Abstract
Context: Accumulating evidence indicates that Herba Epimedii [Epimedii folium (Berberidaceae)] has anti-osteoporotic effect by stimulating osteoblastic bone formation and reducing osteoclastic bone resorption. However, the effect of Herba Epimedii in regulating the cross-talk between osteogenic and adipogenic differentiation of mesenchymal stem cells (MSCs) remains unclear.
Objective: The present study investigates the effect of total flavonoids of Herba Epimedii (HETF) on the osteogenesis and adipogenesis of primary MSCs.
Materials and methods: HETF were prepared and identified by HPLC-fingerprinting, primary mouse MSCs in the presence of 0.006–6 μg/mL HETF for 2–10 d were subject to morphological, biochemical, and quantitative real-time PCR analysis.
Results: Sixteen chemical components were identified in HETF by HPLC-fingerprinting and account for over 95% of the total area of HPLC peaks. During osteogenesis of MSCs, 0.006–6 μg/mL HETF promoted the proliferation of MSCs from 17% to 22%, increased alkaline phosphatase activity up to 3.7-fold (0.6 μg/mL), and extracellular calcium deposits from 1.2- to 1.4-folds by up-regulating the expression of runt-related transcription factor-2 (Runx-2) and bone morphogenetic protein-2 (BMP-2). Meanwhile, HETF suppressed the adipogenesis of MSCs by reducing the formation of adipocyte-like cells and accumulation of fat droplets by down-regulating the expression of peroxisome proliferator-activated receptor-γ (PPAR-γ). The above biological activities of HETF were mainly through estrogen receptor-mediated pathway, which were blocked by estrogen receptor antagonist, ICI 182,780.
Conclusion: HETF could regulate Runx-2-mediated osteogenesis and PPAR-γ-mediated adipogenesis in MSCs and thus exhibit beneficial effects to bone health, which suggests a new strategy for treating patients with osteoporosis and obesity.
Keywords:
Introduction
Osteoporosis, characterized by low bone mass and high risk of fracture, is a major public health problem in the world and is increasing in prevalence with the aging of the population. It is estimated that in 2010 more than 2.3 million osteoporosis-related hip, clinical vertebral, and wrist fractures occurred in the population aged 50 years and over, costing the Chinese healthcare system US$9.61 billion (International Osteoporosis Foundation, Citation2014). In comparison, osteoporosis and low bone mass are currently estimated to be a major public health threat for almost 44 million U.S. women and men aged 50 years and older (Nieves, Citation2005). The burden of osteoporosis is similar in Europe and is projected to rise around the globe, with aging populations and increasing fracture rates accompanying urbanization (Modi et al., Citation2014). Notwithstanding its high prevalence, osteoporosis is often under diagnosed and under treated.
Recently, mesenchymal stem cells (MSCs; also known as multipotent mesenchymal stromal cells) with the ability of self-renewal and multilineage mesenchymal differentiation (Jackson et al., Citation2012), have shown promising results for treating bone fractures (Egermann, et al., Citation2005), which remain in a non-proliferative, quiescent state until stimulated by the signals triggered by tissue renewal, damage and remodeling processes. Therefore, MSCs can serve as precursors to a variety of mature mesenchymal cell types, including osteoblasts, chondrocytes, myocytes, and many other types of cells (James, Citation2013). It is important to notice that with the progressive differentiation toward a mature cell phenotype, often the capacity for differentiation into a competing lineage is lost. In normal bone marrow, osteoblastic and adipocytic differentiation of MSCs occurs in favor of bone formation, which was disrupted in several bone diseases. In the process of osteoporosis, increased bone marrow adipocyte production is counterbalanced by diminished production of osteogenic cells, and the deficiency in osteogenesis from MSCs resulted in various bone diseases (Rodriguez et al., Citation2008).
Accumulating evidence suggests that Herba Epimedii [Epimedii folium (Berberidaceae)] has the potential benefits against osteoporosis, which was traditionally widely used for preventing and treating osteoporosis in Asia. Total flavonoids of Herba Epimedii (HETF) were found to suppress urinary calcium excretion and improve bone properties in ovariectomized mice (Chen et al., Citation2011; Xie et al., Citation2005). The extracts of Herba Epimedii promoted the proliferation and differentiation of primary osteoblasts (Zhang et al., Citation2008) and osteoblast-like UMR106 cells (Meng et al., Citation2005). Our previous study has shown that HETF significantly inhibited the proliferation and differentiation of RAW264.7 cells, suppressed vitamin D-induced differentiation of osteoclasts in rabbit bone marrow cells, and decreased the bone resorption of rabbit mature osteoclasts in vitro (Zhang et al., Citation2012). Although extensive studies focused on the bone-forming osteoblasts and bone-resorbing osteoclasts have been carried out with Herba Epimedii, the detailed mode and mechanisms of action in mesenchymal stem cells remain unclear. The present study was dedicated to explore the effect of HETF to the osteogenic and adipogenic differentiation of MSCs, and test our hypothesis that whether HETF could protect bone loss by inducing osteogenesis and suppressing adipogenesis of MSCs.
Materials and methods
Reagents and materials
Epimedium koreanum Nakai herb was collected in July 2013, in a valley in Xinbin, Liaoning Province and authenticated by Q. S. Sun, Professor of Pharmacognosy, Shenyang Pharmaceutical University, China. A voucher specimen (No. 20121260-1) has been deposited in the Herbarium of the Shenzhen Research Center of Traditional Chinese Medicine and Natural Products. Female specific pathogen free Kunming (KM) mice were obtained from Laboratory Animal Center, Guangzhou University of Traditional Medicine, China. Dulbecco’s Modified Eagle Medium (DMEM without phenol red) was purchased from Gibco (Scotland, UK). Fetal bovine serum (FBS) was obtained from Hyclone (Logan, UT). Alkaline phosphatase (ALP) activity kit was obtained from Nanjing Jiancheng Biological Engineering Institute (Nanjing, China) and a micro-Bradford assay kit was purchased from Beyotime Biotechnology (Shanghai, China). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 17β-estradiol (ES), ascorbic acid, alizarin red S, β-glycerophosphate, cetylpyridium chloride, dexamethasone, insulin, and oil red O were purchased from Sigma (St Louis, MO). Trizol® reagent was obtained from Invitrogen (Carlsbad, CA), and ExScript™ RT Reagent kit was purchased from TaKaRa (Dalian, China). RT2 SYBR Green/ROX Q-PCR Master Mix Kit was obtained from Applied Biosystems (Foster City, CA). All other chemicals used were of analytical grade or of the highest purity available.
Preparation of HETF extract
HETF extract were prepared according to our previous publication (Zhang et al., Citation2008). Briefly, the dried and powdered aerial parts of E. koreanum Nakai (1000 g) were refluxed for 2 h with water (10 000 mL, two times). Then the extract was concentrated and subjected to macroporous adsorptive resins eluted with 0, 30, 50, and 95% alcohol (V/V) which represented a yield of 5.5, 1.7, 1.2, and 0.2%, respectively. The total flavonoids were concentrated in the 50% fraction identified by coupling the HCl–Mg reaction and UV spectra. Followed by animal studies, 50% alcoholic fraction showed a strong effect on bone mineral density (BMD) in total and cortical bones (Xie et al., Citation2005).
HPLC analysis of HETF extract
Chromatographic analyses were performed on the Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA) and a Gemini C18 column (150 mm × 4.6 mm, 5 μm) with a guard column (Gemini C18, 4 × 3 mm, 5 μm) at 30 °C. HETF extract (5 mg) were diluted in methanol (5 mL) and subjected to sonication (15 min at 30 °C). The extract was then filtered through a 0.22-μm pore size filter, and the filtrate was finally injected in three repeats for HPLC analysis. Separation was achieved using gradient elution of water with methanol at a flow rate of 1 mL/min, and a detection was performed at 254 nm. The mobile phase (MeOH:H2O) and elution time were 30–40% (0–10 min), 40–50% (10–20 min), 50–60% (20–40 min), 60–70% (40–60 min), 70–80% (60–70 min), 80–90% (70–80 min), and 90–100% (80–90 min).
Isolation and culture of MSCs
MSCs were prepared from 6-week-old KM mice following the Kelly method (Kelly & Gimble, Citation1998). Animal care and the experimental protocol in this study were approved by the ethics committees of City University of Hong Kong. In brief, the mice were executed by cervical vertebra. Femora and tibiae were aseptically harvested, and the whole bone marrow was flushed using supplemented DMEM in a 10 mL syringe and a 25-gauge needle. Suspended whole bone marrow was washed by DMEM. The cells were collected and cultured in DMEM supplemented with 10% heat inactivated and charcoal-stripped FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin, for 24 h in a humidified atmosphere of 5% CO2 in air at 37 °C, then media were changed. The culture medium was changed every 3 d during the experiments.
MSCs viability and proliferation assay
The viability and proliferation of MSCs upon treatment with HETF extract were determined by testing the mitochondrial enzyme function according to the colorimetric MTT method. In brief, MSCs were seeded in 96-well tissue culture plates at the density of 4 × 106 cells per well and incubated for 3 d. After the treatment with HETF extract for 72 h, the plates were washed twice with culture medium, and then 20 μL of MTT (5.0 mg/mL) was added and incubated for another 4 h. Cells with ES and cells without HETF extract treatment were used as positive control and negative control, respectively, and wells without cells were set as blanks. The absorbance at 570 nm was recorded on a microplate spectrophotometer. The relative cell viability was expressed as percentage of [ODsample − ODblank]/[ODcontrol − ODblank] × 100. Each experiment was performed in triplicate.
Assay for ALP activity
MSCs (5 × 106 cells per well) were seeded in 48-well culture plates with the osteogenic induction supplements (5.0 mM β-glycerophosphate, 50 μg/mL ascorbic acid and 0.1 μM dexamethasone), and cultured overnight at 37 °C. ES and HETF extract were added to culture medium at final concentrations of 1 μM and 0.006–6 μg/mL, and cultured for 7 and 14 d. The plates were washed twice with ice-cold PBS and lysed by two cycles of freezing and thaw. Aliquots of supernatants were subjected to ALP activity and protein content measurement by an alkaline phosphatase activity kit and a micro-Bradford assay kit. All results were normalized by protein content. Unit definition: one unit will convert 1 g of tissue protein to 1 μg p-nitrophenol and phosphate in 15 min at 37 °C.
Assay for mineralized matrix formation
MSCs (5 × 106 cells per well) were seeded in 48-well tissue culture plates and cultured overnight at 37 °C for 3 d. The medium was then changed to mineralization supplements containing 10 mM β-glycerophosphate and 50 μg mL/L ascorbic acid in the presence or absence of test samples for 21 d. The formation of mineralized matrix nodules was determined by alizarin red S (ARS) staining. Briefly, the cells were fixed in 70% ethanol for 1 h at room temperature. The fixed cells were washed with PBS and stained with 40 mM ARS, pH 4.2, for 30 min at room temperature. Quantitation of ARS staining was performed by elution with 10% (w/v) cetylpyridium chloride for 10 min at room temperature and measuring the absorbance at 570 nm. Results were expressed as moles of ARS per milligram of total cellular protein.
Oil red O staining for adipogenic differentiation of MSCs
MSCs (1 × 107 cells per well) were seeded in 48-well tissue culture plates, and were cultured for 14 d. The adipogenic supplement (10 mg/L insulin, 0.1 μM dexamethasone) and test samples were added to the culture medium. Fat droplets within differentiated adipocyte-like cells (ALCs) from MSCs were evaluated by the oil red O staining. Cell monolayers were washed by PBS twice, then stained by 0.6% (w/v) oil red O solution (60% isopropyl alcohol, 40% water) for 15 min at room temperature. For quantification of oil red O content, the cells were washed with distilled H2O three times, and isopropyl alcohol was added to resolve oil red O. The absorbance at 510 nm was measured on a microplate spectrophotometer.
Quantitative real-time PCR (qPCR)
Total RNA from MSCs was extracted using Trizol® reagent, and then reverse transcribed into first-strand cDNA by using ExScript™ RT Reagent kit. Quantitative assessment of cDNA was performed in triplicated on the ABI 7500 cycler, with the RT2 SYBR Green/ROX Q-PCR Master Mix Kit. The GAPDH gene was used as internal control to normalize the amount of target cDNA. Because the amplification efficiencies of the target genes and internal control were equal, the relative change of target gene expression was expressed as fold change, which was calculated by the comparative CT (2−ΔΔCT) relative to the control group as a reference: 2−ΔΔCT = 1 according to ABI (2001). Sequences of primers used in quantitative real-time PCR are shown in .
Table 1. List of primers used in this study.
Statistical analysis
Data were collected from three separate experiments and expressed as means ± standard deviation (SD). The statistical differences were analyzed by one-way ANOVA using SPSS 16.0 (SPSS Inc., Chicago, IL). p Values less than 0.05 were considered to indicate statistical differences.
Results
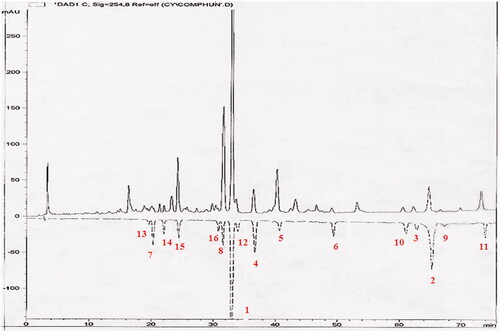
HPLC-fingerprint of HETF
HPLC fingerprinting of HETF extract for quality control is shown in . The HETF extract contained high proportions of icariin (1) and epimedin B (8), moderate and low amounts of baohuoside-I (2), sagittatoside B (3), korepimedoside C (4), caohuoside E (5), baohuoside-II (6), astragalin (7), 3,5,7-trihydroxyl-4′-methoxyl-8-prenylflavone-3-O-α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranoside (9), sagittatoside A (10), acuminatin (11), epimedin C (12), icariside A5 (13), epimedoicarisoside A (14), epimedoside A (15), and hexandraside F (16). The 16 identified components account for over 95% of the total area of HPLC peaks.
Figure 1. HPLC-fingerprint analysis of HETF from E. koreanum Nakai. Chromatographic analyses were performed on the Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, CA) and a Gemini C18 column, flow rate: 1 mL/min, detection wavelength: 254 nm, mobile phase (MeOH:H2O) and elution time: 30–40% (0–10 min), 40–50% (10–20 min), 50–60% (20–40 min), 60–70% (40–60 min), 70–80% (60–70 min), 80–90% (70–80 min), and 90–100% (80–90 min). The data shown are from three independent experiments. Peaks: icariin (1), baohuoside-I (2), sagittatoside B (3), korepimedoside C (4), caohuoside E (5), baohuoside-II (6), astragalin (7), epimedin B (8), 3,5,7-trihydroxyl-4′-methoxyl-8-prenylflavone-3-O-α-L-rhamnopyranosyl-(1→2)-α-L-rhamnopyranoside (9), sagittatoside A (10), acuminatin (11), epimedin C (12), icariside A5 (13), epimedoicarisoside A (14), epimedoside A (15), hexandraside F (16).
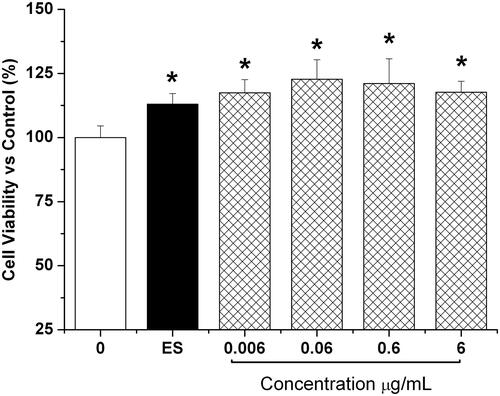
HETF promoted the proliferation of MSCs
As shown in , 0.006–6 μg/mL HETF significantly enhanced the proliferation of MSCs from 17% to 22% in comparison with 13% in the ES group, and no significant difference was found among the tested groups.
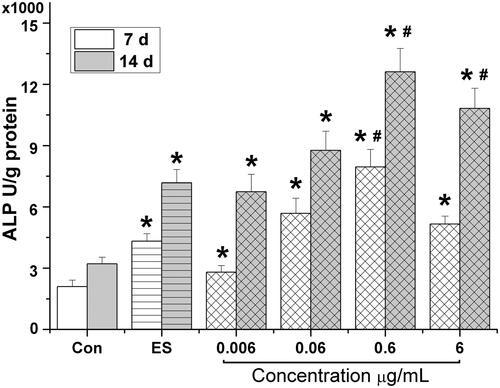
HETF stimulated the osteogenic differentiation of MSCs
As shown in , during osteogenic differentiation of MSCs, ES significantly increased the ALP activity on day 7 to 2.1-fold; 0.006, 0.06, 0.6, and 6 μg/mL HETF significantly increased the ALP activity on day 7 to 2.1-, 3.7-, 2.7-, 1.3-, and 2.5-folds, respectively. The optimal concentration of HETF was 0.6 μg/mL on days 7 and 14. It is noteworthy that the effects of ES and HETF on ALP activity were time dependent and 0.6 μg/mL HETF enhanced the ALP activity up to 3.7-fold on day 7 and 3.9-fold on day 14 much higher than 1 μM ES (p < 0.05).
Figure 3. HETF stimulated the osteogenic differentiation of MSCs by increasing the ALP activity. Values are presented as means ± SD (n = 6 per group). *p < 0.05 compared with control, #p < 0.05 compared with ES (17β-estradiol).
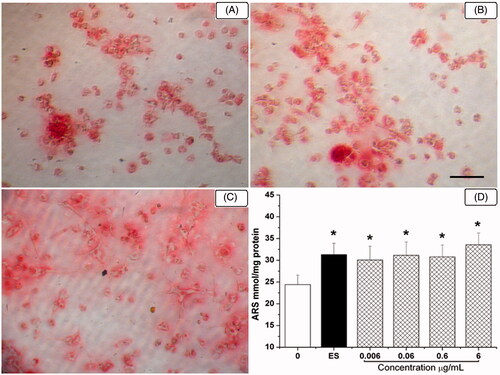
The effect of HETF on extracellular matrix production and mineralization during osteogenic differentiation of MSCs was further investigated by alizarin red S staining. In , red positive stainings of bone nodules were formed by the treatment of mineralization supplements. In comparison, lots of mineralized bone nodules were formed in the presence of 1 μM ES and 0.6 μg/mL HETF (). As shown in , ARS quantification demonstrated elevated calcium content in response to the exposure to ES and HETF, i.e., 31.3 and 30.1–33.6 mol/g protein, respectively, versus 24.4 mol/g protein in the osteogenic control group. The effect of HETF was in the same level of ES, and there is a tendency that the extracellular calcium deposits are increased with the concentration of HETF.
Figure 4. HETF stimulated the osteogenic differentiation of MSCs by increasing bone nodules formation. In vitro matrix mineralization in the presence of HETF and 17β-estradiol stained by alizarin red S (40×, A–C). (A) Mineralization supplement (MS); (B) MS + 17β-estradiol; (C) MS + HETF; scale bar = 20 μm; (D) Quantification of alizarin red S staining. Values are presented as means ± SD (n = 6 per group). *p < 0.05 compared with MS.
HETF suppressed the adipogenic differentiation of MSCs
After the addition of AS, a robust number of fat droplets were found in adipocyte-like cells in . ALCs connected to each other and fat droplets were gradually accumulated. It is notable that the number and size of ALCs were decreased by the treatment of 0.6 μg/mL HETF, suggesting that HETF inhibited the formation and accumulation of fat droplets in ALCs.
Figure 5. HETF suppressed the adipogenic differentiation of MSCs by decreasing adipocytic-like cells formation. Fat droplets within differentiated adipocytes stained by oil red O method (100×, A–C). (A) Adipogenic supplement (AS); (B) AS + 17β-estradiol; (C) AS + HETF; scale bar = 40 μm. (D) Quantification of oil red O staining. Values are presented as means ± SD (n = 6 per group). *p < 0.05 compared with AS.
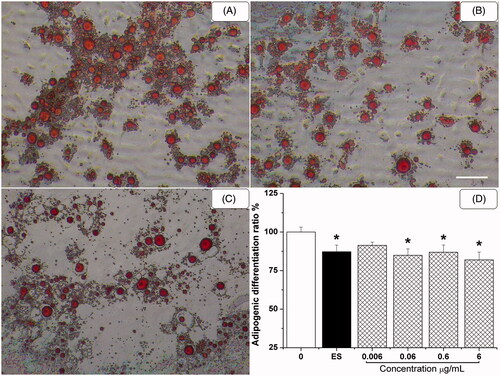
Finally, oil red O was extracted from cells with isopropyl alcohol and the absorbance was measured at 490 nm to quantify staining. The results indicated that ES and HETF inhibited the adipogenic differentiation of MSCs with different degrees of potency (). The inhibitory effect of 0.06–6 μg/mL HETF was in the same level of 1 μM ES (p < 0.05).
HETF regulated the marker genes expression during osteogenic and adipogenic differentiation of MSCs
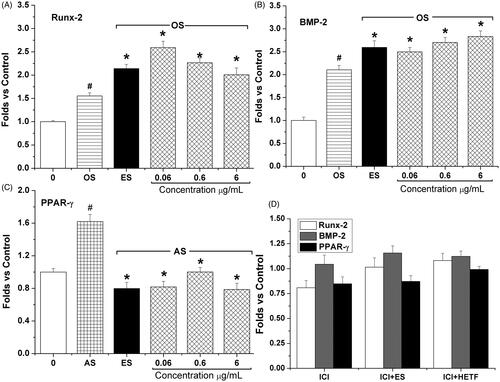
As shown in , the expression of runt-related transcription factor-2 (Runx-2, also called Cbfa1) and BMP-2 was significantly up-regulated in the presence of osteogenic supplement, and the addition of 0.06–6 μg/mL HETF up-regulated Runx-2 mRNA expression to 2.6-, 2.3- and 2.0-folds, respectively, compared with 2.1-fold in the ES group, and dose-dependently up-regulated BMP-2 mRNA expression to 2.5-, 2.7-, and 2.8-folds, respectively, compared with 2.6-fold in the ES group. Besides, the expression level of peroxisome proliferator-activated receptor γ (PPAR-γ) was up-regulated to 1.6-fold in the presence of adipogenic supplement, and decreased by the treatment of 0.06–6 μg/mL HETF and ES to 0.82-, 1.0-, 0.79-, and 0.8-folds, respectively (). However, the addition of 1 μM ICI 182,780 significantly inhibited the expression of Runx-2 and PPAR-γ, and completely blocked the effects of ES on the expression of Run-2, BMP-2, and PPAR-γ ().
Figure 6. The two-way regulation of HETF on the expression of key factors during osteogenic and adipogenic differentiation of MSCs. The gene expression level of Runx-2 and BMP-2 was significantly up-regulated by HETF and ES, while the gene expression level of PPAR-γ was significantly down-regulated by HETF. The antiestrogen ICI 182,780 completely blocked HETF and ES action on the gene expression level of Runx-2, BMP-2, and PPAR-γ. Values are presented as means ± SD (n = 4 per group). #p < 0.05 compared with control, *p < 0.05 compared with OS (osteogenic supplement) or AS (adipogenic supplement).
Discussion
Fingerprint analysis approach using chromatography has become one of the most powerful tools for quality control of herbal medicines (Zeng et al., Citation2008), for instance, identification, authentication, and quality control of herbal drugs, which have been accepted by The Food and Drug Administration (FDA) and World Health Organization (WHO) as a strategy to assess the consistency of botanical drugs. Components of HETF extracts were identified by HPLC fingerprinting with chemical standards isolated by our previous publication (Cheng et al., Citation2006, Citation2007).
Osteoblasts progress through a three-stage process of differentiation, i.e., proliferation, matrix maturation, and matrix mineralization (Malaval et al., Citation1999). In the present study, 0.006–6 μg/mL HETF significantly enhanced the proliferation of MSCs from 17% to 22% (). However, Zhang et al. (Citation2010) reported that Herba Epimedii flavonoids (50 μg/mL) inhibited the proliferation of human MSCs by 8.5% and 11% on days 2 and 3, respectively. The discrepancy with the present study could be caused by different concentrations (50 μg/mL versus 6 μg/mL) and components of the flavonoids extract, which consists of epimedin C (16.8%), icariin (4.9%), and sagittatoside B (9.8%) and quite different from the present study. Besides, our previous study indicated that HETF could stimulate the proliferation and differentiation of primary osteoblasts (Zhang et al., Citation2008). It is also reported that the crude extract, total flavonoids, and main flavonoid constituents from Herba Epimedii (E. brevicornum Maxim and E. koreanum Nakai) have osteoblastic proliferation-stimulating activity toward osteoblast-like UMR106 cells (Meng et al., Citation2005; Xie et al., Citation2005).
Alkaline phosphatase activity and the formation of mineralized bone nodules were used to evaluate the effects of HETF on the early and the final stage of osteoblast differentiation by cytochemical staining and quantification. And the results clearly indicated that 0.6 μg/mL HETF significantly enhanced the ALP activity and extracellular matrix formation ( and ). It is also reported that 50 μg/mL flavonoids from Herba Epimedii (10–75 μg/mL) could stimulated the ALP activity up to 50% on day 6, and increased the mineralization level of human MSCs (Zhang et al., Citation2010). In addition, our previous study using primary osteoblasts also revealed that HETF exhibited a facilitative effect to the alkaline phosphatase activity (Zhang et al., Citation2008).
Obesity and osteoporosis are chronic disorders with increasing prevalence worldwide and the only place in the mammalian organism where bone and fat lie adjacent to each other is in the bone marrow (Devlin & Rosen., 2014). Osteoblasts and adipocytes share a common precursor in adult bone marrow and there is a degree of plasticity between the two cell lineages (Berendsen & Olsen, Citation2014; Song & Tuan, Citation2004). Further studies suggest that a theoretical inverse relationship exists, which is regulated by numerous, intersecting signaling pathways (James, Citation2013). The effect of HETF on the adipogenic differentiation of MSCs was evaluated by the addition of adipogenic supplements and oil red O staining. And the results indicated that HETF significantly inhibited the formation and accumulation of fat droplets in ALCs (). Taken together with the effects on osteogenesis of MSCs, it is safe to conclude that HETF could prevent bone loss by two-way regulating of osteogensis and adipogenesis in MSCs.
Differentiation of osteoblasts or adipocytes from MSCs is regulated by a key transcription factor, Runx-2 (also called Cbfa1) or PPAR-γ, respectively (Harada & Rodan, Citation2003). Osteoblastic maturation is accompanied by induction of expression of Runx-2 and several late marker genes such as osteocalcin, collagen type 1 (Skillington et al., Citation2002). Bone morphogenetic protein-2 up-regulates Runx-2 and Osterix (Osx) expression during osteoblast differentiation, and a lack of BMP-2 and BMP-4 in osteoblast conditional knock-out mice led to a severe impairment of osteogenesis (Bandyopadhyay et al., Citation2006). In the present study, 0.06–6 μg/mL HETF significantly up-regulated Runx-2 and BMP-2 mRNA expressions over 2-fold. The above results were consistent with the ALP activity and ARS staining, suggesting that HETF could stimulate the osteogenic differentiation of MSCs by up-regulating BMP-related regulators.
PPAR-γ is an obligatory key regulator of adipocytic differentiation in vivo as well as ex vivo and involved in the control of fatty acid metabolism (Rosen & Spiegelman, Citation2000). In addition, the activation of PPAR-γ promotes adipogenesis and the suppression of PPAR-γ pathway may inhibit adipogenesis and stimulate osteoblast differentiation (Zhang et al., Citation2015). In the present study, the treatment of 0.06–6 μg/mL HETF decreased the expression level of PPAR-γ to 0.82-, 1.0-, and 0.79-folds, which was consistent with oil red O staining and quantification. It is also reported that other phytochemicals, for instance, kaempferol, have the protective effect against both osteoporosis and obesity by regulating cellular activities (Byun et al., Citation2012). Genistein was also reported to enhance the commitment and differentiation of MSCs to the osteoblast lineage and reduce the adipogenic differentiation of MSCs (Heim et al., Citation2004). Besides, the addition of ICI 182,780, a high affinity estrogen receptor antagonist, completely blocked the effects of HETF and ES on the expression of Run-2, BMP-2, and PPAR-γ (). Although the expression of Runx-2, BMP-2 and PPAR-γ was slightly increased in the presence of HETF compared with ICI 182,780 alone, no significant difference was found (p < 0.05), suggesting the involvement of estrogen receptor pathway.
Conclusion
In summary, the results reported in the present study demonstrate that HETF is capable of promoting osteogenic differentiation by up-regulating the gene expression of Runx-2 and BMP-2, and inhibiting adipogenic differentiation of MSCs by down-regulating the expression of PPAR-γ. Changes in above biological activities are probably mediated predominantly by interaction with nuclear estrogen receptors. To our best knowledge, this is the first report that Herba Epimedii flavonoids have the potential to play an essential role in regulating the balance of bone (osteogenesis) and fat (adipogenesis) in bone marrow. Taken together with our previous study with HETF in osteoclasts differentiated from MSCs (Zhang et al., Citation2012), HETF could prevent bone loss by restoring the balance between bone formation by osteoblasts and bone resorption by osteoclasts, as well as enhancing osteogenesis and reducing adipogenesis in MSCs, which suggests a new strategy for treating patients with osteoporosis and obesity.
Declaration of interest
The authors report that they have no conflicts of interest.
This work was supported by the National Natural Science Foundation of China (No. 81402933), Natural Science Foundation of Guangdong Province (No. 2014A030313539), the Scientific Research Foundation for the Returned Overseas Chinese Scholars[(2014)-1685], State Education Ministry, Research Foundation for Building Strong Province of Chinese Medicine-Traditional Chinese Medicine Bureau of Guangdong Province (No. 20131260), the Project for the Development of Social Science Research of Dongguan (2013108101054), Medical Scientific Research Foundation of Guangdong Province (B2014302), 2014 Project of Teaching Quality and Teaching Reform of Undergraduate Course in Colleges and Universities in Guangdong Province, and the Doctor Scientific Start-up Fund Projects of Guangdong Medical College (B2012008).
References
- Bandyopadhyay A, Tsuji K, Cox K, et al. (2006). Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet 2:e216.
- Berendsen AD, Olsen BR. (2014). Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell Mol Life Sci 71:493–7.
- Byun MR, Jeong H, Bare SJ, et al. (2012). TAZ is required for the osteogenic and anti-adipogenic activities of kaempferol. Bone 50:364–72.
- Chen W F, Mok SK, Wang XL, et al. (2011). Total flavonoid fraction of the Herba Epimedii extract suppresses urinary calcium excretion and improves bone properties in ovariectomised mice. Br J Nutr 105:180–9.
- Cheng Y, Wang N, Wang X, et al. (2006). Chemical constituents from Epimedium koreanum Nakai. J Shenyang Pharm Univ 23:644–7, 657.
- Cheng Y, Wang X, Zhang D, et al. (2007). Nonflavanoid compounds from Epimedium koreanum. Chin Trad Herbal Drugs 38:1135–8.
- Devlin MJ, Rosen CJ. (2015). The bone-fat interface: Basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol 3:141–7.
- Egermann M, Goldhahn J, Schneider E. (2005). Animal models for fracture treatment in osteoporosis. Osteoporos Int 16:S129–38.
- Harada S, Rodan GA. (2003). Control of osteoblast function and regulation of bone mass. Nature 423:349–55.
- Heim M, Frank O, Kampmann G, et al. (2004). The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 145:848–59.
- International Osteoporosis Foundation. (2014). Osteoporotic fractures cost China's healthcare system close to 10 billion USD annually. [Online]. Available from: http://www.eurekalert.org/pub_releases/2014-11/iof-ofc111314.php [last accessed 25 Dec 2014].
- Jackson WM, Nesti LJ, Tuan RS. (2012). Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 1:44–50.
- James AW. (2013). Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo) 2013:684736.
- Kelly KA, Gimble JM. (1998). 1,25-Dihydroxy vitamin D3 inhibits adipocyte differentiation and gene expression in murine bone marrow stromal cell clones and primary cultures. Endocrinology 139:2622–8.
- Malaval L, Liu F, Roche P, et al. (1999). Kinetics of osteoprogenitor proliferation and osteoblast differentiation in vitro. J Cell Biochem 74:616–27.
- Meng FH, L, YB, Xiong ZL, et al. (2005). Osteoblastic proliferative activity of Epimedium brevicornum Maxim. Phytomedicine 12:189–93.
- Modi A, Sajjan S, Gandhi S. (2014). Challenges in implementing and maintaining osteoporosis therapy. Int J Womens Health 6:759–69.
- Nieves JW. (2005). Osteoporosis: The role of micronutrients. Am J Clin Nutr 81:1232S–9S.
- Rodriguez JP, Astudillo P, Rios S, et al. (2008). Involvement of adipogenic potential of human bone marrow mesenchymal stem cells (MSCs) in osteoporosis. Curr Stem Cell Res Ther 3:208–18.
- Rosen ED, Spiegelman BM. (2000). Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16:145–71.
- Skillington J, Choy L, Derynck R. (2002). Bone morphogenetic protein and retinoic acid signaling cooperate to induce osteoblast differentiation of preadipocytes. J Cell Biol 159:135–46.
- Song L, Tuan RS. (2004). Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J 18:980–2.
- Xie F, Wu CF, Lai WP, et al. (2005). The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid Based Complement Alternat Med 2:353–61.
- Zeng Z, Chau FT, Chan HY, et al. (2008). Recent advances in the compound-oriented and pattern-oriented approaches to the quality control of herbal medicines. Chin Med 3:9. doi: 10.1186/1749-8546-3-9.
- Zhang D, Pan B, Cook RL, et al. (2015). Multi-walled carbon nanotube dispersion by the adsorbed humic acids with different chemical structures. Environ Pollut 196:292–9.
- Zhang DW, Cheng Y, Wang NL, et al. (2008). Effects of total flavonoids and flavonol glycosides from Epimedium koreanum Nakai on the proliferation and differentiation of primary osteoblasts. Phytomedicine 15:55–61.
- Zhang DW, Zhang JC, Fong CC, et al. (2012). Herba epimedii flavonoids suppress osteoclastic differentiation and bone resorption by inducing G2/M arrest and apoptosis. Biochimie 94:2514–22.
- Zhang JF, Li G, Chan CY, et al. (2010). Flavonoids of Herba Epimedii regulate osteogenesis of human mesenchymal stem cells through BMP and Wnt/beta-catenin signaling pathway. Mol Cell Endocrinol 314:70–4.