Abstract
Context The bark of Ailanthus altissima (Mill.) Swingle (Simaroubaceae) is traditionally used to treat ascariasis, diarrhoea, spermatorrhoea, bleeding and gastrointestinal diseases.
Objective The objective of this study is to investigate the antitumour activity and mechanism of 2-dihydroailanthone isolated from A. altissima.
Materials and methods The U251 cells were treated with 1.00, 4.00 and 8.00 μg/mL of 2-dihydroailanthone for 48 h and the normal cells treated with 20.00 μg/mL of 2-dihydroailanthone were tested as well. Proliferation inhibition of 2-dihydroailanthone on the cells was tested by MTT. Apoptosis and cell-cycle distribution in U251 cells with 1.00, 3.00 and 5.80 μg/mL of 2-dihydroailanthone for 48 h were determined by flow cytometry, respectively. The expression of the apoptosis-related genes and proteins was analysed by RT-PCR and Western blot method, respectively.
Results MTT assay revealed that 2-dihydroailanthone inhibited U251 cells proliferation. The cell viability of U251 cells was 62.82, 31.34 and 25.58%, and that of three normal cells was 72.75, 82.74 and 44.92%, respectively. Flow cytometry assay showed that 2-dihydroailanthone induced apoptosis and G0/G1 phase cycle arrest towards U251 cells. The late apoptotic cells were 11.37, 21.73 and 33.83%, and the cells cycle distributed in the G0/G1 accounted for 48.85, 62.77 and 64.40%, respectively. The Western blot and RT-PCR assay showed that up-regulation of pro-apoptotic bax protein and down-regulation of anti-apoptotic bcl-2 protein as well as their mRNA on U251 cells might be related to the apoptosis induction and proliferation inhibition.
Conclusion An important bioactive component, 2-dihydroailanthone, has antitumour effects, enlightening a novel source of phytomedicines in tumour therapy.
Keywords:
Introduction
Glioma is the most common primary tumour in the central nervous system (Benesch et al. Citation2006) with a higher incidence, mortality and recurrence, but a lower cure rate (Partap & Fisher Citation2007). The conventional therapies including surgery, radiotherapy and chemotherapy have played important roles in tumour therapy including glioma, whereas there are still many problems in tumour therapy (Sathornsumetee et al. Citation2007). Teniposide, lomustine, cisplatin and semustine capsules have been currently used in the treatment of gliomas in clinic (Robles Irizarry et al. Citation2012). However, the effective concentration of drugs for chemotherapy may be attenuated because of the blood–brain barrier, tumour tissue and peripheral oedema interstitial hydrostatic pressure (Mikuni & Miyamoto Citation2010). The drug resistance of the tumour may reduce the efficiency of chemotherapy and the adverse reactions may also limit its usage (Asthagiri et al. Citation2007). The tolerance of tumour cells to radiation may cause residual lesion recurrence as well.
Despite great progress in medicine, there is still a requirement to develop more effective drugs to cure most tumours with low toxicity and high efficacy without major environmental impact. Some plants have long been used often in tumour therapy (John et al. Citation2002; Efferth et al. Citation2007; Dennis et al. Citation2009; Olaku & White Citation2011; Bishayee Citation2012), such as Ailanthus altissima (Mill.) Swingle (Simaroubaceae). Ailanthus altissima, growing in the northeast and central China, and also found in Europe and the United States, has been used as traditional Chinese medicine for many years. The bark of A. altissima has been used for the treatment of ascariasis, diarrhoea, spermatorrhoea, bleeding, and gastrointestinal diseases in China (Rahman et al. Citation1997); its antitumour effect enables administration for the treatment of colon, cervical and rectal cancers has been described recently (Wang et al. Citation2013). We have isolated an important compound, 2-dihydroailanthone, from the plant quassinoid successfully, but its antitumour activity, especially for glioma, and the underlying mechanism remains to be clarified. We have used U251 glioma cells to evaluate the antitumour activity of 2-dihydroailanthone and the underlying mechanism.
Materials and methods
Annexin V-FITC Apoptosis Detection kit was purchased from Key Gen Corporation (Beijing, China). Propidium iodide (PI) was purchased from Sigma Chemical Company (St. Louis, MO). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was purchased from Promega (Madison, WI). Primary antibodies β-actin (1:500), B-cell lymphoma 2 (Bcl-2) (1:500), and bcl-2-associated X protein (Bax) (1:400) were purchased from Santa Cruz Biotechnology (Santa Cruz County, CA). Dimethyl sulphoxide (DMSO), trypsin, gentamycin sulphate and glycerol were purchased from Amresco (Solon, OH). Foetal bovine serum was purchased from Invitrogen (Carlsbad, CA). Penicillin and streptomycin was purchased from Genview (Galveston, TX). RPMI1640 medium was purchased from Hyclone Company (Cramlington, Northumberland, UK). All the other reagents used were of analytical reagent grade obtained commercially.
Plant material and extraction
The bark of A. altissima (NMC-2012-2) was collected in Anguoshi, Hebei province China in December 2012, and identified by Prof. Chang-Hong Huo (School of Pharmaceutical Sciences, Hebei Medical University, China). The bark of A. altissima was shattered to meal, following 10 times ethanol added to the bark, soaked for 12 h, extracted by hot reflux two times, each time for 2 h, and then filtered and merged to the filtrate. The combined ethanol extracts were concentrated in vacuo, and then added to with saturated salt solution, extracted with petroleum ether and dichloromethane in turn and dried in vacuo. The extract with dichloromethane was separated with 50 times the amount of 200–300 mesh silica gel column chromatographies. Mobile phase was dichloromethane:methanol (50:1, 30:1, 15:1, 10:1, 5:1), each 500 mL was collected, totally 211 fractions were collected. Similar fractions were combined on the basis of TLC to obtain 35 major fractions. After further purification by crystallisation, we obtained 2-dihydroailanthone (Casinovi et al. Citation1983).
Cell culture
The cells (U251 glioma cells and H9C2, PC12, and 293T normal cells) were purchased from Shanghai Institute of Cell Biology (Shanghai, China), and grown in DMEM. DMEM was supplemented with 10% (v/v) FBS, 100 units/mL penicillin and 100 μg/mL streptomycin. Cells were cultured at 37 °C and 5% CO2 in a humidified environment. The cells used in the experiment have reproduced the fourth generation.
Cell viability evaluation
Cell viability was assessed by MTT colorimetric assay (Bishayee et al. Citation2010). U251 and normal cells were seeded into 96-well plates at a density of 1 × 104 cells/well in 0.1 mL medium. U251 cells were treated with various concentrations (0, 1.00, 4.00 and 8.00 μg/mL) of 2-dihydroailanthone for 48 h, and the normal cells were treated with 20.0 μg/mL 2-dihydroailanthone. At the end of the treatment, 50 μL MTT (5 mg/mL) was added to each well and the samples were incubated for an additional 1 h. The culture medium was removed and dissolved by adding 100 μl of DMSO. The absorbance was measured at 490 nm versus 630 nm. The vehicle used to dissolve the 2-dihydroailanthone was DMSO and the final percentage of the vehicle in the cells was 0.1%.
Cell apoptosis
U251 cell apoptosis was quantified by flow cytometry with AnnexinV labelling and PI exclusion staining (for each sample, 10 000 events were collected). Cells undergoing apoptosis were stained with Annexin V and treated as described above. Briefly, U251 cells were collected, washed with PBS and suspended in binding buffer. Then the cells (1 × 106 cells/mL) were stained with 10 μL AnnexinV-FITC and 5 μL PI, incubated in dark at room temperature for 15 min according to the instructions of the manufacturer and subjected to flow cytometry. The data were analysed with Cell Quest software (SAS Inc., Cary, NC). The excitation wavelength was 488 nm, the emission wavelength was 525 nm, and the number of cells was 1 × 106 in apoptosis.
Cell-cycle distributions
The flow cytometric evaluation of the cell-cycle status was performed according to a method described previously (for each sample, 10 000 events were collected). Cells were seeded at a density of 1 × 106/mL in 6-well microplates, grew for 48 h and then treated with different doses of 2-dihydroailanthone for 48 h. Cells were then washed with PBS, trypsinised with 0.25% trypsin and harvested by centrifugation for 5 min at 1000 rpm. The cells were re-suspended with 0.5 mL PBS, fixed overnight with cold 70% ethanol and followed by staining with PI solution containing 50 μg/mL PI and 10 μg/mL RNase A. After incubation at room temperature for 60 min, cells were analysed by flow cytometry (Kim et al. Citation2006).
RT-PCR analysis
Following the extraction of total cellular RNA from the cells using the Trizol protocol, 2 μg of total RNA was reverse transcribed to generate cDNA using the PrimeScript RT reagent Kit (Promega, Madison, WI). RT-PCR was carried out using a SYBR Green method with the Applied Biosystems SDS 7500 instrument (Applied Biosystems Inc., Foster City, CA), according to the protocol of the manufacturer. Samples were amplified using the following PCR variables: 94 °C for 30 s followed by 30 cycles at 94 °C for 30 s, 56 °C for 30 s, and then 72 °C for 45 s, the last at 72 °C extension for 8 min. PCR samples were loaded onto a 2% agarose gel containing ethidium bromide and the bonds were analysed by Ultraviolet spectrophotometer. The primer sequences were as follows: bax (258 bp), F-5-CACCA GCTCTGAGCAGATCA-3, R-5-ATGTCAGCTGCCAC TCGGA-3, bcl-2 (383 bpm), F-5-TACGAGTGGGAT GCGGGAGATGT-3, R-5-CCACCGAACTCAAAGA AGGC-3, gapdh (135 bp), F-CAATGACCCCTTCATTGACC, R-TGGAAGATGGTGATGGGATT.
Western blot
Cells were lysed in lysis buffer: 50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 0.5% NP40, 0.5% sodium deoxycholate, 0.1% SDS, plus protease inhibitor. The proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 0.5% milk in PBS for 1 h at room temperature. After being washed in Tris-buffered saline Tween (TBST), the membranes were incubated for 1 h at room temperature with an appropriate dilution of rabbit polyclonal TRIM28 antibody. After being washed in TBS-T, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG for 1 h and exposed to X-ray film at room temperature. The signal was detected by chemiluminescence using ECL detection kit (Applied Biosystems Inc., Foster City, CA).
Statistical analysis
We used SPSS 16.0 analysis software (SPSS Inc., Chicago, IL) for statistical analysis of the data. Measurement data were expressed as means ± SD. A one-way analysis of variance (ANOVA) followed by Dunnett’s test was used for statistical analysis. Probability (p) values less than 0.05 were considered significant.
Results
Inhibitory effect of 2-dihydroailanthone on the proliferation of tumour cells
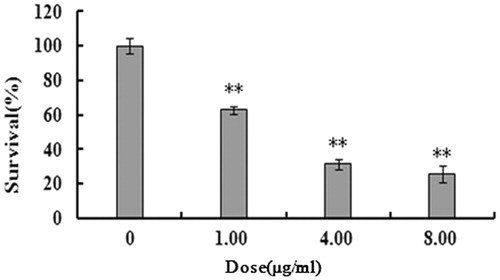
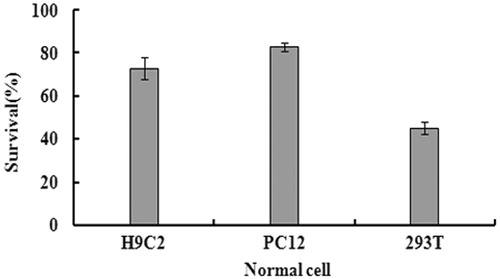
We examined the effect of 2-dihydroailanthone on U251 and normal cells viability by MTT assay. As shown in , compared with untreated control (p <0.05), the cell viability of U251 cells treated with 1.00, 4.00 and 8.00 μg/mL of 2-dihydroailanthone for 48 h was 62.82%, 31.34% and 25.58%, respectively. The viability of H9C2, PC12 and 293T normal cells was 72.75%, 82.74% and 44.92% in , respectively. The data suggest that 2-dihydroailanthone inhibits U251 cell proliferation.
Effects of on apoptosis of U251 cells
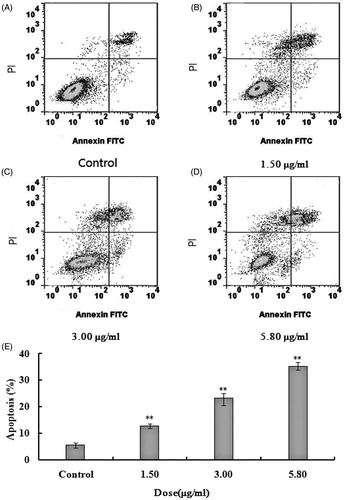
The late apoptotic cells after 48 h were 11.37%, 21.73% and 33.83%, respectively, by the intervention of different doses of 2-dihydroailanthone (control, 1.50, 3.00 and 5.80 μg/mL), all of which were statistically significant, compared with the control (p <0.05) ().
Figure 3. Apoptosis of U251 cells after treatment with different doses of 2-dihydroailanthone. U251 cells were treated with different doses of 2-dihydroailanthone for 48 h and measured by flow cytometry. (A) Control, (B) 1.50 μg/mL, (C) 3.00 μg/mL, (D) 5.80 μg/mL, (E) histogram of apoptosis of U251 cells, *p < 0.05, **p < 0.01 (n = 3).
Effects of 2-dihydroailanthone on cell cycle against U251
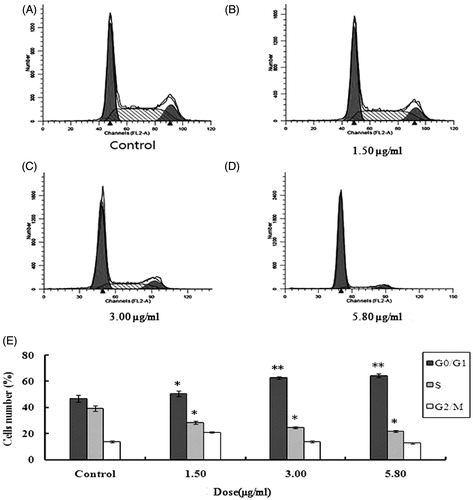
Normal cells mainly distributed in the G0/G1 period accounted for 43.80% of the total cell population and those of stage S and G2/M each accounted for 41.67% and 14.53%. After being exposed to 1.50, 3.00 5.80 μg/mL of 2-dihydroailanthone for 48 h, the experiment group showed statistical significant, compared with the control. After being given different doses of 2-dihydroailanthone, cells increased in the phase G0/G1, and decreased in phase S and G2/M, which shows each doses have statistically significant, compared with the control (p <0.05) ().
Figure 4. Cell cycle of U251 cells after treatment with different doses of 2-dihydroailanthone. U251 cells were treated with different doses of 2-dihydroailanthone for 48 h and the cell cycle were measured by flow cytometry. (A) Control, (B) 1.50 μg/mL, (C) 3.00 μg/mL, (D) 5.80 μg/mL, (E) histogram of cell cycle of U251 cells, *p < 0.05, **p < 0.01 (n = 3).
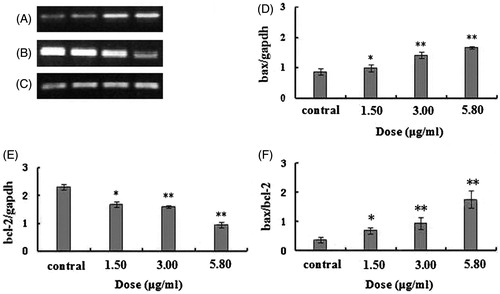
The expression changes of bcl-2 and bax mRNA
After 48 h treatment by different doses of 2-dihydroailanthone, the result showed by RT-PCR, in the U251 cells, the expression of bax gene was promoted and bcl-2 gene was decreased. The doses of 1.50, 3.00 and 5.80 μg/mL of 2-dihydroailanthone were significantly effective, compared with the control (p <0.05) ().
Figure 5. The bax/bcl2 mRNA in U251 cells after treatment with different doses of 2-dihydroailanthone. After 48 h treatment by different doses of 2-dihydroailanthone, bax/bcl2 mRNA in the U251 cells were measured by RT-PCR. (A) Bax, (B) bcl-2, (C) gapdh, (D) histogram of bax, (E) histogram of bcl-2, (F) histogram of bax/bcl-2 ratio, *p < 0.05, **p < 0.01 (n = 3).
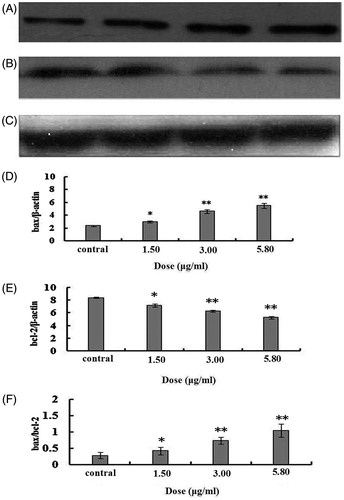
Effects on bcl-2 and bax protein expression level
After 48 h treatment by different doses of 2-dihydroailanthone, the result shown by Western blot that 2-dihydroailanthone promoted the expression of bax protein in the cells (p <0.05), while the bcl-2 protein expression was inhibited. The inhibitory effect on bcl-2 protein expression increased with the increase of drug doses (p <0.05). Correspondingly, the bax/bcl-2 ratio increased with the increase of the doses of 2-dihydroailanthone ().
Figure 6. The bax/bcl2 proteins in U251 cells after treatment with different doses of 2-dihydroailanthone. After 48 h treatment by different doses of 2-dihydroailanthone, bax/bcl2 in the U251 cells were measured by Western blot, (A) bax, (B) bcl-2, (C) β-actin, (D) bax protein content of U251 cells in different doses of 2-dihydroailanthone, (E) bcl-2 protein content of U251 cells in different doses of 2-dihydroailanthone, (F) histogram of bax/bcl-2 ratio, *p < 0.05, **p < 0.01 (n = 3).
Discussion
Phytochemical study showed the presence of quassinoids in A. altissima and the compounds have been known to possess potent antitumour properties. Malignant tumour has long been a serious disease threatening human health. There are three traditional therapies, surgery, chemotherapy and radiotherapy, which have potent effect at the early stage of malignant tumour, and even curative on some cancer patients in early and middle stages. However, they have adverse effects or serious damage on patients. As a result, natural medicines with less damage and side effects have been attracting more and more attention (Harvey Citation2000; Li & Vederas Citation2009; Duan et al. Citation2009). Whereas only limited natural medicines have been used to treat cancer or function as adjuvant therapy for clinical application (Efferth et al. Citation2007; Olaku & White Citation2011; Bishayee Citation2012). New natural antitumour drugs may play more and more important roles in the future. This study showed that 2-dihydroailanthone isolated from A. altissima has inhibited the proliferation of the tumour cells in different degrees, especially for U251 cell. Further investigations on the apoptosis and cell cycle were carried out sequentially.
Most drugs achieve antitumour effects by inducing apoptosis of tumour cells, and the apoptosis is related to cell cycle very closely. The cell-cycle analysis about the distribution of the cells in different phases is one of the important methods to evaluate antitumour effects by indication of tumour cell proliferation in contrast to apoptosis (Yang et al. Citation2009). There are three important cell-cycle regulation points in G1, S and G2 phase which block cell-cycle progression. The tumour cells blocked in G0/G1 is an important target for the development of antitumour therapy (Chen et al. Citation2010). Some molecules such as P53 are important in cell-cycle blockage and apoptosis induction. The cells may be arrested in G1 phase and the apoptosis may be induced by P53. C-MYC promotes cells in G1 phase to come into S and induces apoptosis in the case of no growth factor. In this research, detected by flow cytometry after treatment by different doses of 2-dihydroailanthone, the apoptosis in U251 cells increased with the increase of 2-dihydroailanthone doses. The analysis of the cell cycle by flow cytometry showed the increase of the cell number of U251 cells in G0/G1 phase after being induced and decrease in S phase, suggesting that the cells were arrested in G0/G1 phase by apoptosis induction in U251 cells, so the cells could not enter S phase for DNA synthesis, and the proliferation of the cells was restrained eventually. The cell cycle checkpoint in G2/M also plays a very important role (Zhang et al. Citation2003). It is an important checkpoint of preventing cells with DNA damage from dividing, which may be involved in the effects of 2-dihydroailanthone on the cell cycle of U251 cells as well, but remains to be further revealed in the future.
In this study, flow cytometry assay for apoptosis detection were used with PI staining by which the cellular death can be identified meanwhile. The percentage of dead cells in experimental groups versus control calculated from the flow cytometry assay was less in corresponding to the inhibition of proliferation calculated from MTT assay, illustrating existence of arrest in the cell cycle by 2-dihydroailanthone partially contribute to the proliferation inhibition.
The tumour inhibition and apoptosis, as well as the expression of bcl-2 and bax were examined by flow cytometry and immunoblotting, respectively. The results showed that 2-dihydroailanthone can induce tumour cell apoptosis. Apoptosis is a kind of active cell suicide process regulated by some genes. Bax has the role against bcl-2 and promotes the release of cytochrome in the mitochondria upon cell apoptosis. The effect of the bcl-2 depends on the struggle against bax; therefore, their ratio determines the cells apoptosis or survival upon signal stimulation. Excessive bax expression in the cells may result in apoptosis, whereas excessive bcl-2 expression will survive instead.
The western blot method was used to detect bax and bcl-2 protein and it was found that after treatment with 2-dihydroailanthone at 1.50, 3.00 and 5.80 μg/mL on U251 cells for 48 h, the bax increased, while the bcl-2 dropped significantly. The results suggested that the mechanism of 2-dihydroailanthone inducing U251 cell apoptosis may be related to the adjusting of the bax and the bcl-2 family proteins. The experimental results showed that 2-dihydroailanthone on U251 cell had proliferation inhibitory effect significantly and can induce cell apoptosis. The regulation and activation of caspase apoptosis induced by protease cascade by death receptor pathway and mitochondrial pathway, at the same time, may have their contribution, and the promotion on the bax and the inhibition on the bcl-2 protein may further enhance the antitumour effect.
The intervention on U251 cells by 2-dihydroailanthone showed typical cell apoptosis. With the increase of drug doses, the apoptosis rate increased. The cells in S and G2/M phase were less than that of control, after cells were intervened by different doses of 2-dihydroailanthone for 48 h. The antitumour effects of 2-dihydroailanthone in this experimental study suggested the potential clinical value of 2-dihydroailanthone in the treatment of gliomas. Further investigations may be performed in the future to identify the active principle responsible for the antitumour activity.
Conclusions
This study showed that an important bioactive component, 2-dihydroailanthone, which was isolated from A. altissima had inhibitory effect on U251 cells, can increase cells in G0/G1 phase, decrease cells in S and G2/M phase and promotes cell apoptosis with increased bax and its mRNA and decreased bcl-2 and its mRNA, suggesting that 2-dihydroailanthone has the potential antitumour activity, which enlighten a novel source of phytomedicines in tumour therapy.
Funding information
This work was supported by the National Natural Science Foundation of China (Grant no. 81302664), the colleges and universities in Hebei province scientific research projects (Q2012096).
Acknowledgements
The authors thank Professor Dr. Jun-Qing Liang for providing some necessary facilities to carry out this research work.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Asthagiri AR, Pouratian N, Sheman J. 2007. Advances in brain tumor surgery. Neurol Clin. 25:975–1003.
- Bishayee A. 2012. Editorial: current advances in cancer prevention and treatment by natural products. Curr Pharm Biotechnol. 13:115–116.
- Bishayee A, Haznagy-Radnai E, Mbimba T, Sipos P, Morazzoni P, Darvesh AS, Bhatia D, Hohmann J. 2010. Anthocyanin-rich black currant extract suppresses the growth of human hepatocellular carcinoma cells. Nat Prod Commun. 5:1613–1618.
- Benesch M, Eder HG, Sovinz P. 2006. Residual or recurrent cerebellar low-grade glioma in children after tumor resection: is re-treatment needed? A single center experience from 1983 to 2003. Pediatr Neurosurg. 42:159–164.
- Casinovi CG, Ceccherelli P, Fardella G. 1983. Isolation and structure of a quassinoid from Ailanthus glandulosa. Phytochemistry. 22:2871–2873.
- Chen M, Xu XY, Xu DY, Zhao FZ, Xu QY. 2010. Inhibiting Bcl-2 gene expression enhance radiosensitivity of non-small cell lung cancer NCI-H460 cells. China Oncol. 20:641–645.
- Dennis T, Fanous M, Mousa S. 2009. Natural products for chemopreventive and adjunctive therapy in oncologic disease. Nutr Cancer. 61:587–597.
- Duan JA, Su SL, Qian DW. 2009. Approaches and advances in the resources chemistry of Chinese medicinal material. Chin J Nat Med. 7:333–340.
- Efferth T, Li PC, Konkimalla VS, Kaina B. 2007. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 13:353–361.
- Harvey A. 2000. Strategies for discovering drugs from previously unexplored natural products. Drug Discov Today. 5:294–300.
- John WH, Leung YK, Chun PC. 2002. Herbal medicine in the treatment of cancer. Cur Med Chem Anticancer Agents. 2:209–214.
- Kim MJ, Kim YJ, Park HJ. 2006. Apoptotic effect of red wine polyphenols on human colon cancer SNU-C4 cells. Food Chem Toxicol. 44:898–902.
- Li JW, Vederas JC. 2009. Drug discovery and natural products: end of an era or an endless frontier? Science. 325:161–165.
- Mikuni N, Miyamoto S. 2010. Surgical treatment for glioma: extent of resection applying functional neurosurgery. Neurol Med Chir Tokyo. 9:720–726.
- Olaku O, White JD. 2011. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer. 47:508–514.
- Partap S, Fisher PC. 2007. Update on new treatments and developments in childhood brain tumors. Curr Opin Pediatr. 19:670–674.
- Rahman S, Fukamiya N, Ohno N, Tokuda H, Nishino H. 1997. Inhibitory effects of quassinoid derivatives on Epstein–Barr virus early antigen activation. Chem Pharm Bull. 45:675–677.
- Robles Irizarry L, Hambardzumyan D, Nakano I, Gladson CL, Ahluwalia MS. 2012. Therapeutic targeting of VEGF in the treatment of glioblastoma. Expert Opin Ther Targets. 16:973–984.
- Sathornsumetee S, Rich JN, Reardon DA. 2007. Diagnosis and treatment of high-grade astrocytoma. Neurol Clin. 25:1111–1139.
- Wang Y, Wang WJ, Su C, Zhang DM, Xu LP, He RR, Wang L, Zhang J, Zhang XQ, Ye WC. 2013. Cytotoxic quassinoids from Ailanthus altissima. Bioorg Med Chem Lett. 23:654–657.
- Yang YP, Li M, Xu B. 2009. Allicin induces apoptosis, cell cycle arrest and microtubule disassembly in human nasopharyngeal carcinoma KB cells. J Pharm Sci. 18:114–120.
- Zhang Z, Leonard S, Huang C, Vallyathan V, Castranova V, Shi X. 2003. Role of reactive oxygen species and MAPKs in vanadate-induced G(2)/M phase arrest. Free Radic Biol Med. 34:1333–1342.