Abstract
Context Inulin, a non-digestible carbohydrate isolated from Helianthus tuberosus L. (Asteraceae), has been shown to alter the gut beneficial bacteria including Lactobacillus spp. and Bifidobacteria. Inulin also influences the activities of intestinal microbiota that could prevent the colon cancer development.
Objective This study determines the effect of hydrolysed inulin with different degrees of polymerisation on alteration of intestinal microbiota and their activities on azoxymethane (AOM)-induced preneoplastic aberrant crypt foci (ACF) in rats.
Materials and methods Seventy-two male Sprague–Dawley rats were randomly divided into six groups (three control and three AOM-treated groups) and the animal were fed with either a normal diet or diet containing 10% of long-chain inulin (InuL) or short-chain inulin (InuS), respectively, for 17 weeks. Colon cancer was induced in rats by injecting AOM subcutaneously at the 8th and 9th week of the study period. At the end of the experiment, cecal contents of rats were examined for selected microbiota, organic acids, putrefactive compounds and microbial enzymes. ACF formation was microscopically examined.
Results The inulin diets significantly increased the weight and decreased the pH of the caecal content. The rats fed with InuL-supplemented diet showed approximately 2.9- and 6.8-fold increases in the biomass of Lactobacillus spp. and Bifidobacteria, respectively. Naive and AOM-treated rats fed with inulin-supplemented diet showed ∼1.3- and ∼2.2-fold decreases in the biomass of Escherichia coli and Salmonella enterica serovar Typhi, respectively. Inulins significantly decreased the colonic concentration of phenol, p-cresol and indole. Reduction in the activity of microbial enzymes such as β-glucuronidase, azoreductase and nitroreductase were observed in inulin-treated animals. Reduction in the ACF formation has been observed in inulin-treated groups.
Discussion and conclusion The present study demonstrates that dietary administration of inulin reduces the formation of preneoplastic lesions in the colon, possibly by altering the microecology and microbial activities on carcinogenesis.
Introduction
Colorectal cancer (CRC) is of high incidence in western countries and it is the third most common cancer worldwide. About 1.2 million patients with CRC per annum were estimated (Whitlock et al. Citation2012). Moreover, CRC is one of the top five causes of cancer mortality among the Thai population (Khuhaprema & Srivatanakul Citation2008; Khuhaprema et al. Citation2014). Imperative risk factors for CRC include diet with high fat, especially fat from meat, low-fibre diet, physical inactivity and obesity (Giovannucci et al. Citation1994; Sandhu et al. Citation2001; Larsson & Wolk Citation2006). Scientific evidence revealed that micro-organisms may be a cause of colon cancer, which has aroused the interest of researchers in factors, especially prebiotics, that can modulate the gut microflora and their metabolism (Guinane & Cotter Citation2013; Slavin Citation2013). Reduction in the overall microbial diversity among the CRC patients has been reported (Ahn et al. Citation2013) and microbiome differences were reported in the various sites of the cancer incidence and among different populations (Geng et al. Citation2013).
Prebiotics are defined as discerningly fermented ingredients that positively interferes the composition and activity of the gastrointestinal (GI) microbiota thus conferring benefits to the host (Gibson et al. Citation2010). Inulin is a known component of several edible plants, including Jerusalem artichoke (JA), chicory and onion (Niness Citation1999), which comprise fructose and glucose that are able to reach the colon and stimulate the growth of beneficial bacteria such as Bifidobacteria and Lactobacillus (Gibson & Roberfroid Citation1995; Van Loo et al. Citation1995). Moreover, inulin was considered as an efficient prebiotic using an in vitro study (Pattananandecha et al. Citation2015). Helianthus tuberosus L. (Asteraceae) (Jerusalem artichoke) is an important source of inulin that reaches 50–56% of dry matter or 11.3–14.2% of the fresh mass of tubers (Danilcenko et al. Citation2008).
The well-balanced intestinal microflora actively plays a role in preventing many diseases in humans (Guinane & Cotter Citation2013; Slavin Citation2013). Fermentation of fibre results in the production of organic acids, short-chain fatty acids, which are, further, absorbed by colon and rest of the content released through faeces (Ruppin et al. Citation1980; Wong et al. Citation2006). The acids content in faeces could be used as a biomarker for the physiological processes of an organism as well as for the effect of nutritional interventions (Pang et al. Citation2014). The fermentation of fibre also inhibits the growth of pathogenic bacteria, which contribute to colon cancer by the activated genotoxic and carcinogenic substances such as phenol, p-cresol and phenolic compounds (Burn & Rowland Citation2000) and bacterial enzymes (β-glucuronidase, nitroreductase and azoreductase) that mediate the formation of mutagens, carcinogens and tumour promoters in the gastrointestinal tract (Gorbach & Goldin Citation1990; Rowland et al. Citation1998).
Previous study has demonstrated the use of gut microbial composition as a tool to identify the presence of cancerous lesions and has suggested for the more interdisciplinary research on the alterations of microbiome during cancerous and treatment conditions (Zackular et al. Citation2014). Enterobacteriaceae bacterial strains play the critical role in the gut and intestinal healthiness. An increased level of E. coli population was reported in the tumour tissue and level of E. coli load could be one of the co-factors for colon cancer development (Bonnet et al. Citation2014). Mager (Citation2006) has reviewed the involvement of S. Typhi in cancer development.
Thus, the aim of the present study was to investigate the influence of hydrolysed inulin [with different composition and degree of polymerisation (DP)] in microbiota alteration, microbial activities and preneoplastic lesion in azoxymethane (AOM)-induced Sprague–Dawley rats. Primarily, changes in the biomass of selected probiotic (Bifidobacteria and Lactobacillus spp.) and representative Enterobacteriaceae family strains (Escherichia coli and Salmonella enterica serovar Typhi) were assessed along with microbial enzymes to validate the microbial load during inulin treatment.
Materials and methods
Chemicals
Azoxymethane was purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan. p-Nitrophenyl-β-d-glucuronide, Amaranth (trisodium 3-hydroxy-4-(4′-sulphonatonaphthylazo) naphthalene-2,7-disulphonate), p-nitrobenzoic acid, ammonium sulphamate and NEDD [n-(1-naphthyl) ethylenediamine dihydrochloride] were purchased from Sigma-Aldrich Co. LLC, St. Louis, MO. Hydrolysed inulin with different compositions, and DP, high long-chain (InuL) and high short-chain inulin (InuS) were received from Health Innovation Institute, Chiang Mai, Thailand.
Animals, housing and diets
Seventy-two male Sprague–Dawley rats, 4–5 weeks of age, were obtained from National Laboratory Animal Center, Mahidol University Bangkok, Thailand. After 2 weeks of acclimatisation, animals were randomly divided into six groups which consist of 12 rats each (three control groups and three AOM-treated groups). The temperature was maintained at the range of 21–25 °C; 12 h light/dark cycles were maintained. Water and diets were given ad libitum. All animals were fed with control diet for 2 weeks after acclimatisation and then they were assigned to one of the following three diets for 17 weeks of experiment: Normal diet (Control), diet with 10% (w/w) InuL and diet with 10% (w/w) InuS. Body weight and feed intakes were measured weekly. Animal experiments were carried out as per the regulations and approval of ethical committee of Faculty of Veterinary, Chiang Mai University, Chiang Mai, Thailand (I.18/2009).
Carcinogen injection
Rats in the AOM group were subjected to subcutaneous injection of AOM in saline (15 mg/kg body weight) at week 8 and 9 of the study period.
Cecal content weight and pH measurement
The caecum of each rat was excised, weighed and split open, and the pH of the cecal content was measured at the end of the experiments.
Bacterial enumeration
Faecal microflora including Lactobacillus spp., Bifidobacteria, E. coli and S. Typhi were determined. Briefly, samples were inoculated into specific agar by the spread plate method and incubated at 37 °C, in an aerobic or anaerobic condition. A range of selective media were used to enumerate Bifidobacteria (Bifidus selective agar, BSA), Lactobacillus spp. (de Man, Rogosa agar, MRS), E. coli (Eosin methylene blue agar, EMB) and S. Typhi (Salmonella Shigella agar, SS). After incubation for 24–48 h, single colonies were counted.
Detection of microbial enzymes
Bacterial enzymes, β-glucuronidase, azoreductase and nitroreductase, were determined spectrophotometrically.
β-Glucuronidase assay
The activity of β-glucuronidase was determined according to the modified method of Deeptha et al. (Citation2006). Rat cecal contents were mixed with 0.1 M phosphate-buffered saline (pH 7.0) and sonicated at 4 °C for 5 min and then centrifuged at 2000 ×g for 5 min. The enzyme reaction was carried out at 37 °C for 15 min at pH 7.0. The total volume of the reaction mixture was 1.0 mL containing 200 μL of 0.1 M phosphate-buffered saline (pH 7), 100 μL of 0.1 mM EDTA, 60 μL of mM p-nitrophenyl-β-d-glucuronide and 650 μL of sample supernatant. The reaction was terminated by the addition of 4 mL of 0.2 M glycine buffer (pH 10.4), in 0.2 M NaCl. The released p-nitrophenol was measured spectrophotometrically at 405 nm. The amount of p-nitrophenol released was determined by comparison with a standard p-nitrophenol curve.
Azoreductase assay
The activity of azoreductase was determined according to the modified method of Haberer et al. (Citation2003). The faecal suspension was then anaerobically incubated with 2 mL of deoxygenated 0.1 M potassium phosphate buffer (pH 7.5) and 0.1 mL of amaranth at 37 °C for 60 min. An aliquot of 0.1 mL of the reaction mixture was collected and the reaction was stopped by the addition of 0.9 mL of ice-cold tri-chloro acetic acid followed by centrifugation at 5000 × g at 4 °C for 5 min before reading the absorbance at 520 nm. The amount of amaranth reduced was determined by comparison with a standard amaranth curve.
Nitroreductase assay
Nitroreductase activity in the faecal suspension will be assayed anaerobically using p-nitrobenzoic acid as a substrate according to the modified method of Lee & Lee (Citation2001). The faecal suspension was then anaerobically incubated with 1 mL of deoxygenated 6 mM p-nitrobenzoic acid at 37 °C for 60 min. An aliquot of 0.2 mL of the reaction mixture was collected and the reaction was stopped by the addition of 0.9 mL of ice-cold mixture containing 4 mM NaNO2, 0.6 M HCl, 25 mM ammonium sulphamate and 1 mM NEDD. The reaction was then centrifuged at 8000 × g at 4 °C for 10 min; the supernatant was used to measure the amount of p-aminobenzoic at 540 nm and compared with the standard p-aminobenzoic curve.
Analysis of organic acids and putrefactive compounds
The concentrations of organic acids (acetic, propionic, butyric and lactic acid) and putrefactive compounds (phenol, indole and p-cresol) in the rat cecal contents were determined by high-performance liquid chromatography (HPLC) as detailed in Lima and Abdalla (Citation2002) and Nowak and Libudzisz (Citation2007), respectively.
Microscopic observation of aberrant crypt foci (ACF)
The colons were removed and washed with 0.85% normal saline solution (NSS), immersed in 10% buffered formalin for 24 h, then stained with 0.2% methylene blue for 5 min and the number of ACF in the ascending and descending-rectum was counted using microscope (Olympus/Bx60, Olympus Inc., Center Valley, PA) (Bird Citation1987; Verghese et al. Citation2005).
Statistical analysis
All the values were denoted as mean ± standard deviation (SD). Data were analysed using SPSS 17.0 for windows® (2009 SPSS Inc., Chicago, IL) by analysis of one-way analysis of variance (ANOVA) followed by the Duncan standardised range test. Differences were considered significant at p <0.05.
Results
Body weight, feed intake, cecal weight and pH
Animals fed with hydrolysed inulin (InuL or InuS) gained minimal weight compared with animals receiving the control diet (p <0.05). Hydrolysed inulins did not affect the feed intake (p <0.05) and ranged from 21.1 ± 2.6 to 23.1 ± 2.1 g/d. The rats fed with inulin showed a significantly higher (p <0.05) cecal weight and lower (p <0.05) cecal pH compared with the control groups ().
Table 1. Effect of different compositions and DP of hydrolysed inulin on weight gain, feed intake, cecal weight and cecal pH in Sprague–Dawley rats.
Bacterial load
Cecal Lactobacillus load in naive and AOM-treated rats fed with InuL (5.69 ± 1.17, 4.37 ± 0.77 × 108 CFU/g of cecal content, respectively) and InuS (2.53 ± 0.64, 1.83 ± 0.24 × 108 CFU/g of cecal content, respectively) were higher than respective controls. Even though, some slight increase in the amount of Lactobacillus was observed among AOM-treated control groups, it is not statistically significant difference (p = 0.975) with naive control (). Likewise, Bifidobacteria load in naive and AOM-treated rats fed with InuL (28.90 ± 2.56, 20.85 ± 1.55 × 106 CFU/g of cecal content, respectively) and InuS (26.98 ± 2.14, 19.73 ± 2.07 × 106 CFU/g of cecal content, respectively) were found significantly (p < 0.05) higher than respective controls. Even though, some slight increase in the biomass of Bifidobacteria was noticed in the AOM-treated control group, it is not statistically significant difference (p = 0.897) compared with naive control. However, enrichment of Bifidobacteria load in the inulin-treated group (∼ seven-fold increase compare with control) was significantly higher () than control groups.
Figure 1. Impact of inulin supplementation on Lactobacillus spp. (A), Bifidobacteria (B), Escherichia coli (C) and Salmonella spp. (D) load in cecal content of rats. Values are represented as mean ± SD. Bars with different alphabets represent the significant difference among the groups (p <0.05).
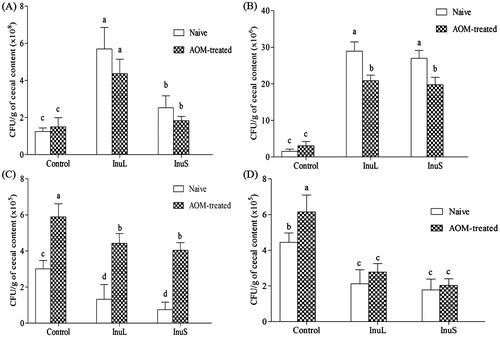
Statistically significant (p < 0.05) level of induction of E. coli (3.01 ± 0.14–5.89 ± 0.07 × 105 CFU/g of cecal content) and S. Typhi (4.45 ± 0.18–6.15 ± 0.27 × 105 CFU/g of cecal content) growth was noticed in AOM-treated control compared with naive control. Interestingly, rats fed on inulin diet significantly (p < 0.05) displayed the decreased cecal E. coli and S. Typhi load in both naive and AOM-treated conditions, when compared with respective controls (). Comparing the results of naive or AOM-treated controls and inulin-supplemented naive or AOM-treated groups suggested that inulin treatment facilitates the growth of selected probiotics and suppresses the growth of E. coli and S. Typhi.
Organic acids and putrefactive compounds
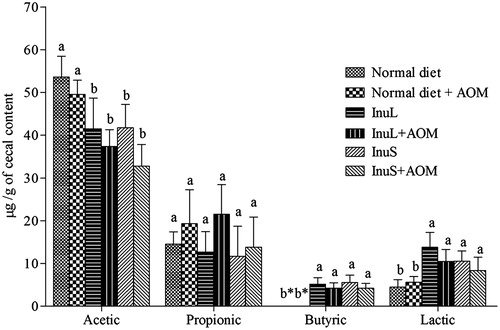
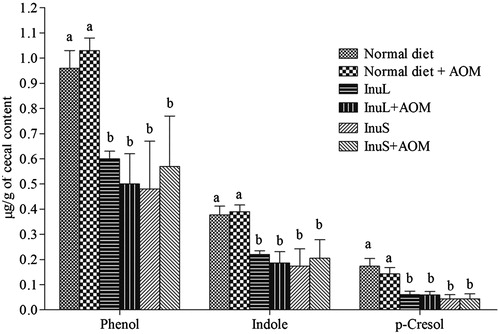
The concentrations of organic acids in cecal contents of normal and AOM-treated rats are shown in . Butyrate was detected only in rats fed with hydrolysed inulins. The concentration of acetate and lactate in rats fed with hydrolysed inulins were significantly (p < 0.05) lower and higher, respectively, compared with the rats fed on the normal diet. Cecal concentration of propionate was not affected by diets. Concentration of cecal phenol, indole and p-cresol was reduced by inulin diet at significant level (p < 0.05), whereas no significant difference between control and AOM-treated group was observed ().
Microbial enzymes
The effect of hydrolysed inulin on microbial enzyme activity is presented in . Rats belonging to the hydrolysed inulin (both InuL and InuS) fed group had significantly (p < 0.05) lower activities of β-glucuronidase, azoreductase and nitroreductase compared with the rats fed with control diet. There were no significant differences in enzyme activity between normal rat and rat treated with AOM.
Table 2. Activity of microbial enzymes of rat cecal content.
Aberrant crypt foci
There was a considerable (p < 0.05) reduction of colonic ACF in AOM-induced rats fed with a diet containing InuL compared with InuS. Aberrant crypt foci were predominantly observed in the descending rectum. The result showed that rats fed on a diet containing hydrolysed inulin appreciably (p < 0.05) reduce the ACF formation in descending rectum compared with the rats fed with the control diet ().
Figure 4. (A) Representative images showing colon of the naive (a) and the AOM-treated (b) rat stained with 0.2% methylene blue. (B) Aberrant crypt foci in colon of rats treated with AOM. Values are the mean ± SD. Bars with different alphabets represent the significant difference among the groups (p <0.05).

Discussion
Our previous study suggested that InuL and InuS are significantly differing with respect to monosaccharides, oligosaccharides content and average DP values. Moreover, InuL was considered as an efficient prebiotic than InuS in in vitro studies (Pattananandecha et al. Citation2015). Current study was performed to evaluate the potential colon cancer preventing activity of hydrolysed inulins from Helianthus tuberosus L. in AOM-induced Sprague–Dawley rats. The rat fed on inulin showed a decreased body mass compared with the control (). The previous studies by Verghese et al. (Citation2002a,Citationb) also reported that the weight of the rat that fed on 10% inulin was reduced. However, some studies have reported no significant consequence of inulin or other prebiotics on body weight or feed intake (Challa et al. Citation1997; Reddy et al. Citation1997; Rowland et al. Citation1998). Increase in cecal weight and decline in pH of the cecal content were observed among inulin-fed rats (), possibly, due to the fermentation of inulin by intestinal bacteria and short-chain fatty acid (SCFA) (Rossi et al. Citation2005). Reduction in the colonic pH has been utilised as a biomarker for the inhibition of bacterial transformation of primary to secondary bile acids (Nagengast et al. Citation1995) and, restraint of carcinogenicity (Zhao et al. Citation2011). Fecal pH has been suggested to be a possible factor in the suppression of colon tumorigenesis (Campbell et al. Citation1997).
The fermentation of inulins by Lactobacillus spp. and Bifidobacteria might have modulated the microbial ecology in the colon. It is known that the inhibitory action of probiotic on pathogenic bacteria includes direct and indirect competition of nutrients, competition for physical attachment sites, production of antimicrobial compounds, enhancement of host immune system activity and synergy between some of these activities (Callaway et al. Citation2012).
Gibson and Roberfroid (Citation1995) reported that intake of oligofructose and inulin significantly increases Bifidobacteria population and decreases the potential pathogenic bacteria. Inulin has more prebiotic effect than oligofructose with respect to fermentation activity as well as bacterial community composition in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) model (Van de Wiele et al. 2007). The prebiotic efficiency of inulin-type fructans not only rely on the dietary dosage but also on the DP (Van Loo Citation2004). Bifidobacteria and some Lactobacillus spp. play a major role in the eco-physiology of the intestinal microbiota, such as resistance to infection and diarrhoeal diseases (Saavedra et al. Citation1994) and stimulation of immune system (Kirjavainen et al. Citation2002). This study indicated that hydrolysed inulins significantly stimulated the growth of Lactobacillus spp. and Bifidobacteria in the colon. InuL showed more growth promotion effect on Lactobacillus spp. than InuS. Only non-significant level of increase in Lactobacillus spp. and Bifidobacteria load has been noticed in the AOM-treated control group. Both InuL and InuS showed the same inhibition effect on the pathogenic strain, E. coli and S. Typhi ().
SCFAs, primarily acetate, propionate and butyrate, are the end-products of microbial fermentation of carbohydrate in the large intestine which depends on the species, amounts of microflora present in the colon, substrate sources and transit time (Wong et al. Citation2006). Different types of non-digestible carbohydrate have been shown to produce different SCFA ratios (Berggren et al. Citation1993; Henningsson et al. Citation2001). Several studies have shown that the acids plays a significant role in the process of inhibiting colon carcinogenesis by stimulating cell proliferation and inducing apoptosis, which in turn affects carcinogenesis (Ruemmele et al. Citation2003; Fung et al. Citation2011).
The cecal acetate concentration was lower in the group fed on inulin while the butyrate concentration was increased (Loh et al. Citation2006). Pan et al. (Citation2009) have reported that intake of fructo-oligosaccharide (FOS) and galacto-oligosaccharide (GOS) improves the concentrations of total cecal SCFAs including butyrate and lactate. Butyrate is an important SCFA and preferred energy source for colonocytes. Butyrate plays a crucial role in maintaining colonic epithelial integrity by regulating epithelial proliferation and differentiation (Zhang & Ohta Citation1993; Siavoshian et al. Citation2000). Butyrate has been reported to induce cell-cycle inhibitors and promoting apoptosis of transformed colonocytes through the mechanisms associated with inhibition of histone deacetylase activity and induction of p21WAF1/Cip1 and cyclin D3 (Boren et al. Citation2003; Comalada et al. Citation2006; Waldecker et al. Citation2008). Butyrate significantly reduces the cellular protein kinase C activities which play a role in the regulation of differentiation in colon cancer cell lines (Duan et al. Citation2005). Lactate and other SCFAs affect the transport processes of colonic epithelial cells, energy metabolism, growth and cellular differentiation (Topping & Clifton Citation2001; Hijova & Chmelarova Citation2007). Lactate has been reported to induce cell migration and cell clusters (Hirschhaeuser et al. Citation2011). Our results indicated that both InuL and InuS increase the production of acetic acid and butyric acid in the colon () that may be the resultant of colonic pH, which lead to the changes in gut environment for stimulation of beneficial bacteria and inhibition of pathogenic bacteria.
Fermented protein products are higher in the distal colon when compared with the proximal colon which implies that protein fermentation is ubiquitous in the distal colon, a common occurring site of colon cancer (Hughes et al. Citation2000). Phenol has an impact on cell permeability in human intestinal epithelial cell line SK-CO15 (McCall et al. Citation2009). p-Cresol also increases the permeability of endothelial cell (Cerini et al. Citation2004). Increasing the permeability of the mucus layer enhances the accessibility of a broad range of carcinogens to the colonic mucosa (Windey et al. Citation2012). Indole is not direct-acting mutagen, but acts as colon cancer promoter and enhances nitrosation (Nowak and Libudzisz Citation2006).
Oligofructose and inulin supplementation reduces the fecal phenols (Propst et al. Citation2003) and fructo-oligosaccharide increases Lactobacillus populations, lowered total fecal indole and phenol concentrations in dogs (Swanson et al. Citation2002). Effect of oligofructose-enriched inulin (OF-IN) and Bifidobacterium breve on colonic nitrogen-protein metabolism was studied in healthy humans and has found a reduction in urinary p-cresol (De Preter et al. Citation2007). In this study, both hydrolysed inulins significantly decreased the colonic concentration of phenol, p-cresol and indole (). The results suggested that inulin supplementation considerably decreased the concentration of putrefactive compounds by selective stimulation of growth of Lactobacillus spp. and Bifidobacteria, which further leads to the inhibition of proteolytic bacterial growth in the colon. Inulin can alter the proteolytic metabolism of the distal colon into a more saccharolytic one, which can influence the composition of the intestinal microbiota.
The microbial activities of the intestinal microflora have been associated with colon cancer through the formation of genotoxic and carcinogenic products (Uccello et al. Citation2012; Schwabe & Jobin Citation2013). The bacterial enzymes include β-glucuronidase, nitroreductase and azoreductase. β-Glucuronidase hydrolyses glucuronide conjugated in the intestine. This process generates potentially toxic and carcinogenic compounds that are detoxified by glucuronide formation in the liver and then enter the bowel via bile (de Moreno et al. 2005). Hydrolysis of enzyme increases endogenous exposure to carcinogenic compounds (Hughes & Rowland Citation2000). Nitroreductase reduces aromatic and heterocyclic nitro compounds to potentially mutagenic and carcinogenic n-nitroso and n-hydroxy compounds before conversion to aromatic amines bile (Hughes & Rowland Citation2000; Lee & Lee Citation2001; de Moreno et al. 2005). Azoreductase reduces azo compound to mutagen (Rafii et al. Citation1990). Effect of inulin supplementation on β-glucuronidase content has been reported (Rowland et al. Citation1998; Hijova et al. Citation2014). Current study indicated that administration of both hydrolysed inulins had the same effect on the reduction of the activities of colon cancer-associated enzymes including azoreductase, nitroreductase and β-glucuronidase (), when compared with the control group, due to the alteration of the colonic microbial load and its activities.
Current study results suggested that the dietary supplementation of inulin suppresses the occurrence of ACF induced by AOM. InuL found to be more efficient than InuS with respect to ACF reduction. ACF are believed to be the possible precursor lesions for colon cancer in rodents and human (Bird Citation1987). Inulin is reported to have an inhibitory effect on the formation of ACF. Oligofructose and inulin have the ability to reduce the ACF formation (Reddy et al. Citation1997), where inulin was considered as superior in this regard with dose-dependent functionality in rat (Femia et al. Citation2002; Verghese et al. Citation2002a,Citationb). Results of the current study indicated that dietary inulin significantly suppresses the AOM-induced ACF formation in both initiation and promotion stages of carcinogenesis (). A mixture of oligofructose and Raftiline HP (DPav of about 25) can reduce the total colonic crypts in Fisher 344 male rats (Verghese et al. Citation2005). The longer chain inulin has deliberating fermentation rate and is being fermented in the distal part of the colon when compared with the short-chain inulin (Reddy et al. Citation1997)
Conclusion
In conclusion, the results of the present study have demonstrated that dietary administration of hydrolysed inulins reduces the formation of preneoplastic lesions in the colon. Especially, InuL with a longer chain of inulin showed the higher inhibition effect on ACF than InuS. The inhibition by inulin might be due to the alteration of the colonic microecology resulting from the fermentation of the fructan. The long-chain oligosaccharides are fermented at slower rate than short-chain oligosaccharides and reach the distal colon, which is the common occurring site of colon cancer. Inulin has altered the metabolic activity of the colonic microbiota, reduced the cecal pH, stimulated the proliferation of Lactobacilli and Bifidobacteria, increased the production of SCFAs and reduced the colon cancer-associated compounds and enzymes. These are the possible mechanisms by which the anti-carcinogenic effect of inulin is exerted.
Declaration of interest
This study was supported by CMU Research Group Grant. T. P. gratefully acknowledges the financial support from Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant no. PHD/0112/2552) and National Research Council of Thailand (NRCT). The authors have declared that there are no conflicts of interest.
References
- Ahn J, Sinha R, Pei Z, Dominianni C, Wu J, Shi J, Goedert JJ, Hayes RB, Yang L. 2013. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst. 105:1907–1911.
- Berggren AM, Björck IME, Nyman EMGL, Eggum BO. 1993. Short-chain fatty acid content and pH in caecum of rats given various sources of carbohydrates. J Sci Food Agric. 63:397–406.
- Bird RP. 1987. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 37:147–151.
- Bonnet M, Buc E, Sauvanet P, Darcha C, Dubois D, Pereira B, Déchelotte P, Bonnet R, Pezet D, Darfeuille-Michaud A. 2014. Colonization of the human gut by E. coli and colorectal cancer risk. Clin Cancer Res. 20:859–867.
- Boren J, Lee WN, Bassilian S, Centelles JJ, Lim S, Ahmed S, Boros LG, Cascante M. 2003. The stable isotope-based dynamic metabolic profile of butyrate-induced HT29 cell differentiation. J Biol Chem. 278:28395–28402.
- Burns AJ, Rowland IR. 2000. Anti-carcinogenicity of probiotics and prebiotics. Curr Issues Intest Microbiol. 1:13–24.
- Callaway TR, Edrington TS, Harvey RB, Anderson RC, Nisbet DJ. 2012. Prebiotics in food animals, a potential to reduce foodborne pathogens and disease. Rom Biotech Lett. 17:7808–7816.
- Campbell JM, Fahey GC Jr, Wolf BW. 1997. Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr. 127:130–136.
- Cerini C, Dou L, Anfosso F, Sabatier F, Moal V, Glorieux G, De Smet R, Vanholder R, Dignat-George F, Sampol J, et al. 2004. P-Cresol, a uremic retention solute, alters the endothelial barrier function in vitro. Thromb Haemost. 92:140–150.
- Challa A, Rao DR, Chawan CB, Shackelford L. 1997. Bifidobacterium longum and lactulose suppress azoxymethane-induced colonic aberrant crypt foci in rats. Carcinogenesis 18:517–521.
- Comalada M, Bailón E, de Haro O, Lara-Villoslada F, Xaus J, Zarzuelo A, Gálvez J. 2006. The effects of short-chain fatty acids on colon epithelial proliferation and survival depend on the cellular phenotype. J Cancer Res Clin Oncol. 132:487–497.
- Danilcenko H, Jarienė E, Aleknaviciene P. 2008. Quality of Jerusalem artichoke (Helianthus tuberosus L.) tubers in relation to storage conditions. Not Bot Hort Agrobot Cluj. 36:23–27.
- de Morenode LeBlanc A, Perdigon G. 2005. Reduction of beta-glucuronidase and nitroreductase activity by yoghurt in a murine colon cancer model. Biocell 29:15–24.
- De Preter V, Vanhoutte T, Huys G, Swings J, De Vuyst L, Rutgeerts P, Verbeke K. 2007. Effects of Lactobacillus casei Shirota, Bifidobacterium breve, and oligofructose-enriched inulin on colonic nitrogen-protein metabolism in healthy humans. Am J Physiol Gastrointest Liver Physiol. 292:G358–G368.
- Deeptha K, Kamaleeswari M, Sengottuvelan M, Nalini N. 2006. Dose dependent inhibitory effect of dietary caraway on 1,2-dimethylhydrazine induced colonic aberrant crypt foci and bacterial enzyme activity in rats. Invest New Drugs 24:479–488.
- Duan H, Heckman CA, Boxer LM. 2005. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol. 25:1608–1619.
- Femia AP, Luceri C, Dolara P, Giannini A, Biggeri A, Salvadori M, Clune Y, Collins KJ, Paglierani M, Caderni G. 2002. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis 23:1953–1960.
- Fung KY, Brierley GV, Henderson S, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove L. 2011. Butyrate-induced apoptosis in HCT116 colorectal cancer cells includes induction of a cell stress response. J Proteome Res. 10:1860–1869.
- Geng J, Fan H, Tang X, Zhai H, Zhang Z. 2013. Diversified pattern of the human colorectal cancer microbiome. Gut Pathog. 5:2.
- Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 125:1401–1412.
- Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, Gareau M, Murphy EF, Saulnier D, Loh G, et al. 2010. Dietary prebiotics: current status and new definition. Food Sci Technol Bull Funct Foods 7:1–19.
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. 1994. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 54:2390–2397.
- Gorbach SL, Goldin BR. 1990. The intestinal microflora and the colon cancer connection. Rev Infect Dis. 12:S252–S261.
- Guinane CM, Cotter PD. 2013. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Therap Adv Gastroenterol. 6:295–308.
- Haberer P, du Toit M, Dicks LM, Ahrens F, Holzapfel WH. 2003. Effect of potentially probiotic lactobacilli on faecal enzyme activity in minipigs on a high-fat, high-cholesterol diet-a preliminary in vivo trial. Int J Food Microbiol. 87:287–291.
- Henningsson A, Bjorck I, Nyman M. 2001. Short-chain fatty acid formation at fermentation of indigestible carbohydrates. Scand J Nutr. 45:165–168.
- Hijova E, Chmelarova A. 2007. Short chain fatty acids and colonic health. Bratisl Lek Listy 108:354–358.
- Hijova E, Szabadosova V, Strojny L, Bomba A. 2014. Changes chemopreventive markers in colorectal cancer development after inulin supplementation. Bratisl Lek Listy 115:76–79.
- Hirschhaeuser F, Sattler UG, Mueller-Klieser W. 2011. Lactate: a metabolic key player in cancer. Cancer Res. 71:6921–6925.
- Hughes R, Magee EA, Bingham S. 2000. Protein degradation in the large intestine: relevance to colorectal cancer. Curr Issues Intest Microbiol. 1:51–58.
- Hughes R, Rowland IR. 2000. Metabolic activities of the gut microflora in relation to cancer. Microb Ecol Health D 12:179–185.
- Khuhaprema T, Sangrajrang S, Lalitwongsa S, Chokvanitphong V, Raunroadroong T, Ratanachu-Ek T, Muwonge R, Lucas E, Wild C, Sankaranarayanan R. 2014. Organised colorectal cancer screening in Lampang Province, Thailand: preliminary results from a pilot implementation programme. BMJ Open 4:e003671.
- Khuhaprema T, Srivatanakul P. 2008. Colon and rectum cancer in Thailand: an overview. Jpn J Clin Oncol. 38:237–243.
- Kirjavainen PV, Arvola T, Salminen SJ, Isolauri E. 2002. Aberrant composition of gut microbiota of allergic infants: a target of bifidobacterial therapy at weaning? Gut 51:51–55.
- Larsson SC, Wolk A. 2006. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer 119:2657–2664.
- Lee SM, Lee WK. 2001. Effects of lactic acid bacteria on intestinal microbial enzyme activity and composition in rats treated with azoxymethane. J Microbiol. 39:154–161.
- Lima ES, Abdalla DSP. 2002. High-performance liquid chromatography of fatty acids in biological samples. Anal Chim Acta. 465:81–91.
- Loh G, Eberhard M, Brunner RM, Hennig U, Kuhla S, Kleessen B, Metges CC. 2006. Inulin alters the intestinal microbiota and short-chain fatty acid concentrations in growing pigs regardless of their basal diet. J Nutr. 136:1198–1202.
- Mager DL. 2006. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 4:14. DOI: 10.1186/1479-5876-4-14.
- McCall IC, Betanzos A, Weber DA, Nava P, Miller GW, Parkos CA. 2009. Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmacol. 241:61–70.
- Nagengast F, Grubben M, van Munster I. 1995. Role of bile acids in colorectal carcinogenesis. Eur J Cancer 31:1067–1070.
- Niness KR. 1999. Inulin and oligofructose: what are they? J Nutr. 129:1402S–1406S.
- Nowak A, Libudzisz Z. 2006. Influence of phenol, p-cresol and indole on growth and survival of intestinal lactic acid bacteria. Anaerobe 12:80–84.
- Nowak A, Libudzisz Z. 2007. Ability of intestinal lactic bacteria to bind or/and metabolize phenol and p-cresol. Ann Microbiol. 57:329–335.
- Pan X, Chen F, Wu T, Tang H, Zhao Z. 2009. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J Zhejiang Univ Sci B. 10:258–263.
- Pang T, Leach ST, Katz T, Day AS, Ooi CY. 2014. Fecal biomarkers of intestinal health and disease in children. Front Pediatr. 2:6. doi:10.3389/fped.2014.00006
- Pattananandecha T, Sirilun S, Sivamaruthi BS, Suwannalert P, Sartjin Peerajan S, Chaiyasut C. 2015. Hydrolyzed inulin with different degree of polymerization as prebiotic for Lactobacillus plantarum. J Pure Appl Microbiol. 9:973–979.
- Propst EL, Flickinger EA, Bauer LL, Merchen NR, Fahey GC Jr. 2003. A dose–response experiment evaluating the effects of oligofructose and inulin on nutrient digestibility, stool quality, and fecal protein catabolites in healthy adult dogs. J Anim Sci. 81:3057–3066.
- Rafii F, Franklin W, Cerniglia CE. 1990. Azoreductase activity of anaerobic bacteria isolated from human intestinal microflora. Appl Environ Microbiol. 56:2146–2151.
- Reddy B, Hamid R, Rao CV. 1997. Effect of dietary oligofructose and inulin on colonic preneoplastic aberrant crypt foci inhibition. Carcinogenesis 18:1371–1374.
- Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D. 2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol. 71:6150–6158.
- Rowland IR, Rumney CJ, Coutts JT, Lievense LC. 1998. Effect of Bifidobacterium longum and inulin on gut bacterial metabolism and carcinogen-induced aberrant crypt foci in rats. Carcinogenesis 19:281–285.
- Ruemmele FM, Schwartz S, Seidman EG, Dionne S, Levy E, Lentze MJ. 2003. Butyrate induced Caco-2 cell apoptosis is mediated via the mitochondrial pathway. Gut 52:94–100.
- Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG Jr. 1980. Absorption of short-chain fatty acids by the colon. Gastroenterology 78:1500–1507.
- Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. 1994. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–1049.
- Sandhu MS, White IR, Mc Pherson K. 2001. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol Biomarkers Prev. 10:439–446.
- Schwabe RF, Jobin C. 2013. The microbiome and cancer. Nat Rev Cancer 13:800–802.
- Siavoshian S, Segain J, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottière HM. 2000. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut 46:507–514.
- Slavin J. 2013. Fiber and prebiotics: mechanisms and health benefits. Nutrients 5:1417–1435.
- Swanson KS, Grieshop CM, Flickinger EA, Bauer LL, Healy HP, Dawson KA, Merchen NR, Fahey GC Jr. 2002. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J Nutr. 132:980–989.
- Topping D, Clifton P. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 81:1031–1064.
- Uccello M, Malaguarnera G, Basile F, D’agata V, Malaguarnera M, Bertino G, Vacante M, Drago F, Biondi A. 2012. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 12:S35.
- Vande Wiele T, Boon N, Possemiers S, Jacobs H, Verstraete W. 2007. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J Appl Microbiol. 102:452–460.
- Van Loo J. 2004. The specificity of the interaction with intestinal bacterial fermentation by prebiotics determines their physiological efficacy. Nutr Res Rev. 17:89–98.
- Van Loo J, Coussement P, de Leenheer L, Hoebregs H, Smits G. 1995. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit Rev Food Sci Nutr. 35:525–552.
- Verghese M, Rao DR, Chawan CB, Shackelford L. 2002a. Dietary inulin suppresses azoxymethane-induced preneoplastic aberrant crypt foci in mature Fisher 344 rats. J Nutr. 132:2804–2808.
- Verghese M, Rao DR, Chawan CB, Williams LL, Shackelford L. 2002b. Dietary inulin suppresses azoxymethane-induced aberrant crypt foci and colon tumors at the promotion stage in young Fisher 344 rats. J Nutr. 132:2809–2813.
- Verghese M, Walker LT, Shackelford L, Chawan CB. 2005. Inhibitory effects of nondigestible carbohydrates of different chain lengths on azoxymethane-induced aberrant crypt foci in Fisher 344 rats. Nutr Res. 25:859–868.
- Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. 2008. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 19:587–593.
- Whitlock K, Gill RS, Birch DW, Karmali S. 2012. The association between obesity and colorectal cancer. Gastroenterol Res Pract. 2012:1–6.
- Windey K, De Preter V, Verbeke K. 2012. Relevance of protein fermentation to gut health. Mol Nutr Food Res 56:184–196.
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. 2006. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 40:235–243.
- Zackular JP, Rogers MA, Ruffin MT 4th, Schloss PD. 2014. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev Res (Phila). 7:1112–1121.
- Zhang X, Ohta Y. 1993. Antimutagenicity of cell fractions of microorganisms on potent mutagenic pyrolysates. Mutat Res. 298:247–253.
- Zhao Y, Hasjim J, Li L, Jane JL, Hendrich S, Birt DF. 2011. Inhibition of azoxymethane-induced preneoplastic lesions in the rat colon by a cooked stearic acid complexed high-amylose cornstarch. J Agric Food Chem. 59:9700–9708.