Abstract
Context Fibrates were reported to have anti-inflammatory effects while the naturally occurring polyphenol resveratrol was traditionally known as a potent antioxidant agent.
Objective The effects of fenofibrate and resveratrol were investigated on complete Freund’s adjuvant (CFA)-induced rheumatoid arthritis (RA) in adult female albino rats.
Materials and methods Rats were divided into a normal control group, an arthritis control group receiving CFA, two reference treatment groups receiving dexamesathone (1.5 mg/kg/day) and methotrexate (1 mg/kg/day), and two treatment groups receiving fenofibrate (100 mg/kg/day) and resveratrol (10 mg/kg/day) for seven consecutive days. Assessment of RA was performed by measuring serum rheumatoid factor (RF), matrix metalloprotinease-3 (MMP-3) and cartilage oligomeric matrix protein (COMP) as specific rheumatoid biomarkers, immunoglobulin G (IgG) and antinuclear antibody (ANA) as immunological biomarkers, tumour necrosis factor-alpha (TNF-α) and interleukin-10 (IL-10) as immunomodulatory cytokines, myeloperoxidase (MPO) and C-reactive protein (CRP) as inflammatory biomarkers and malondialdehyde (MDA) and glutathione (GSH) as oxidative stress biomarkers, supported by a histopathological study on joints and spleens.
Results Serum RF, MMP-3, COMP, IgG, ANA, TNF-α, MPO, CRP and MDA were decreased to about 36, 56, 66, 65, 9, 35, 24, 44 and 31% by fenofibrate, and to about 37, 59, 44, 70, 5, 30, 23, 33 and 28% by resveratrol treatments, respectively. Alternatively, serum IL-10 and GSH were significantly increased to about 215 and 251% by fenofibrate and to about 225 and 273% by resveratrol treatments, respectively.
Discussion and conclusion Fenofibrate and resveratrol protect against RA, possibly through their immunomodulatory, anti-inflammatory and antioxidant potential.
Introduction
Approximately 1% of the population is confirmed to suffer from the disabling autoimmune disorder termed rheumatoid arthritis (RA) (Vivar & Van Vollenhoven Citation2014). RA is a destructive polyarthropathy associated with joint swelling and massive pain and usually leads to disability and even complete immobility. During the course of disease progression, other organs may be involved (Haleagrahara et al. Citation2013), particularly the spleen (Patel & Shah Citation2013; Zhang et al. Citation2014).
The disease progression is characterised by synovial membrane hyperplasia and increased vascularity and inflammatory cell infiltration, primarily with CD4+ T-lymphocytes, which are the main regulators of cell-mediated immune responses (Lubberts Citation2015). RA was earlier suggested to be caused by an unidentified arthritogenic antigen, while recently a number of possible endogenous antigens, including citrullinated protein, human cartilage glycoprotein and heavy-chain binding protein, have been identified (McInnes & Schett Citation2011; Valesini et al. Citation2015). Antigen-activated CD4+ T cells stimulate B cells through cell-surface contact and through binding with specific clusters of differentiation to produce immunoglobulins, including rheumatoid factor (RF) and antinuclear antibody (ANA) (Biesen et al. Citation2014). RF is an antibody directed against the Fc region of immunoglobulin G (IgG) and sometimes immunoglobulin M (IgM), precipitating immune complexes initiating inflammatory cascades through complement fixation and chemotaxis (Ingegnoli et al. Citation2013). ANA is an autoantibody directed against nuclear components and is commonly elevated in RA (Hügle et al. Citation2014).
Antigen-activated CD4+ T cells also stimulate monocytes, macrophages and synovial fibroblasts to produce the cytokines interleukin-1 (IL-1; Lopalco et al. Citation2015), IL-6 (Liu et al. Citation2015), IL-10 (Saxena et al. Citation2014) and tumour necrosis factor-alpha (TNF-α; Edupuganti et al. Citation2015) and to secrete matrix metalloproteinases (MMPs), which are a large family of endonucleases that can degrade any extracellular matrix protein (Mamehara et al. Citation2010; Kizaki et al. Citation2015). Unlike most cytokines, IL-10 is a potent anti-inflammatory cytokine that plays a crucial role in preventing inflammatory and autoimmune pathologies (Iyer & Cheng Citation2012). Persistent inflammation results in the destruction of cartilage and bone through the oxidative and proteolytic breakdown of collagen and proteoglycans. The cartilage oligomeric matrix protein (COMP), a non-collagenous extracellular matrix protein found mainly in cartilage, is released first into the synovial fluid and next diffused into the blood in varying disease conditions of cartilage damage (Kawashiri et al. Citation2010; Andersson et al. Citation2013). Inflammatory cells are rich in a haem enzyme termed myeloperoxidase (MPO), which is therefore present at high concentrations in synovial fluid and blood in RA (Stamp et al. Citation2012). Additionally, C-reactive protein (CRP) is an acute-phase protein of hepatic origin that increases following IL-6 secretion from macrophages and T cells in inflammatory reactions and, therefore, its serum level increases in RA (Medeiros et al. Citation2015). Inflammatory cells cause tissue destruction through the release of proteases and several oxidants like reactive oxygen species (Yu et al. Citation2015), leading to depletion of tissue glutathione (GSH), the most common antioxidant peptide in mammals, and elevation of malondialdehyde (MDA), a final end product of lipid peroxidation (Su et al. Citation2003).
Most currently available treatment lines of RA depend on anti-inflammatory and immunosuppressive agents, like corticosteroids and methotrexate, which unfortunately have many undesired adverse effects (Rau Citation2014; Lampropoulos et al. Citation2015). Therefore, new drug classes for the treatment of RA are claimed. Fenofibrate, a common lipid lowering agent used in the treatment of cardiovascular disorders, was reported to possess anti-inflammatory properties (Castillero et al. Citation2012). It was also reported to be beneficial for the treatment of autoimmunity and inflammatory diseases (Zhou et al. Citation2012; Osada et al. Citation2014). Resveratrol is a natural polyphenol (flavonoid) found in the skin of red grapes which has an effective role in inflammation, oxidation, cancer and aging. Resveratrol was reported to have potent antioxidant (Bellaver et al. Citation2014) and anti-inflammatory (Zhu et al. Citation2011) activities. It was also reported that resveratrol might have an immunomodulatory action in vitro and in vivo (Zou et al. Citation2013).
Based on the aforementioned data, the present investigation aimed to evaluate the possible beneficial effects of fenofibrate and resveratrol, as compared to the standard drugs dexamesathone and methotrexate, on experimental RA in rats. To fulfil this purpose, serum RF, MMP-3 and COMP as specific rheumatoid biomarkers, IgG and ANA as immunological biomarkers, TNF-α and IL-10 as immunomodulatory cytokines, MPO and CRP as inflammatory biomarkers, as well as MDA and GSH as oxidative stress biomarkers were measured, accompanied by a histopathological study of joint and spleen sections.
Materials and methods
Animals
The study was performed on adult female Wistar rats, weighing 200–220 g, obtained from El-Nasr Company, Abo-Zabaal, Egypt and kept in the animal room for 2 weeks for adaptation before being subjected to laboratory experiments. Female rats were used based on the known fact that females are more susceptible to autoimmune diseases, including RA (Ngo et al. Citation2014). Animals were allowed free access to standard forage and tap water ad libitum. Animals were kept under stable temperature and relative humidity in an air-conditioned animal house with specific pathogen-free conditions, with 12 h dark/light cycles. Handling of animals and animal care were done according to the guidelines of Beni-Sueif Animal House approved by the Pharmacology and Toxicology Department, Faculty of Pharmacy, Beni-Sueif University on 20 June 2009, which were based on the guidelines suggested by the recommendations of the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Drugs, chemicals and reagent kits
Complete Freund’s adjuvant (CFA), dexamesathone, methotrexate, fenofibrate and resveratrol were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Serum RF, MMP-3, COMP, IgG and ANA ELISA kits, and MPO and MDA colorimetric kits were purchased from CliniLab Company (Cairo, Egypt). Serum TNF-α ELISA kit was obtained from Glory Science Company (St. Del Rio, TX). Serum IL-10 ELISA kit was obtained from CUSABIO Biotech Co. (Wuhan, China). Serum CRP colorimetric kit was obtained from Assaypro LLC (St. Charles, MO). Serum GSH colorimetric reagent kit was purchased from Biodiagnostic Company (Giza, Egypt). All the used chemicals, solvents and reagents were of analytical grade.
Experimental design
Rats were divided into eight groups, each of 10 rats. Doses of test agents were determined using pilot trials guided with published literature. The first group was kept as a normal control group receiving vehicles only. The second group was kept as arthritis control group and received CFA only. Groups 3 and 4 were kept as reference treatment groups and received 1.5 mg/kg/day dexamesathone (Gretzer et al. Citation2001; Gramoun et al. Citation2014; Wu et al. Citation2014) and 1 mg/kg/day methotrexate (Kim & Kang Citation2015). Groups 5 and 6 were kept as test treatment groups and received 100 mg/kg/day fenofibrate (Osada et al. Citation2014) and 10 mg/kg/day resveratrol (Chen et al. Citation2013). Reference standards or test agents were administered orally for seven consecutive days, starting from day 13 through day 19. Blood, spleen and joint samples were withdrawn 24 h after the last drug administration, i.e., on day 20.
Induction of arthritis
The current study was based on a modified arthritis model, where RA was induced with three subcutaneous doses of CFA, each of 0.4 ml, injected in three different limbs, on days 1, 4 and 7, which we set in a recent study (Fahmy Wahba et al. Citation2015). Most previously reported RA models relied on a single CFA dose (Newbound Citation1963; Kripa et al. Citation2010; Singh et al. Citation2015), or two CFA doses administered 4 d apart (Snekhalatha et al. Citation2013).
Serum sampling
Animals were lightly anaesthetised with ether on the 20th d, and blood was collected from the retro-orbital plexus using heparinised micro-tubes, left to coagulate at room temperature, then centrifuged at 3000 rpm for 10 min using a cooling centrifuge (Sigma 3–30k, St. Louis, MO). The clear serum layer was withdrawn and stored in a −80 °C deep freezer (Als Angelantoni Life Science, Massa Martana, Italy) until the time of assay of serum levels of RF, MMP-3, COMP, IgG, ANA, TNF-α, IL-10, MPO, CRP, MDA and GSH.
Assessment of serum biomarkers
Serum RF and IgG were assessed using ELISA reagent kits according to the methods described by kits manufacturer instructions based on the principles described by Waaler (Citation2007) and Kim et al. (Citation2010). Serum MMP-3 was measured according to ELISA kit manufacturer instructions based on the principles described by Brown (Citation1998), Nagase (Citation1998) and Haro et al. (Citation2000). Serum COMP was assessed using ELISA reagent kits as previously described by Paulsson and Heinegård (Citation1981) and Petersen et al. (Citation2010). Serum ANA level was measured using ANA ELISA kits as indicated by Von Mühlen and Tan (Citation1995). Serum TNF-α was assessed using ELISA reagent kits as described earlier (Brouckaert et al. Citation1993). Serum IL-10 was assessed using ELISA reagent kits as previously described (Khozeimeh et al. Citation2014). Serum MPO was measured colorimetrically according to the method described by Manktelow and Meyer (Citation1986). Serum CRP was measured colorimetrically according to the modified method of Schultz and Arnold (Citation1990). Serum MDA was measured according to the modified method of Esterbauer et al. (Citation1991). Serum GSH was measured colorimetrically according to the principle of Ellman (Citation1959).
Histopathological study
Soon after blood withdrawal, rats are killed by cervical dislocation and whole knee joints, including synovium, adjacent tissues and bones, as well as whole spleen were carefully exposed, separated and preserved in 10% formalin solution in saline in labelled, well-sealed containers. After tissue hardening, joints were transversely sectioned (4–5 μm thick sections) and stained with standard Haematoxylin and Eosin (H&E) stain using standard techniques for photomicroscopic observations as described by Banchroft and Steven (Citation1983). Sections were viewed by the aid of a light microscope attached with a digital camera and reported by two experienced pathologists blinded to the experiment.
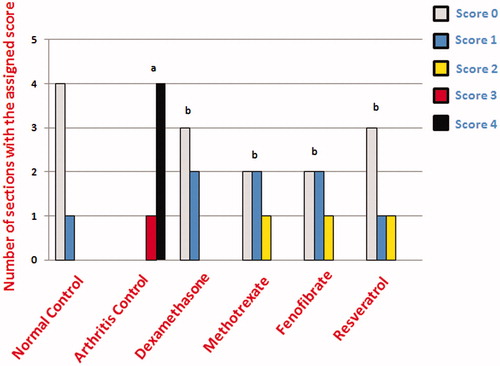
Joint cartilage erosion was evaluated by assigning semi-quantitative scores, on a scale of 0–4, with a score of 0 assigned to joint sections with intact smooth articular surface, a score of 1 to sections with intact but irregular articular surface, a score of 2 to sections with a mildly disrupted articular surface, a score of 3 to sections with moderately disrupted articular surface and a score of 4 to sections with massively disrupted articular surface.
Statistical analysis
Results were expressed as means of 6–10 values ± standard error of the mean. All statistical analyses of biochemical estimations were performed using the one-way analysis of variance test followed by the Tukey–Kramer multiple comparisons test, while statistical analysis of the assigned scores in the histopathological study was performed using the χ2 test by the aid of the statistical package for social sciences (SPSS; version 19.0) computer software program (SPSS Inc., Chicago, IL) with a value of p < 0.05 considered statistically significant.
Results
Biochemical estimations
Specific rheumatoid markers
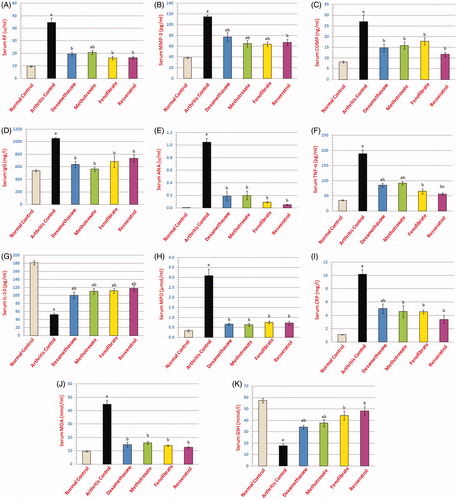
Treatment of rats with dexamesathone, methotrexate, fenofibrate or resveratrol significantly decreased serum levels of both markers. Serum levels of RF were not significantly different from normal levels after treatment of rats with dexamesathone, fenofibrate or resveratrol. Serum levels of MMP-3 were not significantly different from normal levels after treatment of rats with resveratrol. Serum levels of COMP were not significantly different from normal levels after treatment of rats with dexamesathone, methotrexate, fenofibrate and resveratrol. Rats treated with fenofibrate or resveratrol were not significantly different from rats treated with either dexamesathone or methotrexate regarding serum RF, MMP-3 or COMP levels ().
Figure 1. Graphical presentations of serum biochemical estimations in different groups, where (A) rheumatoid factor (RF); (B) matrix metalloproteinase-3 (MMP-3); (C) cartilage oligomeric matrix protein (COMP); (D) Immunoglobulin G (IgG); (E) antinuclear antibody (ANA); (F) Tumour necrosis factor-alpha (TNF-α); (G) interleukin-10 (IL-10); (H) myeloperoxidase (MPO); (I) C-reactive protein (CRP); (J) malondialdehyde (MDA); (K) glutathione reduced (GSH). aSignificantly different from normal control group, bsignificantly different from arthritis control group, csignificantly different from methotrexate treatment group at p < 0.05.
Immunological markers
Treatment of rats with dexamesathone, methotrexate, fenofibrate or resveratrol showed significant decreases in both levels of serum IgG and serum ANA when compared to arthritis control rats, being not significantly different from normal levels. No significant difference was present between either fenofibrate or resveratrol with dexamesathone or methotrexate regarding both markers ().
Cytokines
Treatment of rats with dexamesathone, methotrexate, fenofibrate or resveratrol showed a significant decrease in serum TNF-α and a significant increase in serum IL-10 levels compared with arthritis control rats. Serum level of TNF-α was not significantly different from normal level after treatment of rats with fenofibrate. Rats treated with fenofibrate showed no significant difference when compared with either dexamesathone- or methotrexate-treated rats regarding both markers, while resveratrol-treated rats showed significantly better improvement in serum TNF-α compared to methotrexate-treated rats ().
Inflammatory markers
Treatment of rats with dexamesathone, methotrexate, fenofibrate or resveratrol restored their levels back to normal. No significant difference was observed for fenofibrate or resveratrol when compared with either dexamesathone- or methotrexate-treated rats ().
Oxidative markers
Treatment of rats with dexamesathone, methotrexate, fenofibrate or resveratrol corrected both MDA and GSH levels compared to arthritis control rats, restoring serum MDA levels to values not significantly different from the normal level. Concerning GSH, fenofibrate and resveratrol restored the serum levels back to normal. Neither fenofibrate nor resveratrol could show significant difference with either dexamesathone or methotrexate rats regarding both markers ().
Histopathological study
Joint histopathology
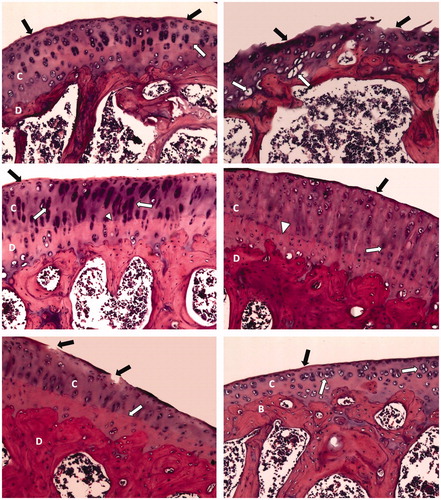
Treatment with dexamethasone or methotrexate conserved a smooth articular surface with somewhat thickened articular cartilage with chondrocyte hypercellularity and cloning. Fenofibrate-treated arthritic rat articular sections showed just a narrow area of disrupted articular surface and thickened articular cartilage. Resveratrol treatment restored smooth articular surface with some articular cartilage hypercellularity and aggregation of chondrocytes ().
Figure 2. Photomicrographs of articular cartilages of different sections (H&E; 200×). Normal control rat section (top left) shows a smooth continuous articular surface (black arrow) and a regular tide mark (white arrow) separating the articular cartilage (C) from the underlying subchondral bone (D). Arthritis control rat section (top right) shows disrupted articular surface (black arrow) with chondrocytes showing degeneration with pyknotic nuclei (white arrow). Dexamethasone-treated arthritic rat section (middle left) shows smooth articular surface (black arrow) and thickened articular cartilage (C) and subchondral bone (D). The chondrocytes (white arrow) show hypercellularity and cloning, while regular tide mark can be seen (arrowhead). Methotrexate-treated arthritic rat section (middle right) shows smooth articular surface (black arrow) with thickened articular cartilage (C) and subchondral bone (D). The chondrocytes (white arrow) show hypercellularity and cloning, and regular tide mark can be seen (arrowhead). Fenofibrate-treated arthritic rat section (bottom left) shows a narrow area of disrupted articular surface (black arrow). Thickened articular cartilage (C) and subchondral bone (D) can be observed. Regular tide mark can also be observed (white arrow). Resveratrol-treated arthritic rat section (bottom right) shows a smooth articular surface (black arrow). The articular cartilage (C) shows hypercellularity and aggregation of chondrocytes (white arrow). Subchondral bone (B) can be observed.
Spleen histopathology
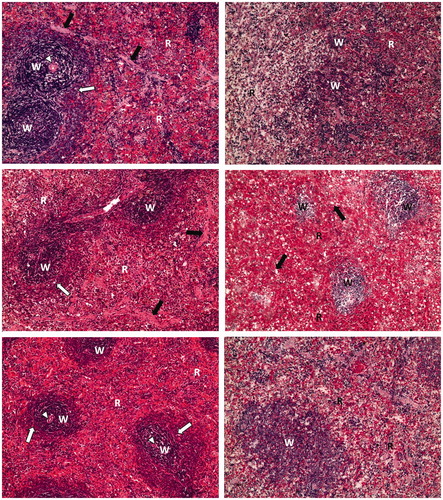
Splenic parenchyma sections obtained from dexamethasone-treated arthritic rats showed white pulp surrounded with red pulp, where lymphatic follicles showed central arteriole and well-defined marginal zone. Methotrexate-treated arthritic rat splenic sections showed slight atrophy of white pulp with the appearance of many foamy cells within red pulp. The splenic parenchyma of fenofibrate-treated arthritic rats showed more or less restored architecture and well-defined marginal zone. Similarly, resveratrol-treated arthritic rat splenic parenchymal sections showed normal white pulp but somewhat atrophic red pulp ().
Figure 3. Photomicrographs of splenic parenchyma of different sections (H&E; 100×). Normal control rat section (top left) shows lymphatic follicles (W) named white pulp surrounded with red pulp (R). Lymphatic follicles show central arteriole (arrowhead) and well-defined marginal zone (white arrow). Trabeculae can be observed (black arrow). Arthritis control rat section (top right) shows atrophy of white pulp (W) and red pulp (R). Dexamethasone-treated arthritic rat section (middle left) shows lymphatic follicles (W) named white pulp surrounded with red pulp (R). Lymphatic follicles show central arteriole (arrowhead) and well-defined marginal zone (white arrow). Trabeculae can be observed (black arrow). Methotrexate-treated arthritic rat section (middle right) shows slight atrophy of white pulp (W) with the appearance of many foamy cells (black arrow) within red pulp (R). Fenofibrate-treated arthritic rat section (bottom left) shows lymphatic follicles (W) named white pulp surrounded with red pulp (R). Lymphatic follicles show central arteriole (arrowhead) and well-defined marginal zone (white arrow). Resveratrol-treated arthritic rat section (bottom right) shows normal white pulp (W) and atrophic red pulp (R).
Regarding joint scoring, fenofibrate and resveratrol sections showed significantly lower cartilage erosion scoring values compared with arthritis control sections ().
Discussion
In the current investigation, the possible protective effects of fibrates, represented by fenofibrate and polyphenols represented by resveratrol were investigated against CFA-induced RA in rats compared with the standard agents dexamethasone and methotrexate.
Results of the present investigation revealed that fenofibrate administration significantly ameliorated RA progression, evidenced by significant reductions in serum RF, MMP-3 and COMP levels compared with arthritis control values. Histopathological examination of joint and spleen sections strongly supported these findings. In the agreement, Koufany et al. (Citation2014) reported that fenofibrate could protect against adjuvant-induced arthritis in rats. Since MMPs are released from activated macrophages during the course of RA, leading to joint destruction and release of COMP into circulation, suppression of MMP-3 and COMP levels strongly indicates inhibition of cartilage destruction (Andersson et al. Citation2013; Kizaki et al. Citation2015). In disagreement, however, Wei et al. (Citation2014) reported that fibrates could not prevent cartilage destruction in mice with osteoarthritis.
Concerning our results, the beneficial effect of fenofibrate may be explained by its immunomodulatory effects evidenced by suppressed plasma levels of IgG and ANA. In support, Márk et al. (Citation2003) reported that fenofibrate suppressed plasma levels of anti-chlamydial antibodies in patients with pneumonia. Interestingly, Zhou et al. (Citation2012) reported an ability of fenofibrate to inhibit T-helper cell differentiation in vitro, while Krysiak et al. (Citation2013) concluded that fenofibrate could suppress the production of proinflammatory cytokines from activated lymphocytes in patients with atherosclerosis. This may also explain our observation that fenofibrate significantly ameliorated plasma TNF-α levels in arthritic rats. On the other hand, we reported that fenofibrate could upregulate plasma level of the anti-inflammatory cytokine IL-10. This comes in agreement with the results of Maruyama et al. (Citation2002) reporting that fenofibrate could protect rats against autoimmune myocarditis through stimulation of IL-10 pathway based on its well-known agonist effect on peroxisome proliferator activated receptor alpha (PPAR-α). Meanwhile, fenofibrate was reported to improve colitis in IL-10-deficient mice (Lee et al. Citation2007).
The anti-inflammatory effect of fenofibrate, evidenced in the current study by suppressed serum MPO and CRP levels, may be another explanation for its beneficial anti-arthritic effect. Recently, Ann et al. (Citation2015) reported that PPAR-α stimulation could inhibit inflammatory activation of macrophages through upregulation of the anti-inflammatory peptide β-defensin while Yang et al. (Citation2015) suggested that the anti-inflammatory potential of fenofibrate in a model of renal ischemia/reperfusion injury could be related to enhancement of phosphoinositide 3 kinase/protein kinase B signalling.
Antioxidant potential of fenofibrate may be a third mechanism accounting for its beneficial anti-arthritic effect, evidenced in the current study as restoration of the oxidative markers MDA and GSH in arthritic animals back to normal levels. This comes in agreement with the results of Moran et al. (Citation2014) showing a potent antioxidant effect of fenofibrate in a model of the ischemic retina, again attributed to its PPAR-α agonist activity causing suppression of NADPH oxidase activity. Side by side, it was reported that attenuation of oxidative stress may have beneficial effects on the modulation of experimental arthritis (Sharma et al. Citation2014).
Regarding resveratrol, an anti-arthritic effect was evident from our results of both specific and non-specific rheumatoid markers coupled with histopathological improvements. In the agreement, Glehr et al. (Citation2013) reported that resveratrol could affect the synovial expression of MMP’s in vitro while Tian et al. (Citation2013) reported that resveratrol could inhibit TNF-α-induced cytokine and MMP-3 production in synoviocytes. Additionally, Mobasheri and Shakibaei (Citation2013) reported a good osteogenic effect for resveratrol in vitro.
Immunomodulatory and anti-inflammatory activities seem to be behind the beneficial anti-arthritic effects of resveratrol. Chen et al. (Citation2013) demonstrated that resveratrol might have an immunomodulatory potential mediated by suppressed production of cytokines like TNF-α, coming in agreement with our results. Cianciulli et al. (Citation2015) also agreed our results and reported that resveratrol immunomodulatory activity could be related to a stimulatory action on IL-10. Interestingly, opposite to most common immunomodulators used in the treatment of RA, resveratrol was reported not to suppress the adaptive immunity in experimental animal models (Yuan et al. Citation2012).
The anti-inflammatory effect of resveratrol was previously attributed to its stimulatory effect on the endogenous anti-inflammatory anti-TNF-α molecule sirtulin-1 or sirt-1 (Zhu et al. Citation2011; Nicoletti et al. Citation2014), which agreed our results concerning the effect of resveratrol administration on serum TNF-α levels in arthritic rats. Chen et al. (Citation2014) reported that the anti-inflammatory effect of resveratrol may be attributed to its inhibitory effect on cyclooxygenase-2 enzyme activity. In another study, the anti-inflammatory effect of resveratrol was attributed to its suppressant action on nuclear factor kappa B and inducible nitric oxide synthase in chondrocytes (Lei et al. Citation2012).
The antioxidant effect of resveratrol was evident in the current study from the significant improvements of serum MDA and GSH levels in arthritic rats receiving resveratrol. This was also well-established on different models in vitro and in vivo (Al Maruf & O’Brien Citation2014; Pangeni et al. Citation2014; Suwalsky et al. Citation2015), and may account for its beneficial anti-arthritic effect observed in the current study.
It could be concluded that fibrates represented by fenofibrate and polyphenols represented by resveratrol may offer promising lines for the treatment of RA. Further trials are needed to evaluate such effects clinically.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Al Maruf A, O’Brien P. 2014. Flutamide-induced cytotoxicity and oxidative stress in an in vitro rat hepatocyte system. Oxid Med Cell Longev. 2014:Article ID 398285.
- Andersson ML, Svensson B, Petersson IF, Hafström I, Albertsson K, Forslind K, Heinegård D, Saxne T. 2013. Early increase in serum-COMP is associated with joint damage progression over the first five years in patients with rheumatoid arthritis. BMC Musculoskelet Disord. 14:229–236.
- Ann SJ, Chung JH, Park BH, Kim SH, Jang J, Park S, Kang SM, Lee SH. 2015. PPARα agonists inhibit inflammatory activation of macrophages through upregulation of β-defensin 1. Atherosclerosis 240:389–397.
- Banchroft GD, Steven A. 1983. Theory and practice of histological technique. 4th ed. London, UK: Churchill Livingstone Publications.
- Bellaver B, Souza DG , Souza DO, Quincozes-Santos A. 2014. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult and aged Wistar rats. Toxicol in Vitro. 28:479–484.
- Biesen R, Burmester GR, Hiepe F. 2014. [Rheumatoid factor or antinuclear antibodies as incidental finding]. Internist (Berl). 55:1157–1164.
- Brouckaert P, Libert C, Everaerdt B, Takahashi N, Cauwels A, Fiers W. 1993. Tumor necrosis factor, its receptors and the connection with interleukin 1 and interleukin 6. Immunobiology 187:317–329.
- Brown PD. 1998. Synthetic inhibitors of matrix metalloproteinases. In: Parks WC, Mecham RP, editors. Matrix metalloproteases. San Diego (CA): Academic Press. p. 243–261.
- Castillero E, Martín AI, Nieto-Bona MP, Fernández-Galaz C, López-Menduiña M, Villanúa MA, López-Calderón A. 2012. Fenofibrate administration to arthritic rats increases adiponectin and leptin and prevents oxidative muscle wasting. Endocr Connect. 1:1–12.
- Chen X, Lu J, An M, Ma Z, Zong H, Yang J. 2014. Anti-inflammatory effect of resveratrol on adjuvant arthritis rats with abnormal immunological function via the reduction of cyclooxygenase-2 and prostaglandin E2. Mol Med Rep. 9:2592–2598.
- Chen XY, Wang ZC, Li J, Liu XL, Sun YH. 2013. Regulation of synoviocyte activity by resveratrol in rats with adjuvant arthritis. Exp Ther Med. 6:172–176.
- Cianciulli A, Dragone T, Calvello R, Porro C, Trotta T, Lofrumento DD, Panaro MA. 2015. IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int Immunopharmacol. 24:369–376.
- Edupuganti SR, Eder V, Ternant D, Courtehoux M, Tranquart F, Goupille P, Paintaud G, Mulleman D. 2015. F-18 fluorodeoxyglucose positron emission tomography can detect early response to adalimumab, a tumor necrosis factor-α antagonist, in rheumatoid arthritis: a prospective pilot study. Joint Bone Spine. 82:381–383
- Ellman GL. 1959. Tissue sulfhydryl groups. Arch Biochem Biophys. 82:70–77.
- Esterbauer H, Schaur RJ, Zollner H. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 11:81–128.
- Fahmy Wahba MG, Shehata Messiha BA, Abo-Saif AA. 2015. Ramipril and haloperidol as promising approaches in managing rheumatoid arthritis in rats. Eur J Pharmacol. 765:307–315
- Glehr M, Breisach M, Walzer S, Lohberger B, Fürst F, Friesenbichler J, Rinner B, Avian A, Windhager R, Leithner A. 2013. The influence of resveratrol on the synovial expression of matrix metalloproteinases and receptor activator of NF-kappaB ligand in rheumatoid arthritis fibroblast-like synoviocytes. Z Naturforsch C. 68:336–342.
- Gramoun A, Crowe LA, Maurizi L, Wirth W, Tobalem F, Grosdemange K, Coullerez G, Eckstein F, Koenders MI, Van den Berg WB, et al. 2014. Monitoring the effects of dexamethasone treatment by MRI using in vivo iron oxide nanoparticle-labeled macrophages. Arthritis Res Ther. 16:R131–R146.
- Gretzer B, Maricic N, Respondek M, Schuligoi R, Peskar BM. 2001. Effects of specific inhibition of cyclo-oxygenase-1 and cyclo-oxygenase-2 in the rat stomach with normal mucosa and after acid challenge. Br J Pharmacol. 132:1565–1573.
- Haleagrahara N, Tudawe D, Chakravarthi S, Kutty Radhakrishnan A. 2013. Amelioration of collagen-induced arthritis in female dark agouti rats by glucosamine treatment. ISRN Pharmacol. 2013: Article ID 562905.
- Haro H, Crawford HC, Fingleton B, MacDougall JR, Shinomiya K, Spengler DM, Matrisian LM. 2000. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J Clin Invest. 105:133–141.
- Hügle B, Hinze C, Lainka E, Fischer N, Haas JP. 2014. Development of positive antinuclear antibodies and rheumatoid factor in systemic juvenile idiopathic arthritis points toward an autoimmune phenotype later in the disease course. Pediatr Rheumatol Online J. 12:28–32.
- Ingegnoli F, Castelli R, Gualtierotti R. 2013. Rheumatoid factors: clinical applications. Dis Markers. 35:727–734.
- Iyer SS, Cheng G. 2012. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 32:23–63.
- Kawashiri SY, Kawakami A, Ueki Y, Imazato T, Iwamoto N, Fujikawa K, Aramaki T, Tamai M, Nakamura H, Origuchi T, et al. 2010. Decrement of serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis (RA) patients achieving remission after 6 months of etanercept treatment: comparison with CRP, IgM-RF, MMP-3 and anti-CCP Ab. Joint Bone Spine. 77:418–420.
- Khozeimeh F, Savabi O, Esnaashari M. 2014. Evaluation of interleukin-1α, interleukin-10, tumor necrosis factor-α and transforming growth factor-β in the serum of patients with Pemphigus vulgaris. J Contemp Dent Pract. 15:746–749.
- Kim S, Kim JH, Lee JH, Kim HS. 2010. Evaluation of three automated enzyme immunoassays for detection of anti-cyclic citrullinated peptide antibodies in qualitative and quantitative aspects. Rheumatology (Oxford). 49:450–457.
- Kim YH, Kang JS. 2015. Effect of methotrexate on collagen-induced arthritis assessed by micro-computed tomography and histopathological examination in female rats. Biomol Ther (Seoul). 23:195–200.
- Kizaki K, Yoshizumi Y, Takahashi T, Era S. 2015. Elevated oxidative stress monitored via the albumin-thiol redox state is correlated with matrix metalloproteinase-3 elevation in patients with rheumatoid arthritis. Clin Lab. 61:175–178.
- Koufany M, Jouzeau JY, Moulin D. 2014. Fenofibrate vs pioglitazone: comparative study of the anti-arthritic potencies of PPAR-alpha and PPAR-gamma agonists in rat adjuvant-induced arthritis. Biomed Mater Eng. 24:81–88.
- Kripa KG, Chamundeeswari D, Thanka J, Uma Maheswara Reddy C. 2010. Effect of hydroalcoholic extract of aerial parts of Leucas aspera (Willd.) link on inflammatory markers in complete Freund’s adjuvant induced arthritic rats. Int J Green Pharm. 4:281–287.
- Krysiak R, Gdula-Dymek A, Okopien B. 2013. The effect of fenofibrate on lymphocyte release of proinflammatory cytokines and systemic inflammation in simvastatin-treated patients with atherosclerosis and early glucose metabolism disturbances. Basic Clin Pharmacol Toxicol. 112:198–202.
- Lampropoulos CE, Orfanos P, Bournia VK, Karatsourakis T, Mavragani C, Pikazis D, Manoussakis MN, Tzioufas AG, Moutsopoulos HM, Vlachoyiannopoulos PG. 2015. Adverse events and infections in patients with rheumatoid arthritis treated with conventional drugs or biologic agents: a real world study. Clin Exp Rheumatol. 33:216–224.
- Lee JW, Bajwa PJ, Carson MJ, Jeske DR, Cong Y, Elson CO, Lytle C, Straus DS. 2007. Fenofibrate represses interleukin-17 and interferon-gamma expression and improves colitis in interleukin-10-deficient mice. Gastroenterology 133:108–123.
- Lei M, Wang JG, Xiao DM, Fan M, Wang DP, Xiong JY, Chen Y, Ding Y, Liu SL. 2012. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur J Pharmacol. 674:73–79.
- Liu X, Teichtahl AJ, Wicks IP. 2015. Interleukin-6 in rheumatoid arthritis – from the laboratory to the bedside. Curr Pharm Des. 21:2187–2197.
- Lopalco G, Cantarini L, Vitale A, Iannone F, Anelli MG, Andreozzi L, Lapadula G, Galeazzi M, Rigante D. 2015. Interleukin-1 as a common denominator from autoinflammatory to autoimmune disorders: premises, perils, and perspectives. Mediators Inflamm. 2015:Article ID 194864.
- Lubberts E. 2015. Role of T lymphocytes in the development of rheumatoid arthritis. Implications for treatment. Curr Pharm Des. 21:142–146.
- Mamehara A, Sugimoto T, Sugiyama D, Morinobu S, Tsuji G, Kawano S, Morinobu A, Kumagai S. 2010. Serum matrix metalloproteinase-3 as predictor of joint destruction in rheumatoid arthritis, treated with non-biological disease modifying anti-rheumatic drugs. Kobe J Med Sci. 56:E98–E107.
- Manktelow A, Meyer AA. 1986. Lack of correlation between decreased chemotaxis and susceptibility to infection in burned rats. J Trauma. 26:143–148.
- Márk L, Márki-Zay J, Orosz I, Kondacs A, Erdei F, Katona A. 2003. [Pleiotropic effect of the micronized fenofibrate: the reduction of plasma chlamydia pneumoniae antibody levels in patients with coronary artery disease]. Orv Hetil. 144:765–768.
- Maruyama S, Kato K, Kodama M, Hirono S, Fuse K, Nakagawa O, Nakazawa M, Miida T, Yamamoto T, Watanabe K, et al. 2002. Fenofibrate, a peroxisome proliferator-activated receptor alpha activator, suppresses experimental autoimmune myocarditis by stimulating the interleukin-10 pathway in rats. J Atheroscler Thromb. 9:87–92.
- McInnes IB, Schett G. 2011. The pathogenesis of rheumatoid arthritis. N Engl J Med. 365:2205–2219.
- Medeiros MM, Oliveira BM, Cerqueira JV, Quixadá RT, Oliveira ÍM. 2015. Correlation of rheumatoid arthritis activity indexes (Disease Activity Score 28 measured with ESR and CRP, Simplified Disease Activity Index and Clinical Disease Activity Index) and agreement of disease activity states with various cut-off points in a Northeastern Brazilian population. Rev Bras Reumatol. 55:477–484.
- Mobasheri A, Shakibaei M. 2013. Osteogenic effects of resveratrol in vitro: potential for the prevention and treatment of osteoporosis. Ann N Y Acad Sci. 1290:59–66.
- Moran E, Ding L, Wang Z, Cheng R, Chen Q, Moore R, Takahashi Y, Ma JX. 2014. Protective and antioxidant effects of PPARα in the ischemic retina. Invest Ophthalmol Vis Sci. 55:4568–4576.
- Nagase H. 1998. Matrix metalloproteinases. In: Barrett AJ, Rawlings ND, Woessner JF, editors. Handbook of proteolytic enzymes. San Diego (CA): Academic Press. p. 1172–1178.
- Newbound BB. 1963. Chemotherapy of arthritis induced in rats by mycobacterial adjuvants. Br J Pharmacol Chemother. 21:127–136.
- Ngo ST, Steyn FJ, McCombe PA. 2014. Gender differences in autoimmune disease. Front Neuroendocrinol. 35:347–369.
- Nicoletti NF, Rodrigues-Junior V, Santos AA Jr, Leite CE, Dias AC, Batista EL Jr, Basso LA, Campos MM, Santos DS, Souto AA. 2014. Protective effects of resveratrol on hepatotoxicity induced by isoniazid and rifampicin via SIRT1 modulation. J Nat Prod. 77:2190–2195.
- Osada M, Sakai T, Kuroyanagi K, Kohno H, Tsuneoka H. 2014. Treatment of experimental autoimmune uveoretinitis with peroxisome proliferator-activated receptor α agonist fenofibrate. Mol Vis. 20:1518–1526.
- Pangeni R, Sharma S, Mustafa G, Ali J, Baboota S. 2014. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 25:Article ID 485102.
- Patel SS, Shah PV. 2013. Evaluation of anti-inflammatory potential of the multidrug herbomineral formulation in male Wistar rats against rheumatoid arthritis. J Ayurveda Integr Med. 4:86–93.
- Paulsson M, Heinegård D. 1981. Purification and structural characterization of a cartilage matrix protein. Biochem J. 197:367–375.
- Petersen SG, Saxne T, Heinegard D, Hansen M, Holm L, Koskinen S, Stordal C, Christensen H, Aagaard P, Kjaer M. 2010. Glucosamine but not ibuprofen alters cartilage turnover in osteoarthritis patients in response to physical training. Osteoarthritis Cartilage. 18:34–40.
- Rau R. 2014. Glucocorticoid treatment in rheumatoid arthritis. Expert Opin Pharmacother. 15:1575–1583.
- Saxena A, Khosraviani S, Noel S, Mohan D, Donner T, Hamad AR. 2014. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine 74:27–34.
- Schultz DR, Arnold PI. 1990. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 20:129–147.
- Sharma S, Gupta R, Thakur SC. 2014. Attenuation of collagen induced arthritis by Centella asiatica methanol fraction via modulation of cytokines and oxidative stress. Biomed Environ Sci. 27:926–938.
- Singh S, Kumar R, Jain H, Gupta YK. 2015. Anti-inflammatory and antiarthritic activity of UNIM-301 (a polyherbal unani formulation) in Wistar rats. Pharmacognosy Res. 7:188–192.
- Snekhalatha U, Anburajan M, Venkatraman B, Menaka M. 2013. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Comparison of thermography and histopathology. Z Rheumatol. 72:375–382.
- Stamp LK, Khalilova I, Tarr JM, Senthilmohan R, Turner R, Haigh RC, Winyard PG, Kettle AJ. 2012. Myeloperoxidase and oxidative stress in rheumatoid arthritis. Rheumatology (Oxford). 51:1796–1803.
- Su JF, Guo CJ, Wei JY, Yang JJ, Jiang YG, Li YF. 2003. Protection against hepatic ischemia-reperfusion injury in rats by oral pretreatment with quercetin. Biomed Environ Sci. 16:1–8.
- Suwalsky M, Villena F, Gallardo MJ. 2015. In vitro protective effects of resveratrol against oxidative damage in human erythrocytes. Biochim Biophys Acta. 1848:76–82.
- Tian J, Chen JW, Gao JS, Li L, Xie X. 2013. Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human rheumatoid arthritis fibroblast-like synoviocytes via modulation of PI3kinase/Akt pathway. Rheumatol Int. 33:1829–1835.
- Valesini G, Gerardi MC, Iannuccelli C, Pacucci VA, Pendolino M, Shoenfeld Y. 2015. Citrullination and autoimmunity. Autoimmun Rev. 14:490–497.
- Vivar N, Van Vollenhoven RF. 2014. Advances in the treatment of rheumatoid arthritis. Hand Clin. 6:31–39.
- von Mühlen CA, Tan EM. 1995. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum. 24:323–358.
- Waaler E. 2007. On the occurrence of a factor in human serum activating the specific agglutintion of sheep blood corpuscles. APMIS 115:422–438.
- Wei W, Clockaerts S, Bastiaansen-Jenniskens YM, Gierman LM, Botter SM, Bierma-Zeinstra SM, Weinans H, Verhaar JA, Kloppenburg M, Zuurmond AM, et al. 2014. Statins and fibrates do not affect development of spontaneous cartilage damage in STR/Ort mice. Osteoarthritis Cartilage. 22:293–301.
- Wu J, Liu X, Chan CO, Mok DK, Chan SW, Yu Z, Chen S. 2014. Petroleum ether extractive of the hips of Rosa multiflora ameliorates collagen-induced arthritis in rats. J Ethnopharmacol. 157:45–54.
- Yang FJ, He YH, Zhou JH. 2015. Fenofibrate pre-treatment suppressed inflammation by activating phosphoinositide 3 kinase/protein kinase B (PI3K/Akt) signaling in renal ischemia-reperfusion injury. J Huazhong Univ Sci Technol Med Sci. 35:58–63.
- Yu Y, Li S, Liu Y, Tian G, Yuan Q, Bai F, Wang W, Zhang Z, Ren G, Zhang Y, et al. 2015. Fibroblast growth factor 21 (FGF21) ameliorates collagen-induced arthritis through modulating oxidative stress and suppressing nuclear factor-kappa B pathway. Int Immunopharmacol. 25:74–82.
- Yuan J, Lu L, Zhang Z, Zhang S. 2012. Dietary intake of resveratrol enhances the adaptive immunity of aged rats. Rejuvenation Res. 15:507–515.
- Zhang W, Zhang J, Zhang M, Nie L. 2014. Protective effect of Asarum extract in rats with adjuvant arthritis. Exp Ther Med. 8:1638–1642.
- Zhou Z, Sun W, Liang Y, Gao Y, Kong W, Guan Y, Feng J, Wang X. 2012. Fenofibrate inhibited the differentiation of T helper 17 cells in vitro. PPAR Res. 2012:Article ID 145654.
- Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu X, Zou H, Qiu J. 2011. Activation of Sirt1 by resveratrol inhibits TNF-α induced inflammation in fibroblasts. PLoS One 6:e27081.
- Zou T, Yang Y, Xia F, Huang A, Gao X, Fang D, Xiong S, Zhang J. 2013. Resveratrol inhibits CD4+ T cell activation by enhancing the expression and activity of Sirt1. PLoS One 8:e75139.