Abstract
Context: Patrinia villosa (Thunb.) Juss (Valerianaceae) is an important ancient herbal medicine widely used for inflammation, wound healing, and abdominal pain. But little is known of the phytochemical constituents of this herbal plant.
Objective: The objective of this study is to isolate and identify the bioactive components from P. villosa.
Materials and methods: A 70% EtOH extract of P. villosa was subjected to normal-phase silica, ODS silica gel column chromatography, and semi-preparative HPLC chromatography after partitioned successively with light petroleum, dichloromethane and n-BuOH. Chemical structures of the compounds were elucidated by spectroscopic methods including UV, 1D-NMR, 2D-NMR, HR-ESI-MS, and CD spectra. The cytotoxic activity of the new component was determined with the SMMC-7721 cell line using the MTT method after incubation for 48 h.
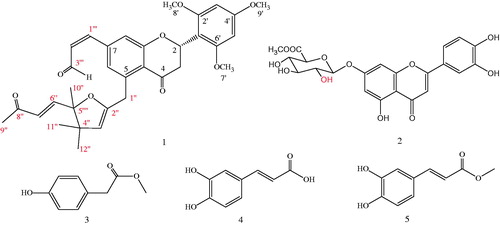
Results: A new flavonoid named patriniaflavanone A (1) along with four known compounds was isolated from P. villosa. The four known compounds were identified as luteolin 7-O-glucuronide-6″-methyl ester (2), p-hydroxyphenylacetic acid methyl ester (3), trans-caffeic acid (4), and trans-caffeic acid methylate (5) by comparison of their spectral data with the reported data. The IC50 value of patriniaflavanone A (1) on SMMC-7721 was 61.27 μM.
Discussion and conclusion: This is the first report on the isolation and identification of patriniaflavanone A (1), and compounds 2–5 were isolated for the first time from the title plant. Patriniaflavanone A (1) exhibited moderate cytotoxic activity.
Introduction
Patrinia (Valerianaceae) is a widely distributed plant grown in East Asia and North America. The genus includes more than 20 species, 10 of which are growing in China (Xie et al., Citation2008). Usually, Patrinia species are used as leaf vegetables in some areas of China, and research also revealed that its leaves have pharmacological properties, especially the species of Patrinia villosa (Thunb.) Juss.
Patrinia villosa is an important ancient herbal medicine widely used for more than 2000 years from ShenNongBenCaoJing, a famous ancient Chinese medicinal literary. It has been used in traditional Chinese medicine for inflammation, wound healing, and abdominal pain. Previous research on the chemical constituents of P. villosa revealed that it contains several compound classes. Pentacyclic triterpenoids, iridoids, and flavonoids are the dominant bioacitve constituents in the leaves of P. villosa, which displayed potential ability of antitumor and anti-inflammatory (Chiu et al., Citation2006; Peng et al., Citation2005; Zhang et al., Citation2008). Other components, such as sterols, fatty acids, and essential oil, were also confirmed in P. villosa (Peng et al., Citation2006; Taguchi et al., Citation1973).
As part of our search for new natural compounds with antitumor activity, we carried out phytochemical investigations on the leaves of P. villosa collected in China. A new flavonoid (compound 1) and four known compounds () were isolated from the plant. The present study reports the isolation and structural elucidation of these components.
Materials and methods
General experimental procedures
Optical rotations were measured on a Perkin-Elmer digital polarimeter (Perkin Elmer Inc., Hanover, MD). CD spectra were recorded by a Bio-Logic (MOS 450) spectrophotometer (Perkin Elmer Inc., Hanover, MD). The NMR spectra, including 1H, 13C, DEPT, and 2D-NMR, were recorded with TMS as an internal standard on a Brucker AVANCE 400 FT-NMR spectrometer (BRUCK USA Inc., Houston, TX). HR-ESI-TOF-MS experiments were performed on a Micro TOF spectrometer (Bruker Co., Karlsruhe, Germany). Column chromatography was performed on silica gel (200–300 mesh) (Marine Chemical Factory, Qingdao, China) and ODS (Ultimate XB-C18, 40–70 μm, Welch Materials, Inc., Ellicott City, MD). HPLC separation was performed with an Agilent 1100 chromatograph apparatus using an ODS column (Ultimate XB-C18, 10 × 250, 5 μm, Welch Materials, Inc.) and detected with a UV detector.
Materials and chemicals
The P. villosa leaves were purchased in from Hebei Qixin Traditional Chinese Medicine Pellets Co., Ltd., PR China in May 2013, and were identified by Prof. Kang Tingguo, College of Pharmacy, Liaoning University of Traditional Chinese Medicine. A voucher specimen (No. PVJ20130821) has been deposited at the Pharmacognosy Laboratory, Harbin University of Commerce. Organic solvents (analytical grade or HPLC grade) for the experiment were purchased from Kermel Chemical Co. (Tianjin, China).
Extraction and isolation
The air-dried leaves of Patrinia villosa (15 kg) were extracted three times with 70% EtOH under reflux. Evaporation of the solvent under reduced pressure gave a condensed extract (2.8 kg), which was suspended in H2O and then partitioned successively with light petroleum, dichloromethane and n-BuOH. The dichloromethane portion (110 g) was then subjected to normal-phase silica gel column chromatography and eluted with a gradient of CH2Cl2–MeOH (100:0 → 0:100, v/v) to give 10 fractions. Fraction 2 (CH2Cl2–MeOH, 80:1, v/v) was subjected to further separation using ODS silica gel column chromatography eluted with 20%, 36%, 44%, 60%, and 80% MeOH in water. Subfraction 2.2 (36% methanol elution) was purified by semi-preparative HPLC (MeCN–H2O, a wavelength of 280 nm) to yield compound 1 (16.5 mg).
The n-BuOH portion (280 g) was subjected to ODS silica gel column chromatography and eluted with a gradient of MeOH–H2O (20% → 100%, v/v) to give five fractions. Fraction 2 (MeOH–H2O, 40%, v/v) was subjected to further separation using ODS silica gel column chromatography to yield compound 2 (32.7 mg). Subfraction 2.1 (MeOH–H2O, 20%, v/v) was purified by semi-preparative HPLC (MeCN–H2O) to yield compounds 3 (169.3 mg), 4 (42.5 mg), and 5 (53.8 mg).
Patriniaflavanone A (1)
Yellow solid, [α]24D −7.1° (c 0.1, MeOH), UV (MeOH) λmax 282, 327 nm, IR (KBr): νmax 2945, 2817, 2704, 1659, 1602, 1428 cm−1, 1H and 13C-NMR spectral data (). HR-ESI-MS m/z: 559.2328 [M − H]− (Calcd for C33H36O8, 559.2332, [M − H]−).
Table 1. 1H and 13C-NMR spectroscopic data of 1 (in DMSO-d6, δ in ppm, J in Hz).
Cytotoxic activity assay
SMMC-7721 cell lines purchased from the American Type Culture Collection (Rockville, MD) were used to evaluate the cytotoxic activity of compound 1 in vitro by the method of MTT. 5-Fluorouracil and 0.1% (v/v) DMSO were used as a positive control and a negative control, respectively.
Results and discussion
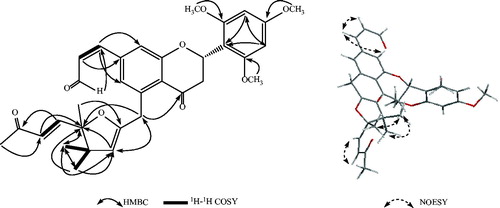
Patriniaflavanone A (1) was obtained as a yellow solid. It had a molecular formula of C33H36O8, based on high-resolution electrospray–ionization mass spectrometry (HR-ESI-MS) and 13C NMR data, implying 16 indices of hydrogen deficiency. The 1H-NMR, 13C-NMR, and DEPT spectroscopic data of 1 revealed the presence of 33 carbon signals including seven methyls, two methylenes, 12 methenyls, and 12 quaternary carbons. The resonances for the proton-attached carbons were designated by analysis of the HSQC spectrum (). The 1H NMR spectrum of compound 1 showed an AMX spin system at δ 5.73 (1H, dd, J = 13.2, 3.2 Hz), 3.07 (1H, dd, J = 17.6, 13.2 Hz), and 2.61 (1H, dd, J = 17.6, 3.2 Hz), corresponding to H-2, H-3a, and H-3b, respectively, of a flavanone skeleton (Cui et al., Citation2008). The carbon resonances at δ 79.1, 39.7, and 196.9 in the 13C NMR spectrum and a complete three proton spin system from the 1H–1H COSY spectrum supported this observation.
The HMBC correlations are between C-3′ (δ 107.1) and H-5′, C-5′ (δ 107.1) and H-3′, C-2′ (δ 148.1) and H-8′, C-6′ (δ 148.1), and H-7′, C-1′ (δ 111.5) and H-3′, H-5′. Taking into consideration the three chemically equivalent carbons, including one methoxyl, one methenyl, and one quaternary carbon, found in 13C-NMR and DEPT spectra, the three methoxyls (C-7′, −8′, and −9′) were linked to the C-2′, C-6′, and C-4′ of the B-ring, respectively.
Four proton signals at δ 7.91 (1H, d J = 9.6 Hz, H-1‴), δ 6.25 (1H, d J = 9.6 Hz, H-2‴), δ 6.98 (1H, d J = 16.0 Hz, H-6″), and δ 6.28 (1H, d J = 16.0 Hz, H-7″), together with the correlations between the proton signals in 1H–1H COSY spectrum, revealed the presence of two –HC=CH– fragments with (Z) and (E)-configuration as indicated by the coupling constants, respectively. In addition, correlations between C-6 and H-1‴, C-8 and H-1‴, C-1‴ and H-3‴ were observed in HMBC spectrum indicating the presence of an acryl group whose C1‴ is linked to C7 with (Z)-configuration at the C1‴–C2‴ double bond. Correlations were observed between carbon signal at δ 198.2 and proton signals at δ 6.98, 6.28, 2.27, carbon signal at δ 130.5 and proton signal at δ 2.27, carbon signal at δ 78.1 and proton signals at δ 6.98, 6.28 in the HMBC spectrum suggesting that a butenone group whose C6″ is linked to C5″ with (E)-configuration at the C6″–C7″ double bond as indicated by the coupling constant (JH6″–H7″ = 16.0 Hz).
The number of “O” atoms confirmed in this compound with the above information was 7. So, an O atom remained. Taking into consideration the carbon signals at δ 160.6 (C-2″) and δ 78.08 (C-5″), and the correlation is between C-5″ and H-3″, C-3″ and H-1″, C-1″ and H-3″, C-4 and H-1″, C-5″ and H-12″, H-11″ in HMBC spectrum, a dihydrofuran group is confirmed and the methylene (C-1″) is the group which linked the C-2″ of dihydrofuran group and C-5 of C-ring. A complete molecule was built by 1H–1H COSY and HMBC experiments ().
Compound 1 with 2-(S) configuration possessing a conformation with p-helicity of the heterocyclic ring and having a C2 equatorial aryl group exhibited a positive Cotton Effect at 240 nm and a negative Cotton Effect at 315 nm (Han et al., Citation2005; Slade et al., Citation2005). With all the above evidence, 1 was identified as (2S)-2′,4′,6′-trimethoxyl-5-{4″,4″,5″-trimethyl-5″-[(E)-8‴-oxobut-6‴-enyl]-4″,5″-dihydrofuran-2″-yl}methyl-7-(Z)-acryl-flavanone (1) and named patriniaflavanone A.
Compounds 2–5 were identified as luteolin 7-O-glucuronide-6″-methyl ester (2), p-hydroxyphenylacetic acid methyl ester (3), trans-caffeic acid (4), and trans-caffeic acid methylate (5) and on the basis of its spectroscopic data (Duan et al., Citation2012; Feng et al., Citation2009; Yang et al., Citation2007).
The antitumor activity of compound 1 was studied using the SMMC-7721 cell line. As a result, the compound exhibited moderate cytotoxic activity (IC50 value = 61.27 μM) in vitro.
Acknowledgements
The authors gratefully acknowledge Dr. Da-Zhi Zhang, Centre of Analysis and Test, Harbin Institute of Technology, for his kind help in determining the samples.
Declaration of interest
The authors report that they have no conflicts of interest. Thanks for the financial support of this work by the National Natural Science Foundation of China (81303211) and China Postdoctoral Science Foundation (2013M540301).
References
- Cui L, Thuong PT, Lee HS, et al. (2008). Flavanones from the stem bark of Erythrina abyssinica. Bioorg Med Chem 16:10356–62
- Chiu LC, Ho TS, Wong EY, et al. (2006). Ethyl acetate extract of Patrinia scabiosaefolia downregulates anti-apoptotic Bcl-2/Bcl-X(L) expression, and induces apoptosis in human breast carcinoma MCF-7 cells independent of caspase-9 activation. J Ethnopharmacol 105:263–8
- Duan SL, Tang SA, Qin N, et al. (2012). Chemical constituents of Phymatopteris hastate and their antioxidant activity. China J Chin Mater Med 37:1402–7
- Feng WS, Li KK, Zheng XK, et al. (2009). Studies on chemical constituents in Forsythia suspensa (Thunb.) Vahl. Chin Pharm J 44:490–2
- Han J, Kim SY, Jung JY, et al. (2005). Epoxide formation on the aromatic B ring of flavanone by biphenyl dioxygenase of Pseudomonas pseudoalcaligenes KF707. Appl Environ Microb 71:5354–61
- Peng, JY, Fan GR, Wu YT. (2005). Studies on chemical constituents of Patrinia villosa Juss. Chin Mater Med 28:883–4
- Peng JY, Fan GR, Wu YT. (2006). Studies on chemical constituents of Patrinia villosa. China J Chin Mater Med 31:128–30
- Slade D, Ferreira D, Marais JP. (2005). Circular dichroism, a powerful tool for the assessment of absolute configuration of flavonoids. Phytochemistry 66:2177–215
- Taguchi H, Yokokawa Y, Endo T. (1973). Studies on the constituents of Patrinia villosa Juss. Yakugaku Zasshi 93:607–11
- Xie Y, Peng JY, Fan GR, et al. (2008). Chemical composition and antioxidant activity of volatiles from Patrinia villosa Juss obtained by optimized supercritical fluid extraction. J Pharm Biomed Anal 48:796–801
- Yang XW, Teng J. (2007). Chemical constituents of the unripe fuits of Evodia utaecarpa. Chin Pharm J 16:20–3
- Zhang T, Li QW, Li K, et al. (2008). Antitumor effects of saponin extract from Patrinia villosa (Thunb.) Juss on mice bearing U14 cervical cancer. Phytotherapy Res 22:640–5