such as saponins (Rocha et al., Citation2012), β-glycosides (Arthan et al., Citation2008), and alkaloids (Giordani et al., Citation2011) have shown anti-T. vaginalis activity, but until now, no study on the action of A. tsao-ko essential oil and geraniol against T. vaginalis or other protozoa is available. In this study, the anti-T. vaginalis activity of A. tsao-ko essential oil and geraniol against two isolates of T. vaginalis was investigated, showing obvious anti-T. vaginalis activity. Although the activity of A. tsao-ko essential oil and geraniol less active than metronidazole and ornidazole, it is stronger than Jieeryin, a well-known therapeutic agent for killing T. vaginitis in China. These results indicated that a rich variety of A. tsao-ko can be used as potential therapeutic natural resource for the development of antitrichomonal drugs. As far as we know, this is the first demonstration of the potential anti-T. vaginalis activity of A. tsao-ko essential oil and geraniol.
We have identified 24 compounds in A. tsao-ko essential oil by GC-MS, representing 97.00% of the total oil (unpublished data), while geraniol represents only about 13.69% of total oil volume. It is unknown whether A. tsao-ko essential oil contains other anti-T. vaginitis constitute or if interactions exist between geraniol and other constitute. Consequently, further studies are necessary to determine the relationship between chemical composition and anti-T. vaginalis activity, as well as to detail the mechanism of action of those bioactive molecules in A. tsao-ko essential oil. Besides that, the risk of cell toxicity due to essential oil ingestion or skin contact, the role of the delivery systems, and the potential application of A. tsao-ko essential oil and geraniol for the treatment of parasitical infections require further investigation.
Morphological changes of T. vaginalis after A. tsao-ko essential oil or geraniol treatment
At present, limited studies have suggested that essential oils could interfere with biochemical pathways (Lopes et al., Citation1999; Valentin et al., Citation1995). The mechanism of A. tsao-ko essential oil and geraniol against T. vaginalis was preliminarily investigated by TEM after the susceptibility assay in this study. Morphological changes of A. tsao-ko essential oil-treated and geraniol-treated Tv2 cell are shown in and , respectively. Compared with untreated Tv2 cell, severe morphological changes were observed by TEM after A. tsao-ko essential oil treatment for 1 h (), such as a large number of vacuoles, decreased or disappeared ribosome, rough endoplasmic reticulum dilated, chromatin margination, perinuclear space widening, the nuclear envelope disappearing, and nuclei dissolving. Similar morphological changes can be observed in geraniol-treated Tv2 cell (). Partially damaged cytoplasmic membranes and leakage of cytoplasmic contents were also observed. Eventually, the damaged cytoplasmic membrane and organelles might cause Tv2 cells to die. Therefore, we presume that A. tsao-ko essential oil and geraniol induce reduction in the trophozoite viability probably by damaging membrane and organelles.
Besides that, the influence of action time on T. vaginalis of A. tsao-ko essential oil or geraniol was analyzed by TEM among 1 h, 3 h, and 5 h treatments, but no obvious differences were observed. Therefore, the action time was not an important factor in T. vaginalis morphological changes caused by sub-MLC essential oil or geraniol. The drug concentration might rather be the most influential factor, which requires further investigation.
Conclusions
In conclusion, A. tsao-ko essential oil and geraniol demonstrated anti-T. vaginalis activities. Their anti-T. vaginalis activity might be resulted from damaged membrane and organelles. Therefore, A. tsao-ko essential oil and geraniol might be potentially useful as alternative lead compounds for development of antitrichomonal drugs.
Declaration of interest
The authors declare that there are no conflicts of interest. This study was financially supported by the Funds of the Department of Science and Technology of Sichuan Province (No. 2011JYZ004), the Sichuan Provincial Department of Education (No. 11ZB206, 15ZB0238), Research Project of Sichuan Provincial Health Department (130307) and the General Program of Chengdu Medical College (No. CYZ11-002).
References
- Altmann KH. (2001). Microtubule-stabilizing agents: A growing class of important anticancer drugs. Curr Opin Chem Biol 15:424–31
- Arthan D, Sithiprom S, Thima K, et al. (2008). Inhibitory effects of Thai plants beta-glycosides on Trichomonas vaginalis. Parasitol Res 103:443–8
- Barros LA, Yamanaka AR, Silva LE, et al. (2009). In vitro larvicidal activity of geraniol and citronellal against Contracaecum sp (Nematoda: Anisakidae). Braz J Med Biol Res 42:918–20
- Blaha C, Duchene M, Aspock H, Walochnik J. (2006). In vitro activity of hexadecylphosphocholine (miltefosine) against metronidazole resistant and -susceptible strains of Trichomonas vaginalis. J Antimicrob Chemother 57:273–8
- Butler SE, Augostini P, Secor WE. (2010). Mycoplasma hominis infection of Trichomonas vaginalis is not associated with metronidazole-resistant trichomoniasis in clinical isolates from the United States. Parasitol Res 107:1023–7
- Cherpes T, Wiesenfeld H, Melan M, et al. (2006). The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis 33:747–52
- Cotch MF, Pastorek JG, Nugent RP, et al. (1999). Trichomonas vaginalis associated with low birth weight and preterm delivery. The vaginal infections and prematurity study group. Sex Transm Dis 24:353–60
- Delmas F, Di Giorgio C, Robin M, et al. (2002). In vitro activities of position 2 substitution-bearing 6-nitro- and 6-amino-benzothiazoles and their corresponding anthranilic acid derivatives against. Leishmania infantum and Trichomonas vaginalis. Antimicrob Agents Chemother 46:2588–94
- Durel P, Couture J, Bassoullet MT. (1967). The rapid detection of metronidazole in urine. Br J Vener Dis 43:111–13
- Frasson AP, dos Santos O, Duarte M, et al. (2012). First report of anti-Trichomonas vaginalis activity of the medicinal plant Polygala decumbens from the Brazilian semi-arid region. Caatinga Parasitol Res 110:2581–7
- Garduño-Espinosa J, Martínez-García MC, Fajardo-Gutiérrez A, et al. (1992). Frequency and risk factors associated with metronidazole therapeutic noncompliance. Rev Invest Clin 44:235–40
- Giordani RB, Vieira P de B, Weizenmann M, et al. (2011). Lycorine induces cell death in the amitochondriate parasite, Trichomonas vaginalis, via an alternative non-apoptotic death pathway. Phytochemistry 72:645–50
- Goldstein F, Goldman MB, Cramer DW. (1993). Relation of tubal infertility to a story of sexually transmitted diseases. Am J Epidem 137:577–84
- Helms DJ, Mosure DJ, Secor WE, Workowski KA. (2008). Management of Trichomonas vaginalis in women with suspected metronidazole hypersensitivity. Am J Obstet Gynecol 1998:370–7
- Kapoor K, Chandra M, Nag D, et al. (1999). Evaluation of metronidazole toxicity: A prospective study. Int J Clin Pharmacol Res 19:83–8
- Khallaayoune K, Biron JM, Chaoui A, Duvallet G. (2009). Efficacy of 1% geraniol (Fulltec) as a tick repellent. Parasite 16:223–6
- Kim SH, Bae HC, Park EJ, et al. (2011). Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem Biophys Res Commun 407:129–34
- Lapczynski A, Bhatia SP, Foxenberg RJ, et al. (2008). Fragrance material review on geraniol. Food Chem Toxicol 46:160–70
- Lopes NP, Kato MJ, Andrade EHA, et al. (1999). Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiapi Amazon Indians. J Ethnopharmacol 67:313–19
- Lorenzi V, Muselli A, Bernardini AF, et al. (2009). Geraniol restores antibiotic activities against multidrug-resistant isolates from Gram-negative species. Antimicrob Agents Chemother 53:2209–11
- Martin TS, Kikuzaki H, Hisamoto M, Nakatani N. (2000). Constituents of Amomum tsao-ko and their radical scavenging and antioxidant activities. J Am Oil Chem Soc 77:667–73
- Mendonça A de L, da Silva CE, de Mesquita FL, et al. (2009). Antimicrobial activities of components of the glandular secretions of leaf cutting ants of the genus Atta. Anton Van Leeuwenhoek 95:295–303
- Mishell BB, Shiigi SM. (1980). Determination of viability by trypan blue exclusion. In: Mishell BB, Shiigi SM, eds. Selected Methods in Cellular Immunology. San Francisco: Freeman, 16–19
- Moon SS, Cho SC, Lee JY. (2005). Tsaokoarylone, a cytotoxic diarylheptanoid from Amomum tsao-ko fruits. B Ko Chem Soc 26:447–50
- Moon SS, Lee JY, Cho SC. (2004). Isotsaokoin, an antifungal agent from Amomum tsao-ko. J Nat Prod 67:889–91
- Moon T, Wilkinson JM, Cavanagh HM. (2006). Antiparasitic activity of two Lavandula essential oils against Giardia duodenalis, Trichomonas vaginalis and Hexamita inflata. Parasitol Res 99:722–8
- Narcisi EM, Secor WE. (1996). In vitro effect of tinidazole and furazolidone on metronidazole-resistant Trichomonas vaginalis. Antimicrob Agents Chemother 40:1121–5
- Oxberry ME, Thompson RC, Reynoldson JA. (1994). Evaluation of the effects of albendazole and metronidazole on the ultrastructure of Giardia duodenalis, Trichomonas vaginalis and Spironucleus muris using transmission electron microscopy. Int J Parasitol 24:695–703
- Peng C, Dai M, Wan F. (2010). The application of A. tsao-ko essential oil in preparing medicament for treating bacterial infectious diseases. Chinese patent: CN 102058821 A
- Rocha TD, De Brum Vieira P, Gnoatto SC, et al. (2012). Anti-Trichomonas vaginalis activity of saponins from Quillaja, Passiflora, and Ilex species. Parasitol Res 110:2551–6
- Schmid G, Narcisi E, Mosure D, et al. (2001). Prevalence of metronidazole-resistant Trichomonas vaginalis in a gynecology clinic. J Reprod Med 46:545–9
- Upcroft JA, Dunn LA, Wright JM, et al. (2006). 5-Nitroimidazole drugs effective against metronidazole resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob Agents Chemother 50:344–7
- Valentin A, Pelissier Y, Benoit F, et al. (1995). Composition and antimalarial activity of volatile components of Lippia multiflora. Phytochemistry 40:1442–93
- Van Der Pol B, Kwok C, Pierre-Louis B, et al. (2008). Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J Infect Dis 1997:548–54
- World Health Organization (2010). Towards universal access. Progress Report 2010. Available from: www.who.int/hiv/pub/2010progressreport/full_report_en.pdf [last accessed 5 Jul 2011]
- Wright JM, Dunn LA, Kazimierczuk Z, et al. (2010). Susceptibility in vitro of clinically metronidazole-resistant Trichomonas vaginalis to nitazoxanide, toyocamycin, and 2-fluoro-2′-deoxyadenosine. Parasitol Res 107:847–53
- Yang X, Küenzi P, Plitzko I, et al. (2009). Bicyclononane aldehydes and antiproliferative constituents from Amomum tsao-ko. Planta Med 75:543–6
- Yang Y, Yan RW, Cai XQ, et al. (2008). Chemical composition and antimicrobial activity of the essential oil of Amomum tsao-ko. J Sci Food Agric 88:2111–16
- Yu L, Shirai N, Suzuki H, et al. (2010). The effect of methanol extracts of tsao-ko (Amomum tsao-ko Crevost et Lemaire) on digestive enzyme and antioxidant activity in vitro, and plasma lipids and glucose and liver lipids in mice. J Nutr Sci Vitaminol (Tokyo) 56:171–6
