Abstract
Context Pelargonium sidoides DC (Geraniaceae) is an important medicinal plant indigenous to South Africa and Lesotho. Previous studies have shown that root extracts are rich in polyphenolic compounds with antibacterial, antiviral and immunomodulatory activities. Little is known regarding the anticancer properties of Pelargonium sidoides extracts.
Objective This study evaluates the anti-proliferative effects of a Pelargonium sidoides radix mother tincture (PST).
Materials and methods The PST was characterized by LC-MS/MS. Anti-proliferative activity was evaluated in the pre-screen panel of the National Cancer Institute (NCI-H460, MCF-7 and SF-268) and the Jurkat leukaemia cell line at concentrations of 0–150 μg/mL. The effect on cell growth was determined with sulphorhodamine B and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays after 72 h. The effect on cell cycle and apoptosis induction in Jurkat cells was determined by flow cytometry with propidium iodide and Annexin V: fluorescein isothiocyanate staining.
Results Dihydroxycoumarin sulphates, gallic acid as well as gallocatechin dimers and trimers were characterized in PST by mass spectrometry. Moderate anti-proliferative effects with GI50 values between 40 and 80 μg/mL were observed in the NCI-pre-screen panel. Strong activity observed with Jurkat cells with a GI50 value of 6.2 μg/mL, significantly better than positive control 5-fluorouracil (GI50 value of 9.7 μg/mL). The PST arrested Jurkat cells at the G0/G1 phase of the cell cycle and increased the apoptotic cells from 9% to 21%, while the dead cells increased from 4% to 17%.
Conclusion We present evidence that P. sidoides has cancer cell type-specific anti-proliferative effects and may be a source of novel anticancer molecules.
Introduction
Plant extracts have played important roles in anticancer drug discovery with several anticancer drugs in clinical use derived from plant extracts (Balunas & Kinghorn Citation2005; Cragg & Newman Citation2005). Thus, much research has been devoted to analyzing plant extracts for anticancer activity. Inflammation and oxidative stress have been implicated in cancer pathogenesis and are not only initiators of tumourigenesis but also involved in driving the disease progression (Brown & Bicknell Citation2001; Lucia & Torkko Citation2004; Federico et al. Citation2007; Azad et al. Citation2008; Colotta et al. Citation2009; Grivennikov et al. Citation2010; Kundu & Surh Citation2012).
Plant extracts or compounds that have anti-inflammatory and/or antioxidant activity may thus prove useful in cancer chemoprevention. Plant extracts with these properties are rich in phenolic compounds such as phenolic acids, flavonoids, flavonol glycosides, anacardic acids, proanthocyanidins, phenylcoumarins, theaflavins, cannabinoids, phenolic amides, curcuminoids, stilbene oligomers, xanthones, phenolic oils and flavonoligans (Anilkumar Citation2010). Specific phenolic compounds include quercetin (Dajas Citation2012), anacardic acid (Sun et al. Citation2006; Hsieh & Hernández-Ledesma Citation2011), epigallocatechin gallate (EGCg) (Khan & Mukhtar Citation2008), gallic acid (Verma et al. Citation2013), proanthocyanidins (Nandakumar et al. Citation2008), curcumin (Goel et al. Citation2008), cannabinoids (Alexander et al. Citation2009), mangostin (Nakagawa et al. Citation2007; Johnson et al. Citation2012), gossypin (Kim et al. Citation2008; Shi et al. Citation2012), silymarin (Ramasamy & Agarwal Citation2008), gingerols and shagoals (Lee et al. Citation2008; Sang et al. Citation2009). In addition, anticancer effects can also include inhibition of cell growth, cell-cycle arrest, induction of apoptosis, inhibition of topoisomerase enzymes and matrix metalloproteinases as well as angiogenesis. The green tea-derived flavanol EGCg is currently being evaluated in clinical trials as a part of combinational anticancer drug therapy (Saba et al. Citation2015). Plant-derived coumarins have been identified as important scaffolds for anticancer therapies, particularly as lead candidates for the treatment of haematological malignancies (Riveiro et al. Citation2008; Kaur et al. Citation2015).
Pelargonium sidoides DC (Geraniaceae) is indigenous to South Africa and Lesotho and is collected from the wild. Due to overharvesting, commercial farming of P. sidoides is being developed in South Africa as an alternative source of raw material (Brendler & Van Wyk Citation2008; Moyo et al. Citation2013). Plant extracts of the tubers or rhizomes are rich in various polyphenolic compounds such as gallic acid, di- and trihydroxy and methoxycoumarins, coumarin sulphates, catechins and proanthocyanidins (Kolodziej Citation2007). Its traditional uses include the treatment of diarrhoea, dysentery, colic, anaemia and tuberculosis and reported medicinal properties including antibacterial, antifungal, antiviral and immunomodulatory activities (Brendler & Van Wyk Citation2008). Based on these medicinal properties commercial formulations such as Linctagon and EPs® 7630 have been developed for the treatment of upper respiratory tract infections (Matthys et al. Citation2007; Brendler & Van Wyk Citation2008).
Based on the previous reports on the types of polyphenolics found in P. sidoides root extracts, it can be expected that P. sidoides will also have anticancer properties (Kolodziej Citation2007). An extensive search of scientific literature only found a patent by Kong et al. (Citation2009), claiming that a P. sidoides extract exhibited cytotoxicity towards GLC4 human lung cancer and COLO320 human colon cancer cell lines. The aim of this study was to evaluate the anti-proliferative effects of a P. sidoides radix mother tincture (PST) in the anticancer pre-screen panel of the National Cancer Institute (NCI) as well as the Jurkat cell line. This is the first report to our knowledge describing the anticancer activity of P. sidoides extracts.
Materials and methods
Chemicals
The PST prepared according to the German Homeopathic Pharmacopoeia HAB4a was purchased from Parceval Pharmaceuticals (Pty) Ltd., Wellington, South Africa. Positive controls, doxorubicin (DOX) and 5-fluoruracil (5FU) were obtained from Sigma-Aldrich Company, Atlasville, South Africa. All other reagents were obtained either from Sigma-Aldrich Company, Atlasville, South Africa or Merck, Johannesburg, South Africa.
Cell cultures
The NCI-H460 human lung large cell carcinoma, SF-268 human gliomablastoma and the MCF-7 human breast adenocarcinoma cell lines were obtained from the NCI, Frederick, MD. The Jurkat E6.1 human leukaemic T-cell lymphoblast cell line was obtained from the European Collection of Cell Cultures supplied by Sigma-Aldrich Company, Atlasville, South Africa. Cell lines were maintained in RPMI 1640 (Sigma-Aldrich Company, Atlasville, South Africa) and Penicillin/Streptomycin/Fungizone formulation (Highveld Biological, Lyndhurst, South Africa) and maintained at 37 °C, 5% CO2.
Liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis of PST
Analysis of the PST was performed on a Waters ultra-performance liquid chromatography (UPLC) Acquity system fitted with a Waters Acquity Photo Diode Array (PDA) detector and coupled to a Waters Synapt G2 mass spectrometer (Waters, Milford, MA). The PST was analyzed with a Waters Acquity UPLC BEH C18 column, 2.1 × 100 mm with 1.7 μm particle size coupled with a Waters Acquity UPLC BEH C18 pre-column, 2.1 × 5 mm with 1.7 μm particle size (Waters, Milford, MA). The column temperature was set at 55 °C and the solvents used were HPLC grade water (solvent A) and HPLC grade acetonitrile (solvent B). Two μL of the PST was injected, and the flow rate was set at 0.2 mL/min. The chromatographic conditions were as follows: 5% B for first 5 min, 5–95% solvent B over 45 min, 95% solvent B was maintained for 5 min followed by column re-equilibration over 5 min. The mass spectrometer was operated in the negative ionization mode and mass data were acquired from 50 to 1500 amu. Conditions were as follows: capillary voltage 2.5 kV, sampling cone voltage 15, desolvation temperature 275 °C, desolvation gas flow 650 L/h and cone gas flow of 50 L/h. A low energy function with trap collision energy of 4 V and a high energy function with trap collision energy that ramped from 15 to 60 V were used to acquire the mass data. Data were processed with Waters MassLynx V4.1 software (Waters, Milford, MA) and compounds were tentatively identified based on their fragmentation patterns and previous literature.
Anticancer activity
The anticancer activity of the PST was evaluated with NCI-H460, SF-268, MCF-7 and Jurkat cell lines. Briefly, cells were plated in 96-well culture plates in 100 μL of RPMI medium with 10% FBS at the following cell densities: 7.5 × 104/mL for the NCI-H460, 1.5 × 105/mL for the SF-268, 1 × 105/mL for the MCF-7 and 2 × 105/mL for the Jurkat cells. The cells were left to settle for 24 h at 37 °C and 5% CO2. Different concentrations of the PST was then added in 100 μL of RPMI medium with 10% FBS and the cells were left for a further 72 h at 37 °C and 5% CO2. DOX and 5FU were used as positive controls, while cells in the absence of any drug were used as the negative control. Following the 72 h exposure period, the anticancer activity was evaluated with the sulphorhodamine B (SRB) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays.
For the SRB assay, cells were fixed with 50 μL of a 50% cold trichloroacetic acid solution (w/v) for 1 h at 4 °C. Thereafter, plates were washed with water and left to dry. Plates were stained with a 0.4% SRB solution (w/v) in 1% acetic acid solution (v/v) for 10 min, washed with 1% acetic acid solution and left to dry. The dye was then solubilised with 200 μL of a 10 mM Tris base solution pH 10.5, and the absorbance was determined at 550 nm with 690 nm as the reference with a Multiscan Ascent plate reader (AEC Amersham, Kelvin, South Africa).
For the MTT assay, 50 μL of a 1 mg/mL MTT solution in phosphate-buffered saline (PBS) was added to each well. Plates were allowed to react at 37 °C and 5% CO2 for 4 h. The medium was removed, and the dye was solubilised with 200 μL of dimethylsulphoxide. The absorbance was determined at 550 nm with 690 nm as the reference with a Multiscan Ascent plate reader (AEC Amersham, Kelvin, South Africa).
The concentration that inhibits the cell growth by 50% (GI50) was calculated as follows: (T–T0)/(C−T0) × 100 = 50, where T is the absorbance of the cell growth in the presence of a drug concentration, T0 the initial cell growth before any drugs were added and C is the control cell growth (Boyd & Paull Citation1995).
Flow cytometric analyses
The effect of the PST on cell cycle and apoptosis was determined with the Jurkat cell line. Briefly, 4 × 105 Jurkat cells in 5 mL of RPMI medium with 10% FBS were plated in 25 cm2 cell culture flasks and left to settle for 24 h at 37 °C and 5% CO2. Cells were exposed to 10 μg/mL of the PST for 72 h.
For the cell-cycle analysis, cells were harvested, washed with Dulbecco’s phosphate-buffered saline (DPBS) and resuspended in 1 mL of 70% cold ethanol solution (v/v) for 1 h at 4 °C. Cells were pelleted and washed with DPBS staining solution containing 2% FBS and 0.01% sodium azide. Cells were resuspended in 1 mL of the DPBS-staining solution and treated with 100 μL of 1 mg/mL RNase solution for 30 min at 37 °C. Cells were stained with 10 μL of 1 mg/mL propidium iodide solution for 30 min at room temperature.
The apoptosis analysis was performed with Annexin V: fluorescein isothiocyanate (FITC) assay kit (AbDSerotec, Oxford, UK) and cells were treated as per the manufacturer’s instructions. Three controls were used to determine the gates for the viable, dead and apoptotic cells. For the viable cell’s gate, unstained cells were used. Cells were fixed with 70% cold ethanol for 1 h at 4 °C and stained with propidium iodide were used to gate for the dead cells. Cells treated with a 3% (v/v) formaldehyde solution in 1 × DPBS for 30 min at 4 °C were used to gate the apoptotic cells.
Cell cycle and apoptosis analysis was performed using the BD FACSAria flow cytometer (BD Biosciences, San Diego, CA). Cell-cycle data were analyzed with the FlowJo software version 7.6.5, while apoptosis data were analyzed with the FACSDiva software version 6.1.3 (BD Biosciences, San Jose, CA). Two independent experiments were conducted for cell cycle and apoptosis analysis, and results are expressed at means together with the standard error of the mean (SEM).
Statistical analysis
The statistical significance of each sample’s GI50 value was determined with the Tukey honestly significant difference test calculated with the JMP (version 9.0.0) software (BD Biocsiences, San Jose, CA) at the 95% level of confidence.
Results
LC-MS/MS analysis of PST
A commercial formulation prepared from the roots of P. sidoides was evaluated for its potential anticancer activity. This formulation is prepared according to German Homeopathic Pharmacopoeia standards namely procedure HAB 4a that involves the extraction of 10 parts fresh P. sidoides roots with 100 parts of alcohol, with a final alcohol concentration of 60%. The concentration was 0.14 ± 0.01 g dry mass/10 mL. LC-MS/MS analysis of the PST () revealed that it was complex in nature, and many compounds could be tentatively identified based on the fragmentation patterns, UV–vis spectrum and previous literature. Twenty-two phenolic compounds were identified; 10 hydroxy, methoxy or sulphated coumarin derivatives, nine flavan-3-ols (catechins and proanthocyanidins) as well as the phenolic acid, gallic acid and flavonoid derivative eriodictyol sulphate (). Three unknown compounds with molecular masses of 321 m/z, 305 m/z and 523 m/z were also found. The presence of the coumarin umckalin (peak 24) confirmed that the tincture was prepared from the roots of P. sidoides and not from closely related species Pelargonium reniforme Curt. which does not contain umckalin (Kolodziej, Citation2007).
Figure 1. Base peak chromatogram of PS extract obtained in negative ionisation mode. Number of peaks refer to compounds tentatively identified in .
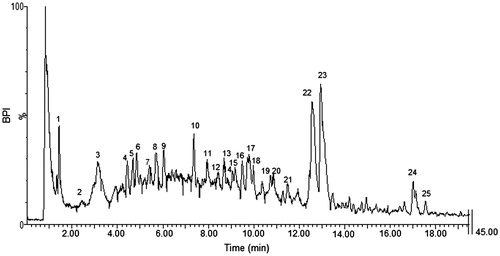
Table 1. Proposed phenolic compounds identified from the PST.
Anticancer activity
Effect on cell growth and viability
The effect of 72 h exposure of PST on cell viability and number was determined with the SRB and MTT assays, respectively. Two known anticancer drugs, DOX and 5FU, were included as positive controls (). Good correlation was found between the SRB and MTT assays. Treatment of these cell lines with the PST showed a dose-dependent response effect over the concentration range evaluated, i.e., 0–150 μg/mL for the NCI-H460, MCF-7 and SF-268 cells, and 0–30 μg/mL for the Jurkat cell line. The GI50 of PST was compared with the positive controls (). The PST was the most effective in the Jurkat cell line with a GI50 value of 6.2 μg/mL followed by the MCF-7 cell line with a GI50 value of 43 μg/mL. The SF-268 and NCI-H460 cell lines were more resistant with GI50 values of 60 and 80 μg/mL, respectively.
Figure 2. Effect of PST on NCI-H460, MCF-7, SF-268 and Jurkat cell lines as determined with the MTT and SRB assays. Cells were exposed to various concentrations of PST for 72 h. Three independent experiments were conducted and error bars represent the standard error of the mean.
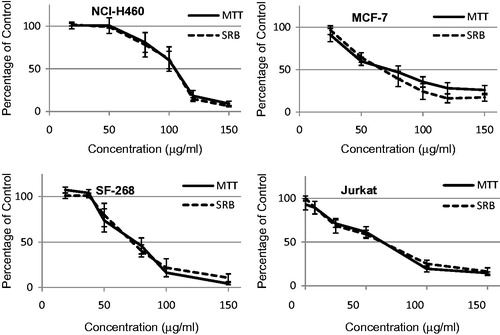
Table 2. GI50 values for PST and positive controls as determined with the SRB assay.
Effect on cell cycle and apoptosis induction
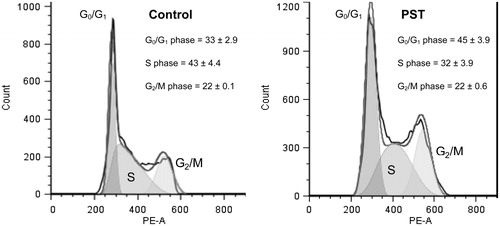
Jurkat cells were exposed to 10 μg/mL PST for 72 h () and in the control cell population, 33%, 43% and 22% of cells were in the G0/G1, S and G2/M phases of the cell cycle, respectively. In the presence of PST, an increased accumulation of cells in the G0/G1 phase to 45% with a concomitant decrease in the S phase to 32% was observed. This indicated that treatment of Jurkat cells with PST results in a G0/G1 cell-cycle arrest.
Figure 3. Cell-cycle analyses of Jurkat cells treated with PST for 72 h. Percentages of each phase are the results of two independent experiments with standard error of the mean.
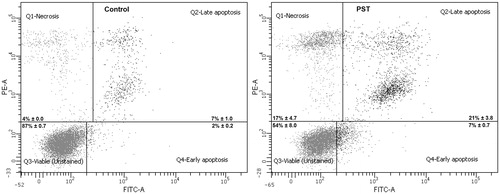
The ability of the PST to induce apoptosis and cell death at 10 μg/mL in the Jurkat cells was also evaluated (). The number of viable cells decreased from 87% in the control cells to 54% in the treated cells while the number of cells undergoing apoptosis following treatment increased 3-fold from 9% to 21% (early and late apoptosis). The percentage dead cells also increased from 4% in the control to 17% in the treated cells. The PST not only disrupts the cell cycle but also induces apoptosis and cell death of Jurkat cells.
Figure 4. Apoptosis induction in Jurkat cells treated with PST for 72 h. Unstained cells were used to gate for the viable cells, cells fixed with 70% ethanol and stained with propidium iodide for the dead cells and cells treated with 3% formaldehyde to gate the apoptotic cells. Percentages of each phase are the results of two independent experiments with standard error of the mean.
Discussion
Inflammation and oxidative stress have been implicated in cancer pathogenesis and thus plant extracts or compounds that have anti-inflammatory and/or antioxidant activity may thus prove useful in cancer chemoprevention (Brown & Bicknell Citation2001; Lucia & Torkko Citation2004; Federico et al. Citation2007; Azad et al. Citation2008; Colotta et al. Citation2009; Grivennikov et al. Citation2010; Kundu & Surh Citation2012). Extracts prepared from the roots of P. sidoides have shown in previous studies that they are rich in polyphenolic compounds, and have antioxidant and immunomodulatory activities (Brendler & Van Wyk Citation2008; Moyo et al. Citation2013). This suggests that P. sidoides should also show anticancer activity.
Mass spectrometry analysis revealed that the PST was rich in sulphated coumarins and flavonoids that were identified based on the loss of 80 mass units to yield major fragments of 193 m/z for dihydroxycoumarin (Gödecke et al. Citation2005), 303 m/z for gallocatechin (GC)/epigallocatechin (EGC) and 287 m/z for eriodictyol (Callemien & Collin Citation2008; Sudjaroen et al. Citation2005). Sulphated coumarins were also found by Latté et al. (Citation2000) and Gödecke et al. (Citation2005) in extracts prepared with 80% acetone from the dried roots and aerial parts of P. sidoides, respectively. These sulphated coumarins were 5,6-dimethoxycoumarin 7-sulphate, 6-hydroxy-5,7-dimethoxycoumarin 8-sulphate, 8-hydroxy-5,7-dimethoxycoumarin 6-sulphate and 6,7-dihydroxycoumarin 8-sulphate. Two pyranocoumarin sulphates and a furanocoumarin sulphate have been isolated from an aqueous extract of the roots of Seseli libanotis, a perennial herb of the Umbelliferae family (Lemmich & Shabana Citation1984). Sulphated flavanols have previously been described by Schötz and Nöldner (Citation2007) in oligomeric fractions prepared from P. sidoides medicine Umckaloabo. Sulphated flavonoids appear to be more widespread in nature and can be found in plants such as Flaveria bidentis, Polygonum hydropiper, Brickellia californica and Malva sylvestris (Barron et al. Citation1988). Sulphation is an important element of the mammalian metabolic pathways, however, little is known about the bioactivity of these molecules compared with the parent compound. This is an aspect that should be addressed in future research.
Standardized evaluation of molecules and plant extracts involves initial screening using the NCI-pre-screen panel consisting of the NCI-H460 human lung large cell carcinoma, SF-268 human gliomablastoma and the MCF-7 human breast adenocarcinoma cell lines (Takimoto Citation2003). The NCI adopted this pre-screen panel, since a large number of compounds submitted to the 60 cell-line panel showed very little activity. Only compounds that can inhibit the growth of the pre-screen panel are then further evaluated in the 60 cell-line panel. A limitation in this pre-screen panel is that it excludes cancer cell types that represent the haematological system and, therefore, the Jurkat T lymphocyte leukaemia cell line was included in this study. The SRB assay is the standard cytotoxicity assay employed by the NCI to evaluate anticancer activity of compounds/plant extracts (Takimoto Citation2003), the MTT assay was included as this assay is widely employed by various authors to assess anticancer activity and as a result data generated in this study can be compared with other studies. Two positive controls were included in this study namely DOX and 5FU. The anticancer activity of DOX is via the generation of hydrogen peroxide and the subsequent formation of reactive oxygen species and the inhibition of DNA topoisomerase II (Mizutani et al. Citation2005). Positive control, 5FU is an anti-metabolite that prevents DNA synthesis by inhibiting the enzyme thymidylate synthetase (Pinedo & Peters Citation1988).
The anticancer activity observed for the PST was found to be similar to that of other polyphenolic rich plant extracts. Green tea extracts have shown anticancer activity ranging from 5 to 70 μg/mL on lung, colon and T-cell leukaemia cancer cells (Yang et al. Citation1998; Li et al. Citation2000). Grape seed extracts on different lung, breast and colorectal cancer cells have shown activity ranging from 30 to 100 μg/mL (Sharma et al. Citation2004; Kaur et al. Citation2008; Akhtar et al. Citation2009). Berry extracts also rich in polyphenolic compounds have shown anticancer activity on oral, colon, breast and prostate cancer cells ranging from 30 to 200 μg/mL (Seeram et al. Citation2006).
The PST was most effective in inhibiting cancer cell growth in the Jurkat cell line with a GI50 value of 6.2 μg/mL. Polyphenolic compounds similar to those found in the PST have shown anti-proliferative effects in leukaemia type cancer cells. Gallic acid was observed to dose-dependently inhibit the growth of HL-60 promyelocytic leukaemia cells in vitro with an IC50 value of 24 μM (4.1 μg/mL) (Madlener et al. Citation2007). Similarly, dihydroxycoumarins esculetin (6,7-dihydroxycoumarin) and 7,8-dihydroxycoumarin have been shown to inhibit the growth of U937 leukaemia cells with IC50 values of 31 μM (5.5 μg/mL) and 48 μM (8.6 μg/mL), respectively (Riveiro et al. Citation2008).
For crude plant extracts to be considered for further evaluation, they need to have a GI50 value of 30 μg/mL or less (Itharat et al. Citation2004; Steenkamp & Gouws Citation2006). As the GI50 value for the PST in the Jurkat cell line was less than 30 μg/mL, the effect of PST on the cell cycle and the ability of PST to induce apoptosis were further evaluated. Cancer cells proliferate uncontrollably and bypass cell-cycle checkpoints (Stewart et al. Citation2003). Molecules that block the cell cycle of cancer cells and prevent their uncontrollable proliferation are considered to be promising cancer chemotherapeutics (Owa et al. Citation2001).
The PST was observed to arrest Jurkat cells in the G0/G1 phase of the cell cycle. Abnormal G1 phase regulation has been implicated as a crucial factor in tumour development and progression (Owa et al. Citation2001). G1 phase block will prevent the cancer cells from undergoing S phase DNA synthesis and subsequent cell division. Progression through the G1 phase is regulated by the cyclin D/cyclin-dependent kinase (CDK) 4 and 6 complexes. Grape seed proanthocyanidins were shown to induce a G1 phase block of the cell cycle in human epidermoid carcinoma A431 cells (Meeran & Katiyar Citation2007). The block was associated with a decrease in cyclin D1, D2 and E as well as CDKs 2, 4 and 6. An increase in CDK inhibitors p21 and p27 was also observed. Treatment of human leukaemia HL-60 cell with dihydroxycoumarin esculetin was also associated with a G1 phase block in a study by Wang et al. (Citation2002). Decreased levels of cyclin D1 and E, CDK 4 and hyperphosphorylated retinoblastoma protein were observed as well as increased levels of p27. Huang et al. (Citation2012) reported that Toona sinensis derived gallic acid caused G1 arrest in HL-60 human promyelocytic cells. The PST was shown to contain gallic acid, proanthocyanidins as well as dihydroxycoumarins, and it is suspected that these compounds may be involved in the G0/G1 phase block of the cell cycle observed in the Jurkat cell line.
Apoptosis maintains the balance between cell proliferation and cell death and eliminates all unnecessary, old, damaged and infected cells from tissues (Schulze-Bergkamen & Krammer Citation2004). Cancer cells have developed mechanisms to avoid apoptosis and continue proliferating uncontrollably. This is due to alterations in p53 function, upregulation of anti-apoptotic proteins, increased activation of the NF-κβ and the PI3K/AKT pro-survival pathway as well as the downregulation of death receptors and of pro-apoptotic proteins. Induction of apoptosis by anticancer drugs is an important mechanism to eliminate cancer cells. The PST was observed to induce both apoptosis and necrosis in the Jurkat cell line. The increase in the number of dead cells was most likely due to a dose-dependent effect. At low concentrations apoptosis, will predominately be seen while at higher concentrations (GI50 and above) both apoptosis and necrosis will be observed. The results reflect the treatment of the Jurkat cells with the GI50 concentration of the extract. Similar results have been observed with platinum-containing compounds (Pèrez et al. Citation2003) and piplartine (Bezerra et al. Citation2007). It has been suggested that the pathways leading to apoptosis and necrosis are interconnected and that factors like energy availability as well as metabolic condition of the cells may play a role in deciding apoptotic or necrotic cell death (Fuertes et al. Citation2003). Further studies are needed to evaluate the mechanisms of apoptosis and necrosis induction by PST in leukaemia cells.
Polyphenolics like gallic acid, proanthocyanidins and dihydroxycoumarins when evaluated individually have been observed to induce apoptosis in cancer cells with little effect on normal cells. These compounds can trigger apoptosis through either the intrinsic mitochondrial pathway or the death receptor extrinsic pathway depending on the cancer cell type (Chu et al. Citation2001; Mantena et al. Citation2006; Meeran & Katiyar Citation2007; Kok et al. Citation2009; Ji et al. Citation2009; You et al. Citation2010).
Synergism between drugs can increase efficacy, reduce toxicity and resistance, and measured effects are a function of cell and target and pathway specificity, concentration and bio-availability. Synergistic effects have been reported between drugs and polyphenolics such that reported among EGCg, green tea catechins and anticancer compounds/drugs (Fujiki et al. Citation2014). Likewise, in plant extracts, synergistic bioactive effects between polyphenolics can occur. Del Follo-Martinez et al. (Citation2013) reported that resveratrol and quercetin in combination had increased anticancer activity in colon cancer cells.
Commercial medicinal formulations of P. sidoides are based on the reported antibacterial, antioxidant and immunomodulatory activities of P. sidoides (Brendler & Van Wyk Citation2008) and is clinically used for the treatment of upper respiratory tract infections (Matthys et al. 2003, Citation2007; Brendler & Van Wyk Citation2008). Inflammation and oxidative stress are two important elements in cancer pathogenesis (Brown & Bicknell Citation2001; Lucia & Torkko Citation2004; Federico et al. Citation2007; Azad et al. Citation2008; Colotta et al. Citation2009; Grivennikov et al. Citation2010; Kundu & Surh Citation2012). Taken in conjunction with the findings of this study, the anticancer effect of PST would be multi-factorial and includes anti-inflammatory, antioxidant activity as well as the inhibition of cellular proliferation.
Conclusion
A commercial extract of P. sidoides reduces the cell growth and viability of human cancer cell lines, in particular leukaemia type cells. In the Jurkat leukaemia cell line, the anti-proliferative effects are associated with G0/G1 cell-cycle arrest and the induction of apoptosis. The polyphenolic compounds identified in the P. sidoides extract are likely responsible for the observed activity. Further studies will be needed to isolate and identify these bio-active compounds as well as their mechanisms of action. The added scientific information provided by this study enables further development and identification of PST as a chemo-preventative product or as a source of new and novel anticancer molecules. It also further highlights the importance of P. sidoides as a commercial medicinal plant in South Africa.
Acknowledgements
The authors would like to acknowledge Wayne Barnes for assistance with flow cytometry analysis and Dr. Marietjie Stander for assistance with LC-MS/MS.
Declaration of interest
The authors report that they have no conflicts of interest. The authors would like to acknowledge the National Research Foundation of South Africa for funding this project.
References
- Akhtar S, Meeran SM, Katiyar N, Katiyar SK. 2009. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clin Cancer Res. 15:821–831.
- Alexander A, Smith PF, Rosengren RJ. 2009. Cannabinoids in the treatment of cancer. Cancer Lett. 285:6–12.
- Anilkumar M. 2010. Ethnomedicinal plants as anti-inflammatory and analgesic agents. In: Chattopadhyay D, editors. Ethnomedicine: a source of complementary therapeutics. Trivandrum: Research Signpost. p. 267–293.
- Azad N, Rojanasakul Y, Vallyathan V. 2008. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 11:1–15.
- Balunas MJ, Kinghorn AD. 2005. Drug discovery from medicinal plants. Life Sci. 78:431–441.
- Barron D, Varin L, Ibrahim RK, Harborne JB, Williams CA. 1988. Sulphated flavonoids – an update. Phytochemistry 27:2375–2395.
- Bezerra DP, Militão GCG, De Castro FO, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, Costa-Lotufo LV. 2007. Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol In Vitro 21:1–8.
- Boyd MR, Paull KD. 1995. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res. 34:91–109.
- Brendler T, Van Wyk B. 2008. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae). J Ethnopharmacol. 119:420–433.
- Brown NS, Bicknell R. 2001. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 3:323–327.
- Callemien D, Collin S. 2008. Use of RP-HPLC-ESI (−)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts. J Am Soc Brew Chem. 66:109–115.
- Chu C, Tsai Y, Wang C, Lin W, Tseng T. 2001. Induction of apoptosis by esculetin in human leukemia cells. Eur J Pharmacol. 416:25–32.
- Chung M, Lai M, Yen M, Wu R, Lin C. 1997. Phenolics from Hypericum geminiflorum. Phytochemistry 44:943–947.
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. 2009. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30:1073–1081.
- Cragg GM, Newman DJ. 2005. Plants as a source of anti-cancer agents. J Ethnopharmacol. 100:72–79.
- Dajas F. 2012. Life or death: neuroprotective and anticancer effects of quercetin. J Ethnopharmacol. 143:383–396.
- Del Follo-Martinez A, Banerjee N, Li X, Safe S, Mertens-Talcott S. 2013. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr Cancer 65:494–504.
- Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. 2007. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer 121:2381–2386.
- Fuertes MA, Castilla J, Alonso C, Pérez JM. 2003. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem. 10:257–266.
- Fujiki H, Sueoka E, Watanabe T, Suganuma M. 2014. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCg, other green tea catechins, and anticancer compounds. J Cancer Res Clin Oncol. 140:1–12.
- Gödecke T, Kaloga M, Kolodziej H. 2005. A phenol glucoside, uncommon coumarins and flavonoids from Pelargonium sidoides DC. Z Naturforsch B. 60:677–682.
- Goel A, Jhurani S, Aggarwal BB. 2008. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 52:1010–1030.
- Grivennikov SI, Greten FR, Karin M. (2010). Immunity, inflammation, and cancer. Cell 140:883–899.
- Hauer H, Germer S, Elsäßer J, Ritter T. 2010. Benzopyranones and their sulfate esters from Pelargonium sidoides. Planta Med. 76:350–352.
- Hsieh C, Hernández-Ledesma BBO. 2011. Cell proliferation inhibitory and apoptosis-inducing properties of anacardic acid and lunasin in human breast cancer MDA-MB-231 cells. Food Chem. 125:630–636.
- Huang P, Hseu Y, Lee M, Kumar KJS, Wu C, Hsu L, Liao J, Cheng I, Kuo Y, Huang S. 2012. In vitro and in vivo activity of gallic acid and Toona sinensis leaf extracts against HL-60 human premyelocytic leukemia. Food Chem Toxicol Toxicol. 50:3489–3497.
- Itharat A, Houghton PJ, Eno-Amooquaye E, Burke PJ, Sampson JH, Raman A. 2004. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J Ethnopharmacol. 90:33–38.
- Ji B, Hsu W, Yang J, Hsia T, Lu C, Chiang J, Yang J, Lin C, Lin J, Suen LW. 2009. Gallic acid induces apoptosis via caspase-3 and mitochondrion-dependent pathways in vitro and suppresses lung xenograft tumor growth in vivo. J Agric Food Chem. 57:7596–7604.
- Johnson JJ, Petiwala SM, Syed DN, Rasmussen JT, Adhami VM, Siddiqui IA, Kohl AM, Mukhtar H. 2012. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 33:413–419.
- Kaur M, Mandair R, Agarwal R, Agarwal C. 2008. Grape seed extract induces cell cycle arrest and apoptosis in human colon carcinoma cells. Nutr Cancer 60:2–11.
- Kaur M, Kohli S, Sandhu S, Bansal Y, Bansal G. 2015. Coumarin: a promising scaffold for anticancer agents. Anticancer Agents Med Chem. 15:1032–1048.
- Kayser O, Kolodziej H. 1995. Highly oxygenated coumarins from Pelargonium sidoides. Phytochemistry 39:1181–1185.
- Khan N, Mukhtar H. 2008. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 269:269–280.
- Kim H, Lee E, Kim J, Jung B, Chong Y, Ahn J, Lim Y. 2008. A flavonoid gossypin binds to cyclin-dependent kinase 2. Bioorg Med Chem Lett. 18:661–664.
- Kim C, Griffiths W, Taylor P. 2009. Components derived from Pelargonium stimulate macrophage killing of Mycobacterium species. J Appl Microbiol. 106:1184–1193.
- Kok S, Yeh C, Chen M, Kuo MY. 2009. Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation in human oral cancer SAS cells. Oral Oncol. 45:1067–1072.
- Kolodziej H. 2007. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo®. Phytomedicine 14:9–17.
- Kong JH, Kim YB, Yoon CW, Cha YJ, Han SS, Rhee CK, Oak M. 2009. Pelargonium sidoides syrup. World Intellectual Property Organisation, WO/2009/011498.
- Kundu JK, Surh Y. 2012. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 52:2013–2037.
- Latté KP, Kayser O, Tan N, Kaloga M, Kolodziej H. 2000. Unusual coumarin patterns of Pelargonium species forming the origin of the traditional herbal medicine umckaloabo. Z Naturforschung C 55:528–533.
- Lee S, Cekanova M, Baek SJ. 2008. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 47:197–208.
- Lemmich J, Shabana M. 1984. Coumarin sulphates of Seseli libanotis. Phytochemistry 23:863–865.
- Li H, Yashiki S, Sonoda J, Lou H, Ghosh SK, Byrnes JJ, Lema C, Fujiyoshi T, Karasuyama M, Sonoda S. 2000. Green tea polyphenols induce apoptosis in vitro in peripheral blood T lymphocytes of adult T-cell leukemia patients. Cancer Sci. 91:34–40.
- Lucia MS, Torkko KC. 2004. Inflammation as a target for prostate cancer chemoprevention: pathological and laboratory rationale. J Urol. 171:S30–S35.
- Madlener S, Illmer C, Horvath Z, Saiko P, Losert A, Herbacek I, Grusch M, Elford HL, Krupitza G, Bernhaus A. 2007. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett. 245:156–162.
- Mantena SK, Baliga MS, Katiyar SK. 2006. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis 27:1682–1691.
- Matthys H, Kamin W, Funk P, Heger M. 2007. Pelargonium sidoides preparation (EPs® 7630) in the treatment of acute bronchitis in adults and children. Phytomedicine 14:69–73.
- Medina S, Domínguez-Perles R, García-Viguera C, Cejuela-Anta R, Martínez-Sanz JM, Ferreres F, Gil-Izquierdo A. 2012. Physical activity increases the bioavailability of flavanones after dietary aronia-citrus juice intake in triathletes. Food Chem. 135:2133–2137.
- Meeran SM, Katiyar SK. 2007. Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, and caspase-3 activation via loss of mitochondrial membrane potential. Exp Dermatol. 16:405–415.
- Mizutani H, Tada-Oikawa S, Hiraku Y, Kojima M, Kawanishi S. 2005. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 76:1439–1453.
- Moyo M, Aremu AO, Gruz J, S˘ubrtová M, Szüc˘ová L, Dolez˘al K, Van Staden J. 2013. Conservation strategy for Pelargonium sidoides DC: phenolic profile and pharmacological activity of acclimatized plants derived from tissue culture. J Ethnopharmacol. 149:557–561.
- Nakagawa Y, Iinuma M, Naoe T, Nozawa Y, Akao Y. 2007. Characterized mechanism of alpha-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem. 15:5620–5628.
- Nandakumar V, Singh T, Katiyar SK. 2008. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 269:378–387.
- Owa T, Yoshino H, Yoshimatsu K, Nagasu T. 2001. Cell cycle regulation in the G1 phase: a promising target for the development of new chemotherapeutic anticancer agents. Curr Med Chem. 8:1487–1503.
- Pèrez JM, Kelland LR, Montero EI, Boxall FE, Fuertes MA, Alonso C, Navarro-Ranninger C. 2003. Antitumor and cellular pharmacological properties of a novel platinum (IV) complex: trans-[PtCl2(OH)2(dimethylamine)(isopropylamine)]. Mol Pharmacol. 63:933–944.
- Phillips MM, Case RJ, Rimmer CA, Sander LC, Sharpless KE, Wise SA, Yen JH. 2010. Determination of organic acids in Vaccinium berry standard reference materials. Anal Bioanal Chem. 398:425–434.
- Pinedo HM, Peters GF. 1988. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 6:1653–1664.
- Ramasamy K, Agarwal R. 2008. Multitargeted therapy of cancer by silymarin. Cancer Lett. 269:352–362.
- Riveiro ME, Moglioni A, Vazquez R, Gomez N, Facorro G, Piehl L, de Celis ER, Shayo C, Davio C. 2008. Structural insights into hydroxycoumarin-induced apoptosis in U-937 cells. Bioorg Med Chem. 16:2665–2675.
- Saba NF, Haigentz M, Vermorken JB, Strojan P, Bossi P, Rinaldo A, Takes RP, Ferlito A. 2015. Prevention of head and neck squamous cell carcinoma: removing the “chemo” from ”chemoprevention”. Oral Oncol. 51:112–118.
- Sang S, Hong J, Wu H, Liu J, Yang CS, Pan M, Badmaev V, Ho C. 2009. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J Agric Food Chem. 57:10645–10650.
- Schötz K, Nöldner M. 2007. Mass spectroscopic characterisation of oligomeric proanthocyanidins derived from an extract of Pelargonium sidoides roots (EPs® 7630) and pharmacological screening in CNS models. Phytomedicine 14:32–39.
- Schulze-Bergkamen H, Krammer PH. 2004. Apoptosis in cancer-implications for therapy. Semin Oncol. 31:90–119.
- Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. 2006. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 54:9329–9339.
- Sharma G, Tyagi AK, Singh RP, Chan DCF, Agarwal R, Chan DCF, Agarwal R. 2004. Synergistic anti-cancer effects of grape seed extract and conventional cytotoxic agent doxorubicin against human breast carcinoma cells. Breast Cancer Res Treat. 85:1–12.
- Shi L, Chen J, Wang Y, Sun G, Liu J, Zhang J, Yan W, Qian C, Liu N, Fu Z. 2012. Gossypin induces G2/M arrest in human malignant glioma U251 cells by the activation of Chk1/Cdc25C pathway. Cell Mol Neurobiol. 32:289–296.
- Steenkamp V, Gouws M. 2006. Cytotoxicity of six South African medicinal plant extracts used in the treatment of cancer. S Afr J Botany 72:630–633.
- Stewart ZA, Westfall MD, Pietenpol JA. 2003. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol Sci. 24:139–145.
- Sudjaroen Y, Haubner R, Würtele G, Hull WE, Erben G, Spiegelhalder B, Changbumrungv S, Bartsch H, Owen RW. 2005. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem Toxicol. 43:1673–1682.
- Sun Y, Jiang X, Chen S, Price BD. 2006. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 580:4353–4356.
- Takimoto CH. 2003. Anticancer drug development at the US National Cancer Institute. Cancer. Chemother Pharmacol. 52:29–33.
- Verma S, Singh A, Mishra A. 2013. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 35:473–485.
- Wang C, Hsieh Y, Chu C, Lin Y, Tseng T. 2002. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 183:163–168.
- Wen-Sheng Y, Li H, Xin-Min C, Yang L. 1992. Two afzelechin glycosides from Arthromeris mairei. Phytochemistry 31:4385–4386.
- Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. 1998. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 19:611–616.
- You BR, Moon HJ, Han YH, Park WH. 2010. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem Toxicol. 48:1334–1340.
