Abstract
Context: Compound K (CK, 20-O-d-glucopyranosyl-20(S)-protopanaxadiol), a novel ginsenoside metabolite, is structurally a member of the dammarane-type triterpene saponins. Several studies have identified the anti-inflammatory activity of CK. Our previous study demonstrated that CK exerted its anti-inflammatory effect via inhibition of abnormal activation and differentiation of T cells. However, its mechanism of action on B cells remains unclear.
Objective: The objective of this study is to investigate the effect and underlying mechanisms of CK’s effects on memory B cells in the setting of adjuvant-arthritis (AA).
Materials and methods: Complete Freund’s adjuvant was used to induce AA in rats. Rats were administered, either CK (10, 40, and 160 mg/kg), once daily for 15 d, or methotrexate (MTX; 0.5 mg/kg) once every 3 d, for a total of six times. To evaluate the anti-inflammatory effect of CK, a global assessment and a swollen joint count of AA rats were performed every 3 d. Spleen index and histopathology were examined. Subsets of B cells including CD45R+IgM+ (total B cells) and CD45R+CD27+ (memory B cells) and expression of CD40 and CD40L were assayed by flow cytometry.
Results: Compared with the AA rats, global assessment scores and swollen joint counts were significantly lower in the treated groups received CK (40 and 160 mg/kg; p < 0.05 and p < 0.01, respectively). CK (40 and 160 mg/kg) decreased the spleen index (p < 0.01), and alleviated hyperplasia of lymph nodes (p < 0.05 and p < 0.01, respectively) and marginal zone (p < 0.05) in the spleen. In addition, CK (40 and 160 mg/kg) suppressed memory B cell subsets (p < 0.05), and suppressed CD40L expression on T cells and CD40 expression on B cells (p < 0.05 and p < 0.01, respectively).
Discussion and conclusion: This study demonstrated that CK downregulated memory B cells in AA rats, and this down-regulation may be T-cell dependent.
Introduction
Rheumatoid arthritis (RA) is a chronic, autoimmune disease characterized by synovial hyperplasia and joint destruction. Numerous studies have demonstrated that B cells are involved in the pathogenesis of RA (Dorner Citation2009; Edwards & Cambridge, Citation2006). B cells appear to play an important immunopathogenic role by providing long-lived memory B cells and autoantibodies in autoimmune conditions, including RA (Hiepe et al., Citation2011; Yoshida et al., Citation2010). The importance of B cells in RA has been corroborated by the successful use of anti-CD20 monoclonal antibodies (mAbs) for the treatment of RA (Edwards et al., Citation2005; Gutierrez-Gonzalez et al., Citation2013). In our previous study, an increased level of antibodies and B cell hyperplasia was observed in animal models of autoimmune arthritis (Song et al., Citation2013; Wang et al., Citation2013).
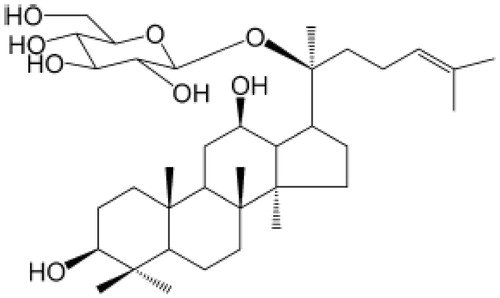
Compound K (CK, 20-O-d-glucopyranosyl-20(S)-protopanaxadiol) () belongs to the class of dammarane-type triterpene saponins by virtue of its structure. CK is a by-product of ginsenoside degradation by intestinal bacteria, and it is the major form of ginsenoside absorbed by the body (Chi & Ji, Citation2005; Tawab et al., Citation2003). It has been reported that CK has an anti-inflammation effect; CK inhibited proinflammatory cytokine production in macrophages (Yang et al., Citation2008). CK also exerts anti-inflammatory effects by inhibiting cyclooxygenase-2 expression (Park et al., Citation2005). Our previous work demonstrated that CK attenuated arthritis in animal models by suppressing T cell activation, inhibiting the production of inflammatory cytokines such as IL-1β, tumor necrosis factor-alpha (TNF-α), IL-2, and IL-17, inhibiting the proliferation of B cells, and decreasing autoantibody levels (Chen et al., Citation2014; Liu et al., Citation2014; Wu et al., Citation2014). However, its effect and mechanism of action on memory B cells remains unclear.
The rat autoimmune arthritis model shares some features with human RA (Hegen et al., Citation2008). Rats are immunized with heat-killed Bacillus Calmette Guerin (BCG) in liquid paraffin and subsequently develop adjuvant-induced arthritis (AA) through a mechanism involving heat shock proteins. Therefore, AA is the most widely used model for studies of RA pathogenesis, and for the screening of new drugs for the treatment of RA (Chang et al., Citation2011; Chen et al., Citation2014; Song et al., Citation2013; Wu et al., Citation2014). Motivated by the aforementioned findings, the present study assessed the effects of CK on memory B cells in AA rats.
Materials and methods
Animals
Sprague–Dawley rats (male, 120 ± 20 g) were obtained from the Animal Department of Anhui Medical University (Hefei, Anhui Province, China). All rats were maintained in a specific pathogen-free animal laboratory at Anhui Medical University. All experiments were approved by the Ethics Review Committee for Animal Experimentation of the Institute of Clinical Pharmacology, Anhui Medical University.
Reagents
Complete Freund’s adjuvant (CFA, 10 mg/ml) was obtained from Chondrex Inc. (Redmond, WA). RPMI-1640 medium was obtained from Gibco Co., Ltd. (Waltham, MA). The fluorophore-conjugated mAbs to CD45R, IgM, CD27, CD40, and CD40L were from Biolegend Co., Ltd (San Diego, CA). CK (20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol, C36H61O8, MW: 621.88) () was provided by Zhejiang Haizheng Medicine Co., Ltd. (Zhejiang, China). CK was separated and purified from protopanaxadiol-type saponins by microbial fermentation and macroporous resin chromatography, and the purity (>98%) was determined by high-performance liquid chromatography. Methotrexate (MTX) was obtained from Shanghai Xinyi Pharmaceutical Co., Ltd. (Kyoto, China).
Induction and treatment of AA
Arthritis was induced by the intradermal injection of 0.1 ml of CFA emulsion into the left hind metatarsal footpad of rats. The control group rats were injected with normal saline. The day of the first immunization was defined as day 0. After the onset of arthritis, on day 15, AA rats were divided into eight groups, including AA, CK (10 mg/kg), CK (40 mg/kg), CK (160 mg/kg), and MTX (0.5 mg/kg). AA rats received CK by intragastric administration, once daily, for 15 d (during the secondary arthritis phase). MTX (0.5 mg/kg) was delivered by intragastric administration, once every 3 d for a total of six times. Simultaneously, the normal and model animals were administered an equal volume of normal saline.
Evaluation of arthritis
To evaluate the severity of arthritis, a global assessment and a swollen joint count of rats were performed every 3 d by two observers blinded to the treatment, according to the methods described in our previous study (Song et al., Citation2013).
Histopathology examination of spleen
Rats were anaesthetized and sacrificed. Spleen histopathology was evaluated by two blinded observers according to the previously described scoring system (Song et al., Citation2013). The spleen’s architecture, including hyperplasia of the lymph nodes (LNs) and marginal zone (MZ), was evaluated by two blinded observers according to the scoring system. Hyperplasia of the LNs and MZ of the spleen was assayed from grade ‘‘0’’ (no effect) to grade ‘‘3’’ (severe effect) according to the scoring system.
Preparation of mononuclear cells
Rats were anaesthetized and sacrificed. A single-cell suspension of splenic tissue was collected by mechanical dissociation of the spleen through nylon mesh. Mononuclear cells from the spleen were purified by density gradient centrifugation. The mononuclear cells were then washed three times with PBS, and suspended in the RPMI-1640 medium at a concentration of 1 × 107 cells/L for the flow cytometry assay.
Flow cytometry assay
B cell subsets and expression of CD40 and CD40L were assayed by flow cytometry. Fluorophore-conjugated antibodies were added to mononuclear cells (100 μl) prepared previously. After gentle mixing, the samples were incubated for 20 min at 4 °C, and then analyzed using flow cytometry.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The analysis of variance (ANOVA) and post-tests were used to determine significant differences between groups. Values of p less than 0.05 were considered significant.
Results
CK attenuated the severity of arthritis in AA rats
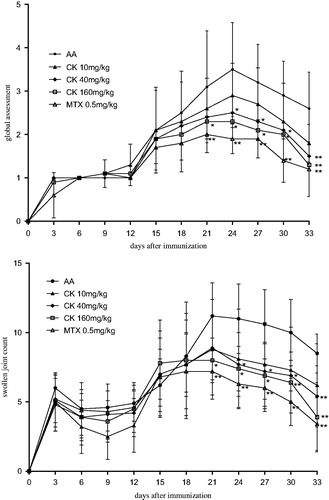
To investigate the anti-inflammatory effect of CK on AA rats, global assessment of arthritis and swollen joint counts were performed. After the onset of arthritis on day 15, a significant increase in the arthritis global assessment scores and swollen joint counts of AA rats was observed. Administration of CK (40 and 160 mg/kg) significantly attenuated the arthritis global assessment scores (p < 0.05 and p < 0.01) () and swollen joint counts of AA rats (p < 0.05 and p < 0.01) ().
Figure 2. CK attenuated severity of arthritis of AA rats. SD rats were immunized with CFA at day 0. After the onset of arthritis, AA rats were divided into five groups and treated intragastrically with CK (once a day) or MTX (once 3 d) from day 15 to day 29. The arthritis global assessment (A) and swollen joint count (B) were evaluated. Data are expressed as mean ± SD from 10 animals for each group. *p < 0.05, **p < 0.01 versus the AA group.
CK alleviated spleen index and histopathology in AA rats
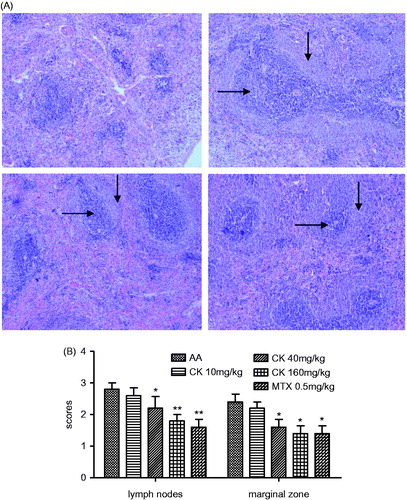
When compared with normal rats, the spleens of AA rats were significantly enlarged, and their spleen indices were increased. CK (40 and 160 mg/kg) significantly decreased the spleen index of AA rats (). The immunomodulatory effect of CK on AA rats was further confirmed by histopathological examination of the spleen. The damage to the spleens of AA rats is characterized by hyperplasia of the LNs and MZ. The MZ in the spleen is the site where B cells interact with helper T cells, and the LN is the site where B cells are activated and differentiate. Administration of CK (40 and 160 mg/kg) attenuated the hyperplasia of the LNs (horizontal arrow) and the MZ (vertical arrow) (). The LNs and MZs of each study group were assessed and given a score according to the degree of hyperplasia. CK (40 and 160 mg/kg) managed to decrease the hyperplasia scores of the LNs (p < 0.05 and p < 0.01) and MZs (p < 0.05) (). These results demonstrated that CK suppressed B cell activation and differentiation.
Figure 3. Effect of CK on spleen index of AA rats. Data are expressed as mean ± SD from 10 animals for each group. **p < 0.01 versus the AA group.
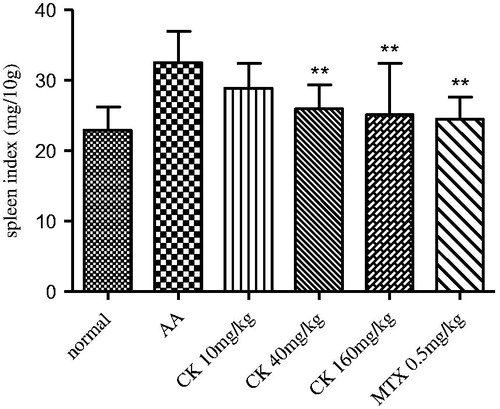
Figure 4. Effect of CK on histopathology of spleen in AA rats. (A) Photomicrograph (original magnification, ×100, H&E stain) of spleen show hyperplasia of LN (horizontal arrow) and MZ (vertical arrow). (B) Histopathological scores of spleen were evaluated. Data are expressed as mean ± SD from six animals for each group. *p < 0.05, **p < 0.01 versus the AA group.
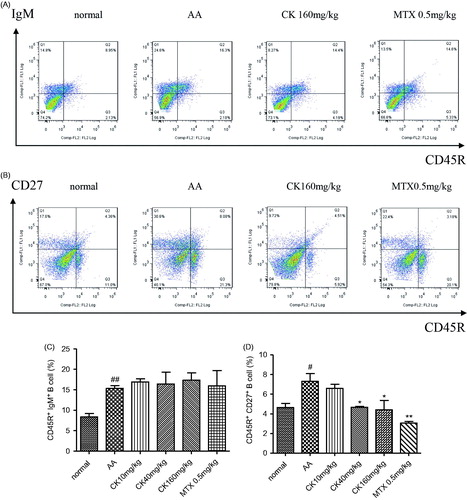
CK downregulated memory B cell subsets in AA rats
Compared with normal rats, splenic CD27+ memory B cells and total B cells increased in AA rats. CK (40 and 160 mg/kg) significantly diminished the percentage of memory B cells in the spleen (), but had no effect on the total number B cells ().
Figure 5. CK regulated B cell subsets in spleen of AA rats. Subsets of B cells including CD45R + IgM + (total B cells) and CD45R + CD27 + (memory B cells) were assayed by flow cytometry. (A and C) CD45R + CD27 + memory B cells, (B, D) CD45R + IgM + total B cells. Data are expressed as mean ± SD from six animals for each group. #p < 0.05, ##p < 0.01 versus the normal group; *p < 0.05, **p < 0.01 versus the AA group.
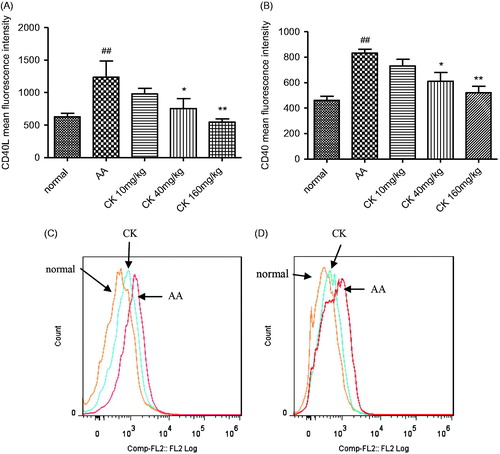
CK suppressed the expression of CD40 and CD40L
CD40 was originally identified as a receptor on B-cells that delivers contact-dependent T helper signals to B-cells through the interaction with CD40 ligands (CD40L and CD154). Compared with normal rats, CD40L expression on CD4+T cells and CD40 expression on CD45R+B cells increased in AA rats. CK (40 and 160 mg/kg) significantly suppressed CD40L expression on T cells () and CD40 expression on B cells (p < 0.05 and p < 0.01) (). These results demonstrated that CK decreased CD40-CD40L signaling between T cells and B cells.
Figure 6. CK suppressed CD40L expression on T cells and CD40 expression on B cells. (A, B) The expression of CD40L on T cell and CD40 expression on B cells was evaluated by flow cytometry. (C, D) The representative flow cytometry histogram. Data are expressed as mean ± SD. ##p < 0.01 versus the normal group; *p < 0.05, **p < 0.01 versus control. The results are representative of at least three independent experiments.
Discussion
B cells have been shown to play a role in the pathogenesis of RA. B cell differentiation to plasma cells that produce rheumatoid factor (RF) and anti-cyclic citrullinated peptide (CCP) antibodies is found in 70–80% of patients with RA (Cambridge et al., Citation2003). In addition to autoantibody production, B-cells can act as antigen-presenting cells providing important co-stimulatory signals required for CD4+T-cell clonal expansion and effector functions (Nanki et al., Citation2009; Takemura et al., Citation2001). B cells also produce cytokines, such as lymphotoxin, which is crucial for the development of tertiary lymphoid structures, as well as IL-10, IL-4, and IL-6, which have both antiinflammatory and proinflammatory capabilities (Fu et al., Citation1998).
LNs in the spleen are the sites where B cells are activated and differentiate. B cells interact with helper T cells at the MZ (B and T cell border), after which antigen-induced T cell-dependent B-cell activation takes place leading to the development of memory B cells in the LNs (Bergmann et al., Citation2013; Klein & Dalla-Favera, Citation2008). Memory B cells, when reactivated, can differentiate into antibody-secreting plasma cells. An abnormal variation in the number of peripheral blood memory B cells has been reported in systemic lupus erythematosus (Odendahl et al., Citation2003), Sjögren's syndrome (Hansen et al., Citation2002), and RA (Souto-Carneiro et al., Citation2009). Antigen-specific memory B cells, residing either in secondary lymphoid organs or the bone marrow, persist for decades (Yu et al., Citation2008), and are responsible for the chronic nature of autoimmune disease (Fekete et al., Citation2007). In fact, compared with naïve B cells, memory B cells easily differentiate into plasma cells (DiLillo et al., Citation2008; Huard et al., Citation2008). Thus, the long-lived humoral immunity observed in autoimmune diseases may be owing to chronic reactivation of memory B cells, thereby constantly generating new plasma cells (Ochsenbein et al., Citation2000).
As an autoimmune arthritis model, AA rats share some features with human RA (Hegen et al., Citation2008) such as swollen joints and an autoimmune response (Caspi et al., Citation2006; Ronnelid et al., Citation1994). The evidence for the anti-inflammatory effect of CK on AA rats included the reduction in arthritis global assessment scores and swollen joint counts, and more importantly, the effect of CK on B cells, as assessed by histopathology of the spleen and B cell subset assays. Our results showed hyperplasia of the LNs and MZ in AA rats. Consistent with this result, flow cytometry data showed that memory B cells increased in the spleens of AA rats. Administration of CK suppressed the hyperplasia of the MZ and LNs and decreased the memory B cells in the spleen. Interestingly, CK did not affect the numbers of total B cells in the spleen. These results suggest that CK inhibits the production of memory B cells in the spleen rather than modifying the levels of total B cells.
Differentiation of B cells into memory B cells could be controlled by signals from T cells, including cytokines secreted by T cells, and contact-dependent signals such as CD40 expressed on T cells. IL-2 is a cytokine that is primarily produced by activated T cells, and has been shown to drive murine B cells to a memory phenotype (Roy et al., Citation2002). The CD40L/CD40 signaling pathway is critically involved in the differentiation of B cells. CD40 is a 50-kDa trans-membrane glycoprotein and is expressed in B cells; its ligand, CD40L, also named CD154, is principally expressed on activated CD4+ T cells. Binding of CD40L to CD40 on B cells is important for the activation of resting B-cells and delivery of anti-apoptotic signals. Following CD40L/CD40 ligation, B cells proliferate, undergo isotype switching and affinity maturation, and can differentiate into plasma cells. We found that CK suppressed CD40 expression on B cells and CD40L expression on T cells. We also found that CK suppressed the activation of T cells as well as production of IL-2 in AA rats (Chen et al., Citation2014). These results suggest that the inhibitory effect of CK on memory B cells may be T cell-dependent.
In autoimmunity and chronic inflammation, memory B cells exhibit resistance to most standard therapies such as cytostatic drugs and conventional immunosuppression drugs (cyclophosphamide and corticosteroids) (Hoyer et al., Citation2004; McMillan et al., Citation1972). Such treatment-refractory, pathogenic memory B cells could be the possible reason underlying the incurable nature of these diseases (Hoyer et al., Citation2008). Elimination of memory B cells has become a well characterized and widely accepted strategy for treating RA; etanercept, a TNF-α decoy receptor, reduces the production of splenic memory B cells in collagen-induced arthritis (Wang et al., Citation2013). These studies suggest that CK plays a key role in regulating humoral immunity in the setting of autoimmune disease and may be helpful for suppressing treatment-refractory, pathogenic memory B cells in these diseases.
Disclosure statement
This work was financially supported by the National Nature Science Foundation of China (Nos. 30973543, 81173075, 81330081, and 31200675), Anhui Province Nature Science Foundation in the University (No. KJ2011Z180), Foundation for Outstanding Young Talents in Higher Education Institutions of Anhui Province (No. 2012SQRL268), and Anhui Provincial Natural Science Foundation (No. 1208085QH158).
References
- Bergmann B, Grimsholm O, Thorarinsdottir K, et al. (2013). Memory B cells in mouse models. Scand J Immunol 78:149–56.
- Cambridge G, Leandro MJ, Edwards JC, et al. (2003). Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum 48:2146–54.
- Caspi D, Anouk M, Golan I, et al. (2006). Synovial fluid levels of anti-cyclic citrullinated peptide antibodies and IgA rheumatoid factor in rheumatoid arthritis, psoriatic arthritis, and osteoarthritis. Arthritis Rheum 55:53–6.
- Chang Y, Wu Y, Wang D, et al. (2011). Therapeutic effects of TACI-Ig on rats with adjuvant-induced arthritis via attenuating inflammatory responses. Rheumatology (Oxford) 50:862–70.
- Chen J, Wu H, Wang Q, et al. (2014). Ginsenoside metabolite compound K alleviates adjuvant-induced arthritis by suppressing T cell activation. Inflammation 37:1608–15.
- Chi H, Ji GE. (2005). Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett 27:765–71.
- DiLillo DJ, Hamaguchi Y, Ueda Y, et al. (2008). Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol 180:361–71.
- Dorner T, Radbruch A, Burmester GR. (2009). B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol 5:433–41.
- Edwards JC, Cambridge G. (2006). B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol 6:394–403.
- Edwards JC, Leandro MJ, Cambridge G. (2005). B lymphocyte depletion in rheumatoid arthritis: Targeting of CD20. Curr Dir Autoimmun 8:175–92.
- Fekete A, Soos L, Szekanecz Z, et al. (2007). Disturbances in B- and T-cell homeostasis in rheumatoid arthritis: Suggested relationships with antigen-driven immune responses. J Autoimmun 29:154–63.
- Fu YX, Huang G, Wang Y, et al. (1998). B Lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med 187:1009–18.
- Gutierrez-Gonzalez LA, Gudiño MA, Ceija IO, et al. (2013). Efficacy and safety of rituximab in biologic-naive patients with rheumatoid arthritis vs anti-TNF therapy failure. Open Rheumatol J 7:81–6.
- Hansen A, Odendahl M, Reiter K, et al. (2002). Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjogren's syndrome. Arthritis Rheum 46:2160–71.
- Hegen M, Keith JC, Collins M, et al. (2008). Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis 67:1505–15.
- Hiepe F, Dorner T, Hauser AE, et al. (2011). Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol 7:170–8.
- Hoyer BF, Moser K, Hauser AE, et al. (2004). Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med 199:1577–84.
- Hoyer BF, Mumtaz IM, Yoshida T, et al. (2008). How to cope with pathogenic long-lived plasma cells in autoimmune diseases. Ann Rheum Dis 67:iii87–9.
- Huard B, McKee T, Bosshard C, et al. (2008). APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118:2887–95.
- Klein U, Dalla-Favera R. (2008). Germinal centres role in B-cell physiology and malignancy. Nat Rev Immunol 8:22–33.
- Liu KK, Wang QT, Yang SM, et al. (2014). Ginsenoside compound K suppresses the abnormal activation of T lymphocytes in mice with collagen-induced arthritis. Acta Pharmacol Sin 35:599–612.
- McMillan R, Longmire RL, Yelenosky R, et al. (1972). Immunoglobulin synthesis by human lymphoid tissues: Normal bone marrow as a major site of IgG production. J Immunol 109:1386–94.
- Nanki T, Takada K, Komano Y, et al. (2009). Chemokine receptor expression and functional effects of chemokines on B cells: Implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther 11:R149.
- Ochsenbein AF, Pinschewer DD, Sierro S, et al. (2000). Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci USA 97:13263–8.
- Odendahl M, Keitzer R, Wahn U, et al. (2003). Perturbations of peripheral B lymphocyte homoeostasis in children with systemic lupus erythematosus. Ann Rheum Dis 62:851–8.
- Park EK, Shin YW, Lee HU, et al. (2005). Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull 28:652–6.
- Ronnelid J, Lysholm J, Engstrom-Laurent A, et al. (1994). Local anti-type II collagen antibody production in rheumatoid arthritis synovial fluid. Evidence for an HLA-DR4-restricted IgG response. Arthritis Rheum 37:1023–9.
- Roy MP, Kim CH, Butcher EC, et al. (2002). Cytokines control of memory B cell homing machinery. J Immunol 169:1676–82.
- Song SS, Huang B, Wang QT, et al. (2013). BF02, a recombinant TNFR2 fusion protein, alleviates adjuvant arthritis by regulating T lymphocytes in rats. Acta Pharmacol Sin 34:414–23.
- Souto-Carneiro MM, Mahadevan V, Takada K, et al. (2009). Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumor necrosis factor. Arthritis Res Ther 11:R84.
- Takemura S, Klimiuk PA, Braun A, et al. (2001). T cell activation in rheumatoid synovium is B cell dependent. J Immunol 167:4710–18.
- Tawab MA, Bahr U, Karas M, et al. (2003). Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos 31:1065–71.
- Wang QT, Wu YJ, Huang B, et al. (2013). Etanercept attenuates collagen-induced arthritis by modulating the association between BAFFR expression and the production of splenic memory B cells. Pharmacol Res 68:38–45.
- Wu H, Chen J, Wang Q, et al. (2014). Ginsenoside metabolite compound K attenuates inflammatory responses of adjuvant-induced arthritis rats. Immunopharmacol Immunotoxicol 36:124–9.
- Yang CS, Ko SR, Cho BG, et al. (2008). The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin-induced lethal shock. J Cell Mol Med 12:1739–53.
- Yoshida T, Mei H, Dorner T, et al. (2010). Memory B and memory plasma cells. Immunol Rev 237:117–39.
- Yu X, Tsibane T, McGraw PA, et al. (2008). Neutralizing antibodies derived from the B cells of 1918 influenza pandemic survivors. Nature 455:532–6.