Abstract
Context Pueraria lobata (Leguminoseae) shows cytotoxic effects against cancer cells; however, its active components remain unclear.
Objective This study investigated the antitumour activity of puerarin 6″-O-xyloside (POS) on the human lung carcinoma A549 cell line.
Materials and methods The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine the cytotoxicity of POS (at 10, 20 and 40 μM) in vitro, and xenograft nude mice were established to evaluate the antitumour effect of POS (at 40 mg/kg/d) in vivo by 15 days intraperitoneal injection (ip). To explore its mechanism of action, flow cytometry was performed to determine the pro-apoptotic effect of POS (at 10, 20 and 40 μM). Subsequently, the expression of caspase-3, caspase-7, caspase-9, Bcl-2 and Bax in A549 cells were determined.
Results POS showed significant cytotoxicity toward A549 cells (p < 0.05) by inducing apoptosis. Treatment with POS significantly upregulated the levels of caspase-3 (p < 0.01), caspase-7 (p < 0.01), caspase-9 (p < 0.01) and Bax (p < 0.01) in A549 cells, and Bcl-2 was downregulated (p < 0.01). Additionally, the in vivo animal study showed that POS significantly inhibited tumour growth in A549 cells (p < 0.01).
Conclusion Our study demonstrated the POS has significant antitumour activities. The mechanisms are related to increased levels of caspase-3, caspase-7, caspase-9 and Bax, and reduced levels Bcl-2.
Introduction
In recent years, lung cancer has been the leading cause of cancer-related deaths worldwide. In addition, it is reported that non-small cell lung cancers (NSCLC) represent more than 85% of the total incidence of lung cancer (Ko et al. Citation2010; Zhao et al. Citation2011; Siegel et al. Citation2015). Although the diagnosis and the treatment of lung cancers have improved greatly, the currently 5-year survival rate of lung cancer patients is still poor (Liu et al. Citation2007; Yang et al. Citation2014). Consequently, identifying novel strategies to cure lung cancer is important and urgent.
Previous investigations have focused on the possible capacities of natural agents derived from plants to prevent and treat lung cancers. The study of the medical uses of plants or herbs may provide useful clues for new effective drug discovery (Li & Vederas Citation2009; Skermana et al. Citation2011; Peng et al. Citation2014; Yang et al. Citation2014). Furthermore, previous investigations demonstrated that the extracts of the radix of Pueraria lobata (Willd.) Ohwi (Leguminoseae), a famous traditional Chinese medicine, possessed notable cytotoxic effect on cancer cells (Du et al. Citation1994; Chen & Hu Citation1997; Du et al. Citation1997; Yuan et al. Citation2007). However, there is little information regarding the active constituents in P. lobata that are responsible for its cytotoxic activity. In the present study, we determined the antitumour activity of puerarin 6″-O-xyloside (POS) (), which is one of the major isoflavones of P. lobata. In addition, the mechanisms of action of its antitumour activity were also investigated.
Materials and methods
Chemicals and regents
Puerarin 6″-O-xyloside was obtained from the Shanghai Tauto Biotech Ltd. Co. (Shanghai, China), and the purity was 98%, as determined by HPLC; 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulphoxide were purchased from Sigma (St. Louis, MO). RPMI 1640 media and foetal bovine serum (FBS) were purchased from Invitrogen (Carlsbad, CA); dimethyl benzene was purchased from Sino Pharm. (Chengdu, China); Annexin V-FITC/PI kit was purchased from BD Bioscience (San Diego, CA); cytochrome c, cleaved-caspase-3 (c-caspase-3), cleaved-caspase-7 (c-caspase-7) and cleaved-caspase-9 (c-caspase-9) antibodies were purchased from Cell Signaling Technology (Beverly, MA); Bcl-2, Bcl-xl, Bax, COX IV and β-actin antibodies were purchased from Abcam Biotechnology (Cambridge, MA); and the BCA protein assay reagent was purchased from Beyotime (Haimen, China).
Animals and ethics statement
Experimental groups consisted of Institute for Cancer Research (ICR) mice (4–5 weeks old) or BALB/C nude mice (5–6 weeks old) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). All animal treatments were strictly in accordance with International Ethical Guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals. The experimental protocols were carried out with approval of the Animal Experimentation Ethics Committee of the Changshu No. 1 People’s Hospital (No. cKY20140201).
Cell culture and determination of cell viability
The A549 cell line was purchased from the American Type Culture Collection (Manassas, MD). The cells were cultured in RPMI-1640 medium supplemented with 10% FBS. The cell lines were cultured in 5% CO2/95% air at 37 °C.
Cell viability was assessed using the MTT assay, as previously described (Wu et al. Citation2014). Cells (5 × 105 cells/mL) were plated to adhere in 96-well plates and cultured for 24 h. Then, POS (10, 20 and 40 μM) was added to the wells, and cells were cultured for 32 h. Subsequently, the absorbance (OD value) was read at 570 nm by a microplate reader (Bio-rad, Hercules, CA) to determine the percentage of cell proliferation inhibition. The inhibition rate was calculated accor-ding to the following formula: [(ODcontrol–ODtreatment)/ODcontrol] × 100%.
Apoptosis assay by flow cytometry
Apoptotic cells were identified by staining with fluorescein isothiocyanate (FITC)-conjugated Annexin V/PI by flow cytometry, according to the instructions of a commercial kit. The percentage of cells in early apoptosis was calculated by Annexin V-positivity and PI-negativity, while the percentage of cells in late apoptosis was calculated by Annexin V-positivity and PI-positivity.
Western blotting
Total proteins of the cells were extracted and the protein concentration was determined by using the BCA protein assay reagent. Equal amounts of proteins (35 μg) were separated by sodium dodecyl sulphate/polyacrylamide gel electrophoresis (SDS/PAGE), blotted onto PVDF membranes and probed with various primary antibodies, followed by incubation with horseradish-peroxidase-conjugated secondary antibodies and chemiluminescence detection. To normalize the protein loading, antibodies directed against β-actin/COX IV were used, and the proteins expression levels were expressed as a relative value to that of β-actin/COX IV.
Xenograft model in nude mice
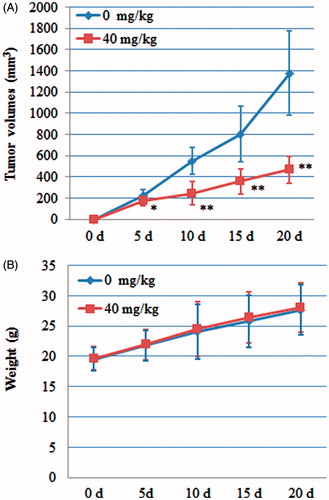
Nude mice were injected in the right flank subcutaneously with A549 cells (2 × 106 cells per mouse). When the tumours grew to approximately 2–3 mm in diameter, the mice were treated with POS (40 mg/kg/d, intraperitoneal injection, ip) or an equal volume of solvent control (0.5% DMSO, ip) as a control, respectively. Mice of the two groups (n = 10) were observed for 15 d, and tumour sizes were measured every 5 d. Tumour diameters were determined by using a vernier caliper, and the tumour volumes were calculated according to the formula: volume = (width2 × length)/2 (He et al. Citation2009; Zhou et al. Citation2013).
Statistical analysis
The significance of the differences between the different groups was determined using Student’s t-test. The results are presented as the mean ± SD, and the differences were considered significant at p < 0.05.
Results
Anti-proliferative effect of POS on A549 cells in vitro
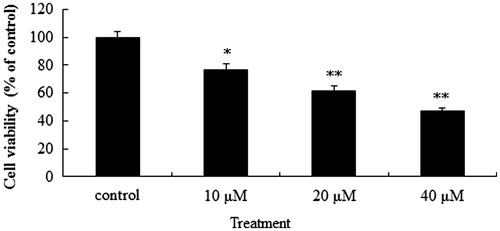
The anti-proliferative activity of POS in vitro was using human lung cancer cell line A549, a classical NSCLC cell line. POS showed obvious anti-proliferative effect on A549 cancer cells (p < 0.05, p < 0.01 and p < 0.01 for 10, 20 and 40 μM POS, respectively), with a significant concentration dependence ().
Figure 2. Anti-proliferative effect of puerarin 6″-O-xyloside (POS) on A549 cells in vitro. A549 cell lines were treated with POS (10, 20 and 40 μM) for 32 h, and cell proliferation was determined by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The data are represented as the mean ± SD (n = 4). *p < 0.05 and **p < 0.01 versus control.
Pro-apoptotic effect of POS on A549 cells
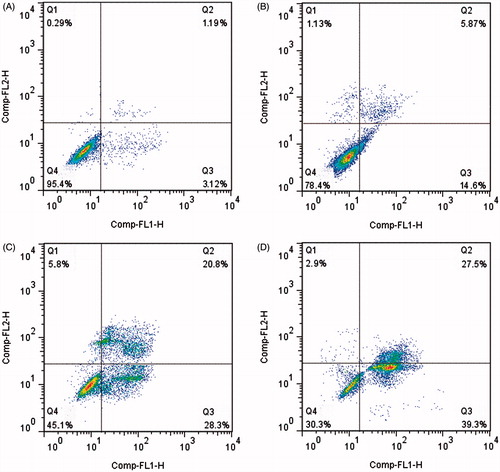
Cytotoxicity assays demonstrated that POS had notable antitumour activity against human lung cancer A549 cells. To determine whether the antitumour activity of POS resulted from apoptosis, A549 cells were further analyzed by staining with Annexin V-FITC/PI followed by flow cytometry (). The results indicated that apoptotic cells increased gradually when treated with increasing concentrations of POS (10, 20 and 40 μM). Thus, POS induced death of human A549 lung cancer cells by inducing apoptosis.
Figure 3. Flow cytometric analysis of A549 cells apoptosis following exposure to puerarin 6″-O-xyloside (POS). A549 cell lines were treated POS (10, 20 and 40 μM) for 32 h. The flow cytometry assay was performed to determine the apoptosis rate by Annexin-V/PI double-staining. (A–D) The control, 10, 20 and 40 μM groups.
Exposure of A549 cells to POS results in the increase of cytochrome c release in cytoplasm
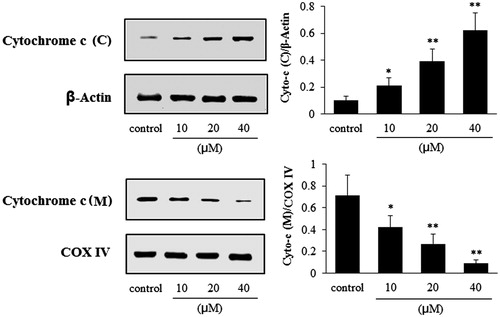
As can be seen from , after cells were exposed to the POS (10, 20 and 40 μM), the release of cytochrome c into the cytosol from the mitochondria was significantly increased in a concentration-dependent manner (p < 0.05, p < 0.01 and p < 0.01, for the 10, 20 and 40 μM, respectively); whereas the cytochrome c levels in the mitochondria decreased (p < 0.05, p < 0.01 and p < 0.01, for the 10, 20 and 40 μM, respectively).
Figure 4. Effects of puerarin 6″-O-xyloside (POS) on the expressions of cytochrome c (cytoplasm and mitochondria) in A549 cells. Cytochrome c (C) and cytochrome c (M) represent the expressions of cytochrome c in the cytoplasm and mitochondria, respectively. A549 cell lines were treated POS (10, 20 and 40 μM) for 32 h, and the levels of the different proteins were measured by western blotting. The data are represented as mean ± SD (n = 4). *p < 0.05 and **p < 0.01 versus control.
Exposure of A549 cells to POS results in the upregulation of C-caspase-3, C-caspase-7, C-caspase-9, bax and downregulation of Bcl-2
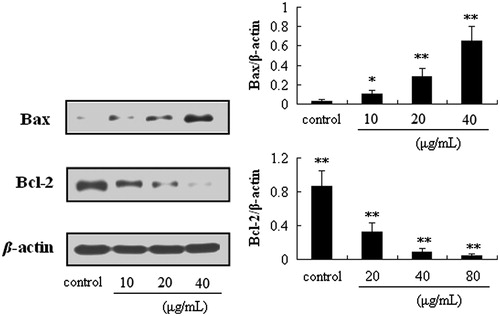
As shown in and , compared with the control group, POS (10, 20 and 40 μM) significantly increased the protein levels of caspase-3 (p < 0.05), caspase-7 (p < 0.05), caspase-9 (p < 0.05) and Bax (p < 0.05), in a concentration-dependent manner; whereas Bcl-2 was obviously decreased (p < 0.01), in a concentration-dependent manner.
Figure 5. Effects of puerarin 6″-O-xyloside (POS) on the expressions of cleaved-caspase-3 (C-capase-3), cleaved-caspase-7 (C-capase-7) and cleaved-caspase-9 (C-caspase-9) in A549 cells. A549 cell lines were treated POS (10, 20 and 40 μM) for 32 h, and the levels of the different proteins were measured by western blotting. The data are represented as the mean ± SD (n = 4). *p < 0.05 and **p < 0.01 versus control.
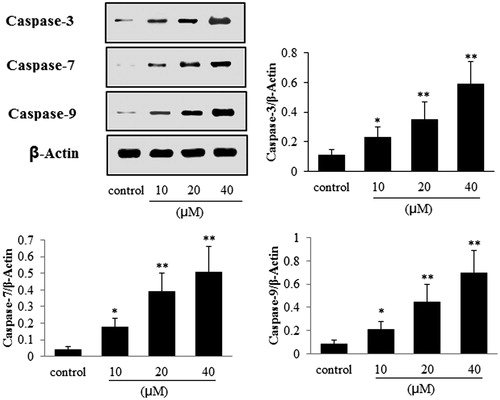
Figure 6. Effects of puerarin 6″-O-xyloside (POS) on the expressions of Bax and Bcl-2 in A549 cells. A549 cell lines were treated with POS (10, 20 and 40 μM) for 32 h, and the levels of the different proteins were measured by western blotting. The data are represented as the mean ± SD (n = 4). *p < 0.05 and **p < 0.01 versus control.
Antitumour activity of POS in vivo
These results from the experiments in vitro indicated that POS possessed cytotoxic effects on A549 cells via inducing mitochondria-mediated apoptosis. Therefore, we studied the antitumour effect of POS in vivo using a transplanted tumour nude mouse model. To achieve better bioavailability, POS was delivered by intraperitoneal injection (i.p.). The results demonstrated that exposure of mice to POS (40 mg/kg) significant suppressed tumour growth during a 15 d observation period (p < 0.05), compared with the control group (). No significant difference in body weight was observed between the POS and control mice (p > 0.05) ().
Discussion
In recent years, increasing evidence has demonstrated that uncontrolled cell proliferation and insufficient apoptosis would eventually lead to cancer (Wu et al. Citation2014). Apoptosis, known as a programmed cell death, is an active process of cell death, the promotion of which is considered as an ideal method of cancer therapy (Yang et al. Citation2007; Chipuk et al. Citation2012). Apoptosis is characterized by distinct morphological changes and biochemical hallmarks, including cell shrinkage, nuclear DNA fragmentation, apoptotic bodies and membrane blebbing, and is regulated by a series of biochemical events that eventually result in cell death (Gao et al. Citation2009). Mitochondria-mediated apoptosis is a major apoptotic pathway induced by the release of cytochrome c into the cytoplasm by mitochondrial outer membrane permeabilization, which activates caspases proteins (Chipuk et al. Citation2012; Xiong et al. Citation2014). Caspases (cysteine-aspartic acid proteases) are key mediators of apoptosis, and the activation of caspase-3 is a biomarker for cells undergoing apoptosis. Caspase-9 is the initiating caspase protein in the caspase cascade, and caspase-3, the crucial activated death proteases, is activated by caspase-9 (Cohen Citation1997; Li et al. Citation2004; Gao et al. Citation2009). The Bcl-2 family is another species of proteins that play a crucial role in the mitochondria-mediated intrinsic apoptosis pathway, and is considered to be the first regulatory step in mitochondrial apoptosis (Kuwana & Newmeyer Citation2003; Xiong et al. Citation2014). In addition, the Bcl-2 family proteins are commonly divided into two types with opposing effects on the regulation of mitochondrial apoptosis. Bax is the pro-apoptotic protein, whereas Bcl-2 is anti-apoptotic protein (Oltvai et al. Citation1993; Antonsson, Citation2001). Our results revealed that POS has significant cytotoxic activity toward human A549 lung cancer cells, which involves the induction of apoptosis. Our results showed that POS induces apoptosis in A549 cells by altering the levels of proteins related to mitochondria-mediated intrinsic apoptosis. Thus, exposure of A549 cells to POS led to a significant increase in the levels of C-caspase-3, C-caspase-7, C-caspase-9 and Bax, whereas the level of Bcl-2 significantly decreased. These findings indicated that POS induced mitochondria-mediated intrinsic apoptosis in the human lung cancer cell line A549. In addition, the in vivo experiments were consistent with the results obtained in vitro, which suggested that POS has antitumour activity toward the human lung cancer A549.
In conclusion, the present study demonstrated that POS has significant antitumour activities in vitro and in vivo. The mechanisms of its antitumour activities involve induction of mitochondria-mediated apoptosis via increased abundances of caspase-3, caspase-7, caspase-9, Bax and reduced levels of Bcl-2. Thus, the results suggested that POS could be regarded as a new candidate to treat lung cancer.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Antonsson B. 2001. Bax and other pro-apoptotic Bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res. 306:347–361.
- Chen XL, Hu Y. 1997. The antitumor effect of Puerariae Radix by MTT method and flow cytometry assay. Pharmacol Clin Chin Mater Med. 13:27–29. (in Chinese).
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR. 2012. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 148:988–1000.
- Cohen GM. 1997. Caspases: the executioners of apoptosis. Biochem J. 326:1–16.
- Du DJ, Shi XF, Ran CQ, Zhao JN, Wen ZJ, Xie DC, Ye YL, Gu YC. 1994. Anticancer activity of Flos Puerariae. Pharmacol Clin Chin Mater Med. 10:16–20. (in Chinese).
- Du DJ, Yang W, Yie YL, Ran CQ, Shi XF, Zhao SY. 1997. Antitumor cytotoxicity of main components from Puerariae (Legeioeaoae). Chin J Cancer. 16:165–167. (in Chinese).
- Gao N, Budhraja A, Cheng S, Yao H, Zhang Z, Shi X. 2009. Induction of apoptosis in human leukemia cells by grape seed extract occurs via activation of c-Jun NH2-terminal kinase. Clin Cancer Res. 15:140–149.
- He ZJ, Chen XF, Cai QH, Yu Y, Tang JM, Yang JY, Cheng CT, Wang JN. 2009. Comparative study of the different calculating and measuring methods in the implanted tumor volume. Chin J Compar Med. 19:47–50. (in Chinese).
- Ko JC, Su YJ, Lin ST, Jhan JY, Ciou SC, Cheng CM, Lin YW. 2010. Suppression of ERCC1 and Rad51 expression through ERK1/2 inactivation is essential in emodin-mediated cytotoxicity in human non-small cell lung cancer cells. Biochem Pharmacol. 79:655–664.
- Kuwana T, Newmeyer DD. 2003. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 15:691–699.
- Li JW, Vederas JC. 2009. Drug discovery and natural products: end of an era or an endless frontier? Science. 325:161–165.
- Li YH, Wang C, Meng K, Chen LB, Zhou XJ. 2004. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 10:1984–1988.
- Liu Y, Sun WY, Zhang KT, Zheng HW, Ma Y, Lin DM. 2007. Identification of genes differentially expressed in human primary lung squamous cell carcinoma. Lung Cancer. 56:307–317.
- Oltvai ZN, Milliman CL, Korsmeyer SJ. 1993. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 74:609–619.
- Peng W, Ming QL, Han P, Zhang QY, Jiang YP, Zheng CJ, Han T, Qin LP. 2014. Anti-allergic rhinitis effect of caffeoylxanthiazonoside isolated from fruits of Xanthium strumarium L. in rodent animals. Phytomedicine. 21:824–829.
- Skermana NB, Joubertb AM, Cronjéa MJ. 2011. The apoptosis inducing effects of Sutherlandia spp. extracts on an oesophageal cancer cell line. J Ethnopharmacol. 137:1250–1260.
- Siegel R, Ma JM, Zou ZH, Jemal A. 2015. Cancer Statistics, 2014. 64:9–29.
- Wu JG, Peng W, Yi J, Wu YB, Chen TQ, Wong KH, Wu JZ. 2014. Chemical composition, antimicrobial activity against Staphylococcus aureus and a pro-apoptotic effect in SGC-7901 of the essential oil from Toona sinensis (A. Juss.) Roem. leaves. J Ethnopharmacol. 154:198–205.
- Xiong S, Mu T, Wang G, Jiang X. 2014. Mitochondria-mediated apoptosis in mammals. Protein Cell. 5:734–749.
- Yang SJ, Meng JP, Liu YB. 2007. The progress on the signal transduction pathways of apoptosis. Chin J Compar Med. 17:297–301. (in Chinese).
- Yang XK, Xu MY, Xu GS, Zhang YL, Xu ZX. 2014. In vitro and in vivo antitumor activity of scutebarbatine A on human lung carcinoma A549 cell lines. Molecules. 19:8740–8751.
- Yuan HB, Mi MT, Chen ZD, Wei ZJ. 2007. Effect of Kudzu flavonoids extracts on proliferation and apoptosis of HL260 cells. Cancer Res Prev Treat. 34:671–736.
- Zhao YY, Jiang WW, Li B, Yao Q, Dong JQ, Cen YY, Pan XC, Li J, Zheng J, Pang XL, et al. 2011. Artesunate enhances radiosensitivity of human non-small cell lung cancer A549 cells via increasing NO production to induce cell cycle arrest at G2/M phase. Int Immunopharmacol. 11:2039–2046.
- Zhou T, Li G, Cao B, Liu L, Cheng Q, Kong H, Shan C, Huang X, Chen J, Gao N. 2013. Downregulation of Mcl-1 through inhibition of translation contributes to benzyl isothiocyanate-induced cell cycle arrest and apoptosis in human leukemia cells. Cell Death Dis. 4:e515.