Abstract
Context: Oxidative stress acts as an essential mediator in the pathophysiology of urolithiasis. Lepidagathis prostrata Dalz. (Acanthaceae) is a Pashanbhed plant that is recommended for the management of urolithiasis; however, no scientific validation has been reported.
Objectives: To evaluate the antiurolithiatic and antioxidant potential of L. prostrata.
Materials and methods: Methanol extract (LPM) and fractions; petroleum ether (LPPE), ethyl acetate (LPEA), n-butanol (LPBU) and aqueous (LPAQ) were prepared. In vitro antiurolithiatic activity was evaluated by the capacity to inhibit calcium oxalate (CaOx) nucleation and aggregation at different concentrations of extract/fractions (0.04–3 mg/mL) for 30 min. Total phenol and flavonoid content and antioxidant potential were determined. A validated HPTLC method was performed to quantify lupeol and β-sitosterol.
Results: LPEA exhibited the highest dose-dependent inhibition of CaOx nucleation (IC50: 336.23 ± 30.79 µg/mL) and aggregation (IC50: 149.63 ± 10.31 µg/mL), which was significantly (p < 0.05) better than standard Cystone®. The polar LPBU fraction was enriched with phenols (47.34 ± 0.19 mg GAE/g) and flavonoids (20.38 ± 0.05 mg QE/g), which correlates with its highest antioxidant potential in DPPH, ABTS, nitric oxide scavenging and iron chelating activities (IC50: 1.18–87.34 µg/mL). To our knowledge, this is the first study reporting the presence of lupeol and β-sitosterol in L. prostrata.
Conclusion: The antiurolithiatic activity of L. prostrata is probably mediated through the inhibition of CaOx crystallization. In addition to its free radical scavenging and antioxidant activities, it would act as an excellent agent for the prevention of urolithiasis.
Introduction
Urinary calculi are the third most prevalent disorder of the urinary tract and nearly 80% of these calculi are composed of calcium oxalate (CaOx) (Patel et al., Citation2012). Current therapies, such as extracorporeal shock wave lithotripsy (ESWL) and percutaneous nephrolithotomy are expensive, pose a threat of recurrence and have serious side effects. There are very few drugs available for the management of urolithiasis. Thiazides, allopurinol and potassium magnesium citrate are beneficial in the secondary management of urolithaisis. Cystone® is a marketed herbal formulation that prevents the formation of lithogenic substances, causes disintegration of urinary calculi, and has antibacterial activity that is beneficial in the prevention of stone-associated urinary tract infections. However, drugs that directly inhibit CaOx crystallization in urine would represent a novel class of agents for the management of urolithiasis (Erickson et al., Citation2011).
Multiple mechanisms involved in the pathogenesis of urolithiasis are one of the most important reasons for failure in the development of antiurolithiatic drugs. Reactive oxygen species (ROS) are known to perturb the oxidant–antioxidant balance in kidney cells resulting in cellular damage (Jyothilakshmi et al., Citation2014). Renal epithelial cells produce ROS when exposed to different crystals like CaOx, calcium phosphate and uric acid (Khan et al., Citation2014). Several macromolecular modulators of CaOx crystallization are also regulated by ROS. Genes encoding for fibronectin, CD 44, fetuin B, osteopontin and matrix-gla protein are up-regulated, while those encoding for inter-α inhibitor 1, 3, and 4, calgranulin B, prothrombin, and Tamm-Horsfall protein are down-regulated in urolithiasis. Hence, an imbalance between the promoters and inhibitors of lithogenesis leads to the formation of kidney stones. Therefore, drugs with multiple targets, such as antioxidant, anti-inflammatory and antispasmodic properties are an obvious choice for the development of antiurolithiatic drugs. Medicinal plants possess multiple constituents that work in a synergistic manner with minimum side effects and are accessible to a large population (Khan et al., Citation2012). It can be postulated that interventions with natural antioxidants could mitigate free radical toxicity, which can be used as an adjunct therapy in urolithiasis. Previous studies have reported that antioxidants from plant sources, particularly, phenols and flavonoids are effective in alleviation of stone formation in animal models as well as in humans (Koide et al., Citation1995; Naghii et al., Citation2014; Saha et al., Citation2014). Plant polyphenols serve as an excellent group of exogenous antioxidants, which act by virtue of the hydrogen-donating properties of their phenolic hydroxyl groups and electron-donating properties to stop free radical chain reactions (John & Shahidi, Citation2010). The antimicrobial property of phenolic and flavonoid compounds are also effective in infectious urolithiasis; therefore, phenolic compounds can serve as potent antiurolithiatic agents.
Natural compounds have long been used for the treatment of kidney stones. Pashanbhed is a commercially available Ayurvedic drug that is widely used as a diuretic and lithotripic. The identity of Pashanbhed is based on the doctrine of signatures, where “Pashanbhed” means a plant that can split and grow inside the crevices of rocks. This property is indicative of their ability to dissolve kidney or bladder stones (Kapoor, Citation1990; Verma et al., Citation2014). Plants of lepidagathis genus were used traditionally for the treatment of urinary calculi, dysuria, polyuria, fever, dysentery and uterine disorders (Madhavan et al., Citation2009). Other medicinal uses of lepidagathis plants include skin infections, malaria, migraine, cardiovascular diseases and gastric problems (Hassan-Abdallah et al., Citation2013; Mollik et al., Citation2009; Ravikanth et al., Citation2001; Richard et al., Citation2011). Previous studies have shown they possess excellent larvicidal, anti-inflammatory, analgesic, antipyretic and cytotoxic properties (Charoenchai et al., Citation2010; Obomanu et al., Citation2006; Richard et al., Citation2011). Despite having a number of traditional claims and uses in management of kidney stones, very few species of this genus have been studied for antiurolithiatic activity.
In the present study, we explored the antiurolithiatic potential of Lepidagathis prostrata that grows abundantly in the Western Ghats region of India. It is a rigid, prostrate undershrub with a woody rootstock and commonly grows in the crevices of exposed laterite rocks (Bhat, Citation2003). Lepidagathis prostrata belongs to the Pashanbhed group of herbs, but there is no scientific validation till date for its use in urolithiasis. Based on the above information and considering the complete lack of any scientific basis in literature of L. prostrata as an antiurolithiatic agent, the present study was designed to investigate: (i) the phytochemistry of L. prostrata; (ii) antiurolithiatic activity by inhibition of CaOx nucleation and aggregation; (iii) free radical scavenging, iron chelating and total antioxidant capacity; (iv) a possible correlation between total phenolic and flavonoid content and antioxidant potential of the extract and solvent fractions of L. prostrata.
Materials and methods
Chemicals
Folin-Ciocalteu reagent, aluminum chloride, gallic acid, quercetin, ascorbic acid, curcumin, β-sitosterol (96% pure), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) were purchased from Sigma Chemicals Co. (St. Louis, MO). Lupeol (98% pure) was purchased from Natural Remedies Pvt. Ltd (Bengaluru, Karnataka). All other reagents used were of analytical grade and purchased from usual sources.
Plant material
The whole plant of L. prostrata was collected from End Point, Manipal, India, in December 2013. The plant was authenticated by Dr K. Gopalkrishna Bhat, Professor and Head (Ret.), Department of Botany, Poornaprajna College, Udupi. A voucher specimen (PP609) has been deposited in the herbarium of our institute, Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal for future reference.
Preparation of extracts
The whole plant of L. prostrata was shade dried and extracted with methanol in a Soxhlet apparatus for 72 h at 68 °C. The extract was dried to a dark brown sticky mass in a rotary evaporator under controlled temperature and pressure. The methanol extract (LPM) was suspended in distilled water and partitioned successively with petroleum ether (60–80 °C) (LPPE), ethyl acetate (LPEA), n-butanol (LPBU), and remaining aqueous fraction (LPAQ).
Phytochemical screening
Preliminary phytochemical screening was carried out for the presence of secondary metabolites; alkaloids, sterols, terpenoids, phenols, flavonoids, glycosides, tannins, saponins, fixed oils and fat by standard methods (Evans, Citation2009; Kokate, Citation1991).
Determination of total phenolic content
Total phenolic content was determined in the methanol extract and fractions of L. prostrata by the Folin-Ciocalteau method. A calibration curve was prepared using gallic acid as standard. The standard/extract solution (1 mL) was mixed with 5 mL Folin-Ciocalteu reagent (diluted ten-fold with water) and 4 mL of 0.7 M sodium carbonate. The absorbance was measured after incubation for 2 h at room temperature at 765 nm with a UV-spectrophotometer. All determinations were carried out in triplicate. The concentration of phenolic compounds in the extracts was determined from the standard gallic acid calibration curve. The total content of phenolic compounds in the extracts was expressed as mg gallic acid equivalents (GAE)/g of dry extract (Slinkard & Singleton, Citation1977).
Determination of total flavonoid content
The total flavonoid content was determined by the aluminum chloride method. A calibration curve was constructed using quercetin. Different concentrations of extract/fractions (0.5 mL) were mixed with 1.5 mL methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL distilled water. The mixture was incubated for 30 min and the absorbance measured at 415 nm with a UV-spectrophotometer. Aluminum chloride was substituted with distilled water in blank. All determinations were carried out in triplicate. The flavonoid content was determined from the standard quercetin curve. The total flavonoid content in the extract and fractions was expressed as mg quercetin equivalents (QE)/g of extract (Chang et al., Citation2002).
Antiurolithiatic activity
Inhibition of CaOx crystallization was assayed according to the methods described by Patel et al. (Citation2012) with slight modifications.
Nucleation assay
A solution of 10 mmol/L calcium chloride and 10 mmol/L sodium oxalate were prepared in a buffer containing 0.05 mmol/L Tris-HCl and 0.15 mol/L sodium chloride solution at pH 6.5. Calcium chloride solution (500 µL) was mixed with 200 µL of extract/fractions of L. prostrata at final concentrations in the range of 0.04–3 mg/mL. Crystallization was started by an addition of 500 µL of sodium oxalate solution. Temperature was maintained at 37 °C and absorbance monitored at 620 nm (0–30 min every 3 s) by a kinetic method. The rate of nucleation was calculated by comparing the induction time of CaOx crystallization in the presence or absence of inhibitors. All samples were assayed in triplicate. Cystone® was used as a positive control. The percentage inhibition of nucleation was calculated using the formula:
Aggregation assay
CaOx crystals were prepared by mixing calcium chloride and sodium oxalate at 50 mmol/L. The solutions were equilibrated to 60 °C in water bath, cooled to 37 °C and kept overnight. The solution was centrifuged to yield CaOx crystals and evaporated at 37 °C. The reaction mixture consisted of CaOx crystals at a concentration of 0.8 mg/mL, 0.05 mol/L Tris-HCl and 0.15 mol/L sodium chloride at pH 6.5. The experiment was conducted at 37 °C in the presence of plant extract/fractions at final concentrations in the range of 0.04–3 mg/mL and incubated for 30 min (Patel et al., Citation2012). All samples were assayed in triplicate. Cystone® was used as positive control. The percentage inhibition of aggregation was calculated using the formula:
Antioxidant assays
ABTS scavenging activity
The free ABTS radical was generated in the solution by reacting 7 mM ABTS solution and 2.45 mM potassium persulfate. This mixture was incubated in dark for 15 h and diluted with methanol to obtain an absorbance of 1.1 ± 0.2 units at 750 nm. Various concentrations of plant extract/fractions were prepared in methanol and 20 µL of extract solution was added to 180 µL of ABTS free radical cation solution. The reaction mixture was incubated for 20 min and absorbance read at 750 nm using a micro plate reader (Tachakittirungrod et al., Citation2007; Thaipong et al., Citation2006). All samples were assayed in triplicate. Ascorbic acid was used as positive control. Percent scavenging was calculated using the formula:
(1)
where At = Absorbance of test solution, A0 = Absorbance of control (without extract).
DPPH radical scavenging assay
DPPH free radical solution was generated by preparing 100 µM DPPH solution in methanol. Various concentrations of the extract and fractions were prepared in methanol, incubated with DPPH solution for 20 min in dark and absorbance read at 517 nm. Ascorbic acid was used as a positive control (Bansal et al., Citation2011). All samples were assayed in triplicate. The percent scavenging was calculated using the formula (Equation1(1) ).
Nitric oxide scavenging assay
The nitric oxide scavenging activity was assessed by the Griess reagent assay with some modifications (Sunil et al., Citation2013). The reaction mixture consisted of 2 mL 10 mM sodium nitroprusside, 0.5 mL phosphate buffered saline and 0.5 mL of various concentrations of extract/fractions. The mixture was incubated for 150 min at 25 °C and 0.5 mL of the mixture was mixed with 1 mL of sulfanilic acid reagent (0.33% in 20% glacial acetic acid). It was allowed to stand for 5 min followed by an addition of 1 mL of 1% naphthyl ethylenediamine dihydrochloride. The mixture was incubated for 30 min and absorbance measured at 540 nm against corresponding blank. Curcumin was used as positive control. All samples were assayed in triplicate. The percent scavenging was calculated using the formula (Equation1(1) ).
Iron chelating activity
Iron chelating activity was estimated using the o-phenanthroline method. The reagent was prepared by mixing 0.198 g of 1,10-phenanthroline monohydrate, 2 mL of 1 M hydrochloric acid, 0.16 g of ferric ammonium sulfate, and diluted with water to 100 mL. Briefly, 0.2 mL extract/fractions were mixed with 0.2 mL 1,10-phenanthroline-iron (III) reagent. The mixture was incubated for 30 min and absorbance was read at 510 nm (Berker et al., Citation2007; Besada, Citation1987). Ascorbic acid was used as positive control. All samples were assayed in triplicate. Percent scavenging was calculated using the formula:
(2)
where A0 = Absorbance of control (without extract), At = Absorbance of test solution.
Total antioxidant capacity
The total antioxidant capacities of the extract/fractions were estimated using the phosphomolybdenum method. The reagent solution consisted of 0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. Briefly 0.1 mL of test samples were mixed with 0.3 mL of reagent solution, incubated at 95 °C for 90 min, cooled to room temperature and absorbance read at 695 nm. A calibration curve was prepared using various concentrations of ascorbic acid. The tests were performed in triplicate. The results were expressed as ascorbic acid equivalents (AAE) (Dasgupta & De, Citation2007).
Quantitative analysis of extract/fractions by HPTLC
A validated HPTLC method was employed to detect and quantify the content of lupeol and β-sitosterol in extract/fractions of L. prostrata (Tandon & Sharma, Citation2008). Standard stock solutions (100 µg/mL) of lupeol and β-sitosterol were prepared in methanol. Extract/fractions of L. prostrata were prepared at a concentration of 2000 µg/mL in methanol and used for analysis.
Pre-coated silica gel GF254 plates (20 cm × 10 cm with 0.2 mm thickness) were used. Sample solutions (10 µL) were applied in duplicate as 8 mm bands using a Camag Linomat 5 applicator under nitrogen gas flow. Toluene:methanol (9:1) was used as the mobile phase for linear ascending development. The chamber was pre-saturated with mobile phase for 20 min. The plate was developed up to 75 mm from the point of application of test samples. Scanning was performed with Scanner-3 with slit dimensions of 6 mm × 0.45 mm. After development, the plate was air-dried and treated with anisaldehyde sulfuric acid reagent at 110 °C for 10 min and scanned at 600 nm. The peak areas were recorded and percentage of lupeol and β-sitosterol were calculated.
Statistical analysis
The results are expressed as mean ± standard error of mean (SEM) of three determinations. Graph pad prism 5 was used to analyze the data. One-way analysis of variance (ANOVA) and post-hoc Tukey’s multiple comparison tests were used to determine the difference between means. Statistical correlation was applied using Pearson’s correlation analysis between total phenolic/flavonoid content and antioxidant activities.
Results and discussion
Urolithiasis is the culmination of a number of events starting with crystal nucleation, growth, aggregation and their retention within the kidneys. CaOx urinary stones are the most common type of urinary stones. Currently, there are no drugs directly inhibiting CaOx crystallization in urine (Erickson et al., Citation2011). Upon exposure to CaOx crystals, renal epithelial cells produce reactive oxygen species (ROS) in addition to crystallization modulators. ROS play a significant role in further crystallization and aggregation of CaOx crystals. Therefore, continued exposure to CaOx causes injury to cells and mitochondria that is known to increase the uptake of calcium in such conditions causing anomalies in the respiratory chain reactions with continued generation of ROS. Renal cells are also injured by oxidative stress that is produced during the attachment of crystals to tubular cells. It is further aggravated by serum-free transition metals like iron that enhances lipid peroxidation and inhibits renal antioxidant defense systems (Zhai et al., Citation2013).
Hence, drugs with inhibitory activity on CaOx crystallization and oxidative stress could serve as potent antiurolithiatic agents. In this study, we evaluated the antiurolithiatic activity by direct inhibition of CaOx crystallization, free radical scavenging and antioxidant capacity of L. prostrata by suitable in vitro models. Plant secondary metabolites like polyphenols and flavonoids are well known for their potential to ameliorate ROS-mediated oxidative stress, which could be beneficial in the management of urolithiasis.
Extraction yield, phytochemical characterization, total phenolic and flavonoid content
The extraction yield, total phenolic and flavonoid content of the extract and fractions of L. prostrata are presented in .
Table 1. Percent yield, total phenolic and flavonoid content of extract and fractions of L. prostrata.
Total LPM showed the presence of sterols, triterpenoids, phenols, flavonoids, alkaloids, saponins and tannins. Liebermann Burchard and Salkowski’s test were strongly positive for LPPE and LPEA demonstrating the presence of sterols and triterpenoids. LPEA and LPBU showed a strong presence of phenols in the ferric chloride test. LPAQ exhibited the presence of tannins in the lead acetate test. The Shinoda test was positive for LPEA and LPBU suggesting the presence of flavonols (isoflavones), flavones, flavonones and flavononols. Dragendorff and Mayer’s tests were positive for LPEA and LPBU confirming the presence of alkaloids. The foam test revealed the presence of saponins in LPEA, LPBU and LPAQ.
The therapeutic effects of a wide range of vascular plants are ascribed to the presence of phenolic and flavonoid compounds because of their wide pharmacological activities. The Folin-Ciocalteu method was used to determine the total phenolic content in plant extract/fractions (Blainski et al., Citation2013). The total phenol content was determined from a linear calibration curve of gallic acid. The highest amount of phenolics was found to be in LPBU (47.34 ± 0.19 mg GAE/g of extract) which was significantly (p < 0.05) higher than the other solvent fractions. The least amount of phenolics was found to be in LPM (24.60 ± 0.22 mg/g of extract). The total phenol content was in the following order: LPBU > LPPE > LPAQ > LPEA > LPM ().
Total flavonoid content was determined by the aluminum chloride method that estimates the sum of total flavones and flavonols (Chang et al., Citation2002; Meda et al., Citation2005). A linear calibration curve of quercetin was constructed to determine the total flavonoid content. The highest content was found in LPBU (20.38 ± 0.05 mg QE/g of extract) that was significantly (p < 0.05) higher than the other fractions. The total flavonoid content was in the following order: LPBU > LPM > LPEA > LPAQ > LPPE ().
Antiurolithiatic activity by inhibition of CaOx crystallization
Increased oxalate acts as a predisposing factor for increase in production of ROS and decrease in endogenous antioxidant enzyme levels, facilitating crystal nucleation, aggregation and retention within the kidneys. Current treatments typically decrease urinary supersaturation by altering the urine composition. However, there are no drugs that inhibit CaOx crystallization directly in urine. Drugs that would be able to inhibit the crystallization process would represent a new class of drugs (Erickson et al., Citation2011). Nucleation is the first step in the formation of CaOx crystals which grow bigger with aggregation and occlude the urinary tract. Therefore, we assessed the percent inhibition of CaOx crystallization by nucleation and aggregation methods.
The nucleation of CaOx crystals in solution was inhibited by the addition of extract/fractions of L. prostrata at different concentrations. The absorbance values were found to decrease with the increase in concentration of extract/fractions indicating the decrease in nucleation of CaOx crystals. As shown in , LPEA demonstrated highest inhibitory activity with an IC50 of 336.23 ± 30.79 µg/mL. The order of activity for the extract/fractions were LPEA > LPM >LPBU > LPPE. The extract/fractions showed significantly (p < 0.05) higher inhibition of CaOx nucleation than the standard drug Cystone®.
Table 2. In vitro antiurolithiatic activity by inhibition of CaOx crystallization: (A) Nucleation; (B) Aggregation by extract and fractions of L. prostrata.
It has been observed previously (Chaudhary et al., Citation2010) that aggregation is an important contributing factor in the formation of CaOx stones. Urine from normal people contains single crystals, while those with recurrent disease possess large aggregates of CaOx crystals. Hence, aggregation aids in the progression and worsening of the disease (Fleisch, Citation1978). Incubation with the extract/fractions of L. prostrata with CaOx crystals (generated from the metastable solutions of Ca2+ and oxalate) resulted in reduced aggregation of CaOx crystals indicated by lower absorbance as compared to the control (absence of extract/fractions) in a dose-dependent manner. LPEA showed the highest percent inhibition of aggregation at 750 µg/mL with an IC50 of 149.63 ± 10.31 µg/mL that was significantly (p < 0.05) better than Cystone®. The order of potency for the extract/fractions were LPEA > LPM > LPPE > LPBU (). LPEA was found to be most active in inhibiting both nucleation and aggregation phases of CaOx crystallization. LPAQ did not show any inhibitory activity on CaOx crystallization.
Antioxidant activities
Free radical scavenging activities of extract and fractions of L. prostrata were evaluated by the ABTS and DPPH free radical decolorization assays. The DPPH radical is a model for a lipophilic radical (Ingold et al., Citation1993), while ABTS radical is a model for both hydrophilic and lipophilic radicals (Alam et al., Citation2012). The ABTS free radical was generated by the reaction of ABTS with potassium persulfate to generate a blue-green ABTS+ chromophore. The ability of extract solutions to decolorize the chromophore was measured as percentage scavenging of ABTS radicals (MacDonald-Wicks et al., Citation2006). ABTS free radical scavenging increased with increasing concentrations of fractions with LPBU and LPEA showing the lowest IC50 values (25.12 ± 0.52 and 68.41 ± 6.15 µg/mL, respectively). The potency of the extract/fractions was found to be in the following order: LPBU > LPEA > LPM > LPPE > LPAQ (). Correlation analysis showed a slightly higher correlation between ABTS scavenging potential and total flavonoid content of the extract/fractions (R = −0.864) indicating that flavonoids present in the plant extract/fractions constitute the major free radical scavenging compounds of L. prostrata ().
Table 3. Free radical scavenging and antioxidant capacity of extract and fractions of L. prostrata.
Table 4. Pearson’s correlation coefficient (R) between total phenol/flavonoid content and antioxidant activities of extract and fractions of L. prostrata.
DPPH is a stable, free radical by virtue of the delocalization of the spare electron over the molecule as a whole. When a solution of DPPH is mixed with the substrate that can donate a hydrogen atom, it gives rise to the reduced form with the loss of violet color (Alam et al., Citation2012). The extract and fractions showed concentration-dependent DPPH scavenging activity with LPBU showing the least IC50 value of 20.82 ± 1.13 µg/mL followed by LPEA with an IC50 value of 64.26 ± 2.02 µg/mL (). Pearson’s correlation analysis revealed a moderate correlation between DPPH scavenging and total flavonoid content (R = −0.817). Lower correlation was observed with total phenolic content (R = −0.595), suggesting that flavonoids were mainly responsible for the DPPH scavenging activity ().
Nitric oxide plays an important role in various inflammatory processes in the body and their toxicity multiplies only when they react with free oxygen radicals to form peroxynitrite, which causes further damage to cellular biomolecules like proteins, lipids and nucleic acids (Gouthamchandra et al., Citation2010). The extract and fractions of L. prostrata inhibited nitrite formation by competing with oxygen to react with nitric oxide formed by the reaction of sodium nitroprusside in physiological solution in a concentration-dependent manner (Razali et al., Citation2008). The aqueous fraction exhibited the highest nitric oxide scavenging activity at 500 µg/mL with an IC50 value of 64.62 ± 3.39 µg/mL (). The potency of the extract/fractions was found to be in the following order: LPAQ > LPBU > LPM >LPPE > LPEA. Correlation analysis did not reveal a strong correlation between total phenolic, flavonoid content and nitric oxide scavenging indicating that components other than phenols that are responsible for the nitric oxide scavenging activity of L. prostrata ().
Iron ions catalyze the conversion of less reactive molecules like hydrogen peroxides, lipid peroxides and thiols to more reactive and deleterious species like hydroxyl, peroxyl/alkoxyl radicals, cytotoxic carbonyls and superoxide radicals. They are powerful catalysts of biochemical reactions. A previous study has reported the accumulation of transition metal ions like iron and copper in hyperoxaluric rat kidneys. In addition, excess iron released by cellular damage tend to accelerate oxidative stress in cells, therefore, compounds with iron chelating activity act as excellent antioxidants and antiurolithiatic agents (Selvam, Citation2002). The extract/fractions of L. prostrata showed excellent iron chelating activity with LPBU and LPEA exhibiting comparable iron chelating activity with ascorbic acid. The IC50 values were found to be 1.18 ± 0.34 µg/mL for LPBU and 2.63 ± 0.13 µg/mL for LPEA. The order of potency of the fractions were found to be LPBU > LPEA > LPPE > LPAQ > LPME (). The results of this assay were in agreement with other antioxidant assays with LPBU and LPEA being most active. A moderate correlation was also observed between iron chelating activity and total phenolic (R = −0.603, p > 0.05) and flavonoid content (R = −0.608, p > 0.05) of plant extract/fractions suggesting the role of phenolics in iron chelating ability of L. prostrata ().
Total antioxidant capacities of extract/fractions were expressed as ascorbic acid equivalents by the phosphomolybdenum method. The assay is based on the reduction of Mo (VI) to Mo (V) by antioxidant compounds and formation of a green phosphate/Mo (V) complex at acidic pH. LPBU demonstrated the highest antioxidant capacity, 236.67 ± 2.22 AAE/mg of extract followed by LPEA, LPAQ, LPPE and LPME (). A moderate correlation was observed between total antioxidant capacity and phenolic content (R = 0.765, p > 0.05) and flavonoid content (R = 0.596, p > 0.05) ().
HPTLC analysis
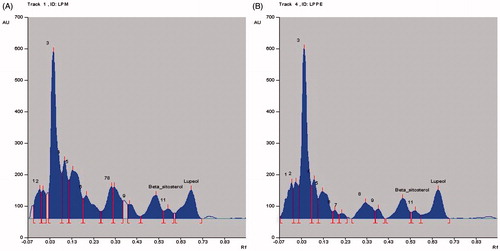
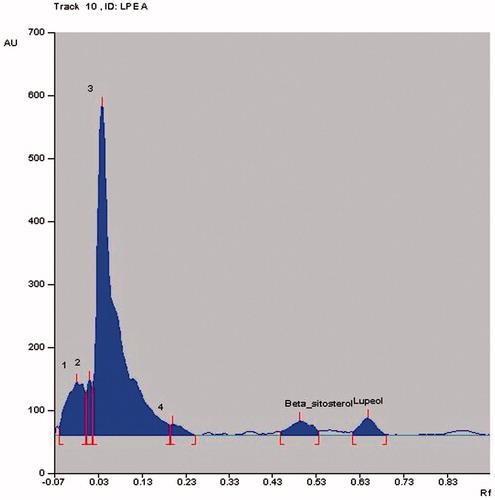
HPTLC is widely used as a routine analytical method for the fingerprinting of compounds in complex herbal extracts and quantification of detectable compounds that can be used as markers in quality control of herbal drugs (Rathee et al., Citation2011). HPTLC analysis of the extract/fractions of L. prostrata confirmed the presence of lupeol and β-sitosterol. Total LPM was found to contain 1.22% lupeol and 1.20% w/w β-sitosterol. The content of lupeol and β-sitosterol in LPPE were found to be 1.23% w/w and 1.0% w/w, respectively ( and ). LPEA showed the lowest lupeol (0.31% w/w) and β-sitosterol (0.41% w/w) content (). To our knowledge, this is the first study confirming the presence of lupeol and β-sitosterol in L. prostrata; hence, these compounds can act as potential markers for L. prostrata.
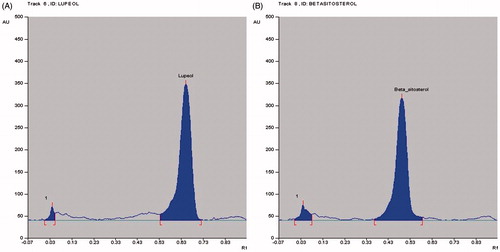
Figure 1. HPTLC chromatographic profile of (A) Standard lupeol (Rf value = 0.65); (B) Standard β-sitosterol (Rf value = 0.49).
Conclusion
Our findings suggest that L. prostrata can serve as an excellent source of antiurolithiatic agents, which is probably mediated through the inhibition of CaOx crystallization. Owing to the presence of phenols and flavonoid compounds, the plant showed significant free radical scavenging and iron chelation activity, which would further strengthen its use to ameliorate urolithiasis-induced oxidative stress.
Acknowledgements
The authors are thankful to the Department of Pharmacognosy, Manipal College of Pharmaceutical Sciences, Manipal University, Manipal, India, for providing the facilities for carrying out this research work.
Declaration of interest
The authors report no declarations of interest.
This work is supported by INSPIRE fellowship (Fellowship No: IF120775) awarded to Raviraj Anand Devkar from the Department of Science and Technology, Government of India.
References
- Alam MN, Bristi NJ, Rafiquzzaman M. (2012). Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm J 21:143–52
- Bansal P, Paul P, Nayak PG, et al. (2011). Phenolic compounds isolated from Pilea microphylla prevent radiation-induced cellular DNA damage. Acta Pharm Sin B 1:226–35
- Berker KI, Guclu K, Tor I, Apak R. (2007). Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, bathophenanthroline, tripyridyltriazine (FRAP) and ferricyanide reagents. Talanta 72:1157–65
- Besada A. (1987). A facile and sensitive spectrophotometric determination of ascorbic acid. Talanta 34:731–2
- Bhat GK. (2003). Flora of Udupi. Manipal, India: Manipal Press Limited
- Blainski A, Lopes GC, Mello JCP. (2013). Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 18:6852–65
- Chang C, Yang MH, Wen HM, Chern JC. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–82
- Charoenchai P, Vajrodaya S, Somprasong W, et al. (2010). Part 1: Antiplasmodial, cytotoxic, radical scavenging and antioxidant activities of Thai plants in the family acanthaceae. Planta Med 76:1940–3
- Chaudhary A, Singla SK, Tandon C. (2010). In vitro evaluation of Terminalia arjuna on calcium phosphate and CaOx crystallization. Indian J Pharm Sci 72:340–5
- Dasgupta N, De B. (2007). Antioxidant activity of some leafy vegetables of India: A comparative study. Food Chem 101:471–4
- Erickson SB, Vrtiska TJ, Lieske JC. (2011). Effect of Cystone® on urinary composition and stone formation over a one year period. Phytomedicine 18:863–7
- Evans WC. (2009). Trease and Evans Pharmacognosy. Edinburgh, UK: Saunders/Elsevier Limited
- Fleisch H. (1978). Inhibitors and promoters of stone formation. Kidney Int 13:361–71
- Gouthamchandra K, Mahmood R, Manjunatha H. (2010). Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L. Environ Toxicol Pharmacol 30:11–18
- Hassan-Abdallah A, Merito A, Hassan S, et al. (2013). Medicinal plants and their uses by the people in the region of Randa, Djibouti. J Ethnopharmacol 148:701–13
- Ingold KU, Bowry VW, Stocker R, Walling C. (1993). Autoxidation of lipids and antioxidation by alpha-tocopherol and ubiquinol in homogeneous solution and in aqueous dispersions of lipids: Unrecognized consequences of lipid particle size as exemplified by oxidation of human low density lipoprotein. Proc Natl Acad Sci USA 90:45–9
- John JA, Shahidi F. (2010). Phenolic compounds and antioxidant activity of Brazil nut (Bertholletia excelsa). J Funct Foods 2:196–209
- Jyothilakshmi V, Thellamudhu G, Chinta R, et al. (2014). Beneficial antioxidative effect of the homeopathic preparation of Berberis vulgaris in alleviating oxidative stress in experimental urolithiasis. Forsch Komplementmed 21:7–12
- Kapoor LD. (1990). Handbook of Ayurvedic Medicinal Plants. Boca Raton (FL): CRC Press LLC
- Khan A, Khan SR, Gilani AH. (2012). Studies on the in vitro and in vivo antiurolithiatic activity of Holarrhena antidysenterica. Urol Res 40:671–81
- Khan SR, Joshi S, Wang E, Peck AB. (2014). Regulation of macromolecular modulators of urinary stone formation by reactive oxygen species: Transcriptional study in an animal model of hyperoxaluria. Am J Physiol Renal Physiol 306:F1285–95
- Koide T, Yamaguchi S, Utsunomiya M, et al. (1995). The inhibitory effect of kampou extracts on in vitro CaOx crystallization and in vivo stone formation in an animal model. Int J Urol 2:81–6
- Kokate CK. (1991). Practical Pharmacognosy. New Delhi, India: Vallabh Prakashan
- MacDonald-Wicks LK, Wood LG, Garg ML. (2006). Methodology for the determination of biological antioxidant capacity in vitro: A review. J Sci Food Agric 86:2046–56
- Madhavan V, Goswami PK, Gurudeva MR, Yoganarasimhan SN. (2009). Pharmacognostical studies on the root of Nothosaerva brachiata: A botanical source of the Ayurvedic drug Pashanabheda. Indian J Tradit Know 9:629–34
- Meda A, Lamien CE, Romito M, et al. (2005). Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem 91:571–7
- Mollik MAH, Faruque MR, Badruddaza M, et al. (2009). Medicianl plants from Sundarbans used for the prevention of cardiovascular diseases: A pragmatic randomized ethno-botanical survey in Khulna division of Bangladesh. Abstracts; Eur J Intgr Med 1:223–60
- Naghii MR, Eskandari E, Mofid M, et al. (2014). Antioxidant therapy prevents ethylene glycol-induced renal CaOx crystal deposition in Wistar rats. Int Urol Nephrol 46:1231–8
- Obomanu FG, Ogbalu OK, Gabriel UU, et al. (2006). Larvicidal properties of Lepidagthis alopecuroides and Azadirachta indica on Anopheles gambiae and Culex quinquefasciatus. Afr J Biotechnol 5:761–5
- Patel PK, Patel MA, Vyas BA, et al. (2012). Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. & Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. J Ethnopharmacol 144:16–70
- Rathee D, Rathee S, Rathee P, et al. (2011). HPTLC densitometric quantification of stigmasterol and lupeol from Ficus religiosa. Arabian J Chem 8:366--71
- Ravikanth V, Reddy N, Ramesh P, et al. (2001). An immunosuppressive tryptophan-derived alkaloid from Lepidagathis cristata. Phytochemistry 58:1263–6
- Razali N, Razab R, Junit SM, Aziz A. (2008). Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem 111:38–44
- Richard SW, Marius L, Noya S, et al. (2011). Anti-inflammatory, analgesic and antipyretic effects of Lepidagathis anobrya Nees (Acanthaceae). Afr J Tradit Complement Altern Med 8:420–4
- Saha S, Shrivastav PS, Verma RJ. (2014). Antioxidative mechanism involved in the preventive efficacy of Bergenia ciliata rhizomes against experimental nephrolithiasis in rats. Pharm Biol 52:712–22
- Selvam R. (2002). CaOx stone disease: Role of lipid peroxidation and antioxidants. Urol Res 30:35–47
- Slinkard J, Singleton VL. (1977). Total phenol analysis: Automation and comparison with manual methods. Am J Enol Vitic 28:49–55
- Sunil C, Duraipandiyan V, Ignacimuthu S, Al-Dhabi NA. (2013). Antioxidant, free radical scavenging and liver protective effects of Friedelin isolated from Azima tetracantha Lam leaves. Food Chem 139:860–5
- Tachakittirungrod S, Okonogi S, Chowwanapoonpohn S. (2007). Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem 103:381–8
- Tandon N, Sharma M. (2008). Quality Standards of Indian Medicinal Plants. New Delhi, India:Indian Council of Medical Research
- Thaipong K, Boonprakob U, Crosby K, et al. (2006). Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compost Anal 19:669–75
- Verma P, Gauttam V, Kalia AN. (2014). Comparative pharmacognosy of Pashanbhed. J Ayuveda Integr Med 5:104–8
- Zhai W, Zheng J, Yao X, et al. (2013). Catechin prevents the CaOx monohydrate induced renal calcium crystallization in NRK-52E cells and the ethylene glycol induced renal stone formation in rat. BMC Complem Altern Med 13:228