Abstract
Context Phyllanthus emblica L. (Euphorbiaceae) (amla), Manilkara zapota L.P. Royen (Sapotaceae) (sapota) and silymarin are reported to contain antioxidant effects. However, information on other biological activities relating to the anti-aging properties is limited.
Objective To compare in vitro antioxidants, anti-collagenase (MMP-1 and MMP-2) and anti-elastase properties as well as the phenolic and flavonoid contents of amla, sapota and silymarin as potential anti-aging ingredients.
Materials and methods The ethanol amla and sapota fruit extracts were prepared by three cycles of maceration with 24 h duration each. The total phenolic (TPC) and flavonoid (TFC) contents were determined. The antioxidant capacity was evaluated by DPPH and ABTS assays. The effects of MMP-1, MMP-2 and elastase inhibitions were determined by using the EnzChek® assay kits (Molecular-Probes, Eugene, OR).
Results Amla exhibited the highest in TPC (362.43 ± 11.2 mg GAE/g) while silymarin showed the highest in TFC (21.04 ± 0.67 mg QE/g). Results of antioxidant activity by DPPH and ABTS methods showed that amla possessed the most potent capacity with IC50 values of 1.70 ± 0.07 and 4.45 ± 0.10 μg/mL, respectively. Highest inhibitions against MMP-1, MMP-2 and elastase were detected for sapota with IC50 values of 89.61 ± 0.96, 86.47 ± 3.04 and 35.73 ± 0.61 μg/mL, respectively.
Discussion and conclusion Test extracts offered anti-aging properties in different mechanisms. Amla showed the highest phenolic content and antioxidant property with moderate anti-collagenase. Silymarin exhibited measurable flavonoid content with anti-elastase effect. Sapota showed the highest collagenase and elastase inhibitions with moderate antioxidant effect. Thus, extracts might be added as a mixture to gain the overall anti-aging effects.
Introduction
Aging of skin is a complex biological phenomenon due to intrinsic and extrinsic factors. Intrinsic factors are caused by time passage whereas extrinsic factors are mainly from sun exposure (Assaf et al. Citation2010). UV exposure causes physical changes of the skin through complex pathways and finally generates reactive oxygen species or ROS, matrix metalloproteinases (MMPs) and elastase secretion (Demeule et al. Citation2000). ROS directly causes skin aging by involving the oxidative damage of lipids, proteins, and DNA of the skin. ROS can also indirectly induce the production of MMPs via MAP-kinase pathway (Fisher et al. Citation2002; Sim et al. Citation2007). MMPs are a group of zinc-dependent extracellular proteinases categorized into five sub-groups based on their substrate: collagenase, gelatinase, stromelysins, membrane-type MMPs (MT-MMPs) and others. Collagenase is responsible for extracellular matrix (ECM) remodelling including collagen breakdown. Elastase, a serine proteinase, is primarily responsible for the breakdown of elastin in ECM. Since collagen and elastin mainly maintain skin structural integrity and elasticity, the depletions of collagen and elastin contribute to undesired wrinkles and aging skin.
Plant extracts, such as white tea, have been widely investigated and found to possess free radical scavenging, anti-collagenase and anti-elastase activities (Thring et al. Citation2009). The tea contains various chemical constituents such as epigallocatechin-3-gallate (EGCG), quercetin, kaempferol and gallic acid, which are classified in the groups of tea catechin, flavonoids and phenolic acid. Plant extracts, containing these structural-related components, therefore, might show anti-aging benefits.
Phyllanthus emblica (L.) (Euphorbiaceae), commonly known as amla, is a medicinal plant that has been reported to be a good source of antioxidants due to being rich in ascorbic acid, polyphenols and phenolic acids such as gallic and ellagic acids (Kim et al. Citation2005; Yokozawa et al. Citation2007). Moreover, recent studies revealed its cosmetic benefits including anti-tyrosinase, anti-wrinkle, antibacterial and anti-inflammatory properties (Joseph et al. Citation2013). Amla extract contains a large amount of functional tannins such as emblicanin, pedunclagin and puniglucoin and, therefore, it may exert its activities through a mechanism similar to that of EGCG (Fujii et al. Citation2008).
Manilkara zapota (L.) P.Royen (Sapotaceae), or sapota, has been used as herbal Indian medicine for a decade. The sapota or sapodilla fruit is known to be a good source of antioxidants. Ethanol pulp extract contains alkaloids, saponins, terpenoids, flavonoids, tannins, leucoanthocyanidins, anthroquinones, glucosides and catechol (Gomathy et al. Citation2013). Ma et al. (Citation2003) found bioactive flavonoids from methanol extract of sapota fruit such as (−)-epicatechin, (+)-gallocatechin, gallic acid, quercetin, myricitrin and (+)-catechin, of which the latter three pure compounds were reported to inhibit enzyme collagenase and elastase activities (Sim et al. Citation2007; Thring et al. Citation2009).
Silymarin is a standardized flavonoid extract from the milk thistle seeds, Silybum marianum (L.) Gaertner. The main active constituent in silymarin is silybin. Silymarin demonstrates antioxidant and anti-inflammatory properties in mouse skin which prevents skin disorders such as photoaging or UV-related skin cancer (Katiyar et al. Citation2008).
The present study determines and compares total phenolic and flavonoid contents together with the investigation of in vitro antioxidants, anti-collagenase and anti-elastase activities of the extracts. Ethanol amla (P. emblica) extract, ethanol sapota (M. zapota) extract and silymarin were selected on the basis of their different phytochemical compositions. These plant extracts might be a candidate for novel natural anti-aging ingredients.
Materials and methods
Materials
Dried amla fruits were supplied by Herbal pharmacy, Chao Phraya Abhaibhubejhr Hospital (Thailand) during May 2013. Sapota fruits were purchased in November 2011 from Thai vegetable and fruit market in Pathum Thani (Thailand). The yellowish standardized extract of silymarin was gifted from Berlin Pharmaceutical Industry (Thailand), which were purchased from IVAX Pharmaceutical s.r.o. (Czech Republic). The voucher specimens were deposited in the Department of Pharmacognosy and Pharmaceutical Botany, Chulalongkorn University, Thailand, where identification of plant specimens was confirmed by Associate Professor Boonchoo Sritularak. Folin-Ciocalteau reagent, standardized gallic acid, quercetin, DPPH (2,2-diphenyl-2-picrylhydrazyl hydrate), EGCG, ABTS (2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid)) and potassium persulphate were purchased from Sigma Aldrich (St. Louis, MO). AR sodium carbonate was obtained from Ajax Finechem Pty Ltd, Taren Point, Australia. Aluminium chloride solution and potassium acetate were purchased from Merck (Darmstadt, Germany) and May & Baker Ltd. (Wandsworth, UK), respectively. Ascorbic acid was obtained from Carlo Erba (Rodano, Italy). EnzChek® collagenase/gelatinase assay kit (E-12055) and EnzChek® elastase assay kit (E-12056) were purchased from Molecular-Probes (Eugene, OR).
Methods
Plant extracts and stock solution preparations
The dried amla fruits were ground and soaked in 95% ethanol (1:4) for 24 h at room temperature. The solvent was collected and the process was repeated over three cycles. All collected solvents were combined and evaporated to dryness under reduced pressure at 40 °C. The crude ethanol amla extract was further purified by dissolving the extract in 95% ethanol. The mixture was concentrated under reduced pressure giving the yield of 17.59%.
Sapota fruits were peeled and the seeds were discarded. The fruit pulp was cut into small slices and soaked in absolute ethanol for 4 d at room temperature. The process was repeated over four cycles. Then, all collected solvents were combined and evaporated to dryness under reduced pressure giving the yield of 11.09%.
Both extracts were kept in tight and light-resistant containers and stored in a refrigerator at 4 °C for further study. Silymarin powder was stored in a desiccator at room temperature.
Total phenolic content
Total phenolic content of all samples was assessed by using a modified Folin–Ciocalteau method (Miliauskas et al. Citation2004). Folin–Ciocalteau (FC) reagent is mixture of phosphomolydate and phosphotungstate, which oxidizes phenolates and reduces the heteropoly acids to a blue complex. Diluted sample (20 μL) in 95% ethanol, 10% FC reagent (100 μL) and 75 g/L of sodium carbonate (80 μL) were mixed in a 96-well plate. Deionized water was used as a blank. After a 60 min incubation period at room temperature and light protected, the absorbance of the reaction mixture was measured at 765 nm with a microplate spectrophotometer (Spectramax M5, Molecular Devices, Sunnyvale, CA). The standard curve for quantifying phenolic contents was prepared by using gallic acid as a reference. All samples were performed in triplicate. The results were expressed as milligrams of gallic acid equivalent (GAE) per gram of the extract.
Total flavonoid content
Total flavonoid content was measured by the aluminium chloride colorimetric assay with slight modification (Chang et al. Citation2002). Diluted sample in 95% ethanol (80 μL) were mixed with 10% aluminium chloride solution (4 μL), 1 M potassium acetate (4 μL) and deionized water (112 μL) in a 96-well plate. 10% Aluminium chloride was substituted by distilled water as a blank. After 30 min of incubation, the absorbance of the reaction mixture was measured at 415 nm. The standard curve for quantifying flavonoid contents was prepared by using quercetin as a reference. All samples were performed in triplicate. The results were expressed as milligrams of quercetin equivalent (QE) per gram of the extract.
Free radical scavenging activity
DPPH and ABTS free radical scavenging activities were determined to assess antioxidant activities for all test samples. DPPH free radical scavenging activities of tested extracts were determined based on a protocol modified from Marinova and Batchvarov (Citation2011). A volume of 0.06 mM DPPH solution in absolute ethanol was prepared. Samples with different concentrations in absolute ethanol were equally mixed with ethanol DPPH solution to obtain a total of 200 μL. After a 30 min incubation period in the dark, the absorbance was measured at 517 nm by using the microplate spectrophotometer. Each measurement was corrected with its background, which was the sample without DPPH solution. l-(+)-ascorbic acid was employed as a standard. All samples were performed in triplicate. The ability to scavenge DPPH radical was calculated as the following:
The antioxidant activities of all extracts were finally expressed as an IC50 value that is defined as the concentration (μg/mL) of extract showing 50% inhibition.
The ABTS radical method (Lee et al. Citation2014) was slightly modified in order to evaluate the antioxidant effect of the samples. The ABTS reagent was prepared by adding 140 mM potassium persulphate (88 μL) in 5 mL of 7 mM ABTS solution. The mixture was light-protected and incubated at room temperature for 12–16 h to allow free radicals to be fully generated, after which, it was diluted with distilled water (1:49 v/v). To determine antioxidant activity, diluted samples (20 μL) and ABTS solution (180 μL) were mixed in 96-well microplate and kept in the dark for 6 min. The absorbance was then measured at 734 nm. Distilled water and l-(+)-ascorbic acid were used as negative and positive controls, respectively. Each absorbance was corrected with its background, which was the sample without ABTS solution. The ability to scavenge ABTS radical was calculated using the same equation as DPPH.
MMP-1 and MMP-2 inhibitions
The in vitro collagenase inhibitions focusing on MMP-1 and MMP-2 were determined using EnzChek® collagenase/gelatinase kits (Molecular Probes, Eugene, OR). The substrates for MMP-1 and MMP-2 inhibition assays were DQTM collagen 1 and DQTM gelatin, respectively. Collagenase (ChC) from Clostridium histolyticum, DQTM substrate and various concentrations of the sample extract were separately dissolved in pH 7.4 Tris-HCL buffer. Diluted extract (80 μL), DQTM gelatin (20 μL) and ChC (100 μL) were mixed in a 96-well plate. The final concentrations of ChC and DQTM substrate were 0.2 units/mL and 12.5 μg/mL, respectively. After 90 min of incubation, light protected at room temperature, the fluorescence intensity was measured with the excitation and the emission wavelength at 485 nm and 538 nm, respectively, using a fluorescent microplate reader (Spectramax M5, Molecular Devices, Sunnyvale, CA). 1,10-Phenanthroline and EGCG were used as a positive inhibitor and a standard, respectively. The ability to inhibit against gelatinase/collagenase was calculated as following:
Collagenase inhibition (%) = {[(A−B)–(C−D)]/(A−B)} × 100, where A is the fluorescent intensity without the test sample (control), B is the fluorescent intensity without the test sample and enzyme (blank of A), C is the fluorescent intensity with the test sample, and D is the fluorescent intensity with the test sample without enzyme. The anti-collagenase activities of all samples were finally expressed as an IC50 value.
Inhibition of elastase
The assay was followed EnzChek® elastase assay kit (Molecular Probes, Eugene, OR). Briefly, porcine pancreatic elastase (PE), DQTM elastin substrate and various concentrations of the sample extract were separately dissolved in pH 8 Tris-HCL buffer. Diluted extract (50 μL) was preincubated with 0.4 U/mL PE (100 μL) for 15 min. 0.1 mg/mL DQTM elastin (50 μL) was then added into the mixture. After 30 min incubation with light protection, the fluorescence intensity was measured with the excitation and the emission wavelength at 485 nm and 538 nm, respectively. EGCG and N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone were used as a standard and a positive inhibitor, respectively. The negative control was Tris-HCl buffer containing the substrate. The ability to inhibit against elastase was calculated using the equation mentioned in the section of MMP-1 and MMP-2 inhibitions. The anti-elastase activities of all samples were finally expressed as an IC50 value.
Results
Total phenolic and flavonoid content
The results of total phenolic content (TPC) and total flavonoid content (TFC) among three extracts; ethanol P. emblica (amla) extract, ethanol M. zapota (sapota) extract and silymarin are summarized in .
Table 1. The total phenolic and flavonoid contents of the test extracts.
Amla extract showed the highest total phenolic content followed by silymarin and sapota extract. Silymarin exhibited the highest total flavonoid content, whereas amla extract contained a lower amount of flavonoid. Total flavonoid content was, however, undetectable in the sapota extract.
DPPH and ABTS free radical scavenging activities
The antioxidant capacities of the test extracts were determined by DPPH and ABTS based methods which are widely used in plant and food research for screening antioxidant activity (Karadag et al. Citation2009; Floegel et al. Citation2011; Jain et al. Citation2011). The study revealed that all extracts inhibited DPPH and ABTS free radicals in a dose-dependent manner with similar order. Amla showed the most potent ability to scavenge DPPH and ABTS free radicals, which was comparable with ascorbic acid. The inhibitory activities on both free radicals of silymarin and sapota were much lower than that of amla. All 50% inhibition concentrations (IC50) against ABTS were higher than that of DPPH, except for silymarin ().
Table 2. Antioxidative capacity of the extracts measured by DPPH- and ABTS-based methods (mean ± SD).
Inhibition of collagenase and elastase
MMP-1 and MMP-2 collagenase and elastase inhibitory effects were performed using the test kits, and the results are shown in . The test extracts inhibited both collagenase and elastase in a dose-dependent manner (). Among the test extracts, amla and sapota showed the most effect in collagenase inhibition at almost one-fold higher than silymarin. However, their effects were 10 times lower than a standard catechin, EGCG. Regarding anti-elastase activity, sapota and silymarin possessed a comparable effect with an IC50 value of 35.73 ± 0.61 μg/ml and 38.57 ± 0.04 μg/ml, respectively. Interestingly, their inhibition activities were almost three times higher than EGCG. Amla showed the poorest anti-elastase activity among the extracts.
Figure 1. Effects of ethanolic amla extract, ethanolic sapota extract and silymarin on collagenase and elastase inhibitions. The percent inhibitions expressed as mean ± SD.
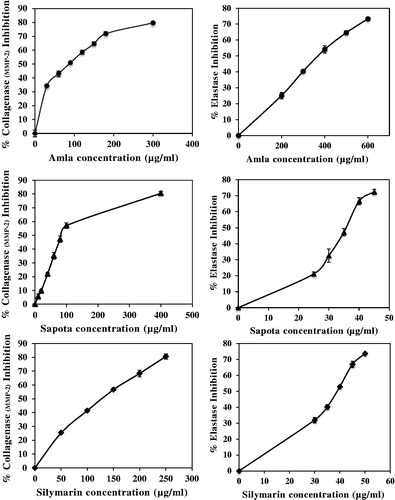
Table 3. Biological activities of the extracts on MMP-1, MMP-2 and elastase inhibitions, data expressed as IC50 (mean ± SD).
Discussion
Test extracts used in the study were selected based on the group of chemical constituents. Major chemical components of amla have been reported as ascorbic acid, and phenolic compounds with some flavonoid content (Majeed et al. Citation2009). Silymarin contained high flavonoid content (Cai et al. Citation2009). Sapota was selected due to the presence of some phenolic compounds, flavonoid and tannin (Gomathy et al. Citation2013).
Phenolic and flavonoid compounds have been reported to present significant antioxidant properties (Miliauskas et al. Citation2004). In the present study, amla extract exhibited the highest total phenolic content. This result might be due to the fact that major components of phenolic compounds in amla are gallic and ellagic acids which are in the group of phenolic compounds (Yokozawa et al. Citation2007; Majeed et al. Citation2009; Amir et al. Citation2011). Amla extract also showed the most potent DPPH and ABTS free radical scavenging activity among the test extracts with an IC50 value of 1.70 and 4.45 μg/mL, respectively. Its effect was comparable with ascorbic acid which is a well-known antioxidant widely used in cosmetic products. The present study revealed a new alternative potent antioxidant for anti-aging application. This finding is in accordance with the findings of Spiridon et al. (Citation2011) and Floegel et al. (Citation2011) presenting a linear correlation between phenolic content and DPPH scavenging activity. Samples with high content of phenolic compound tend to express high DPPH scavenging activity. The factors behind the correlation between TPC and DPPH free radical activity might be due to the same principle that utilizes an electron-transfer mechanism (Karadag et al. Citation2009). Regarding the ABTS-based test system, the results show the same rank order of antioxidant property compared with data obtained from the DPPH method due to similarity of mechanisms. However, silymarin showed moderately higher antioxidant capacity as measured by the ABTS assay relative to the DPPH assay.
With respect to TFC, silymarin unsurprisingly showed the highest total flavonoid content since it is clearly known as a flavonolignan, one of the flavonoid compounds. Such flavonoid compound also showed a DPPH scavenging ability due to its reducing ability and metal-chelating property (Malesev & Kuntic Citation2007; Pallab et al. Citation2013). Total phenolic and total flavonoid content might be screening parameters of the antioxidant activity of test substance. However, the degree of antioxidant property depends not only on the concentration of phenolic or flavonoid content but also on the type of chemical entity as well as its presenting structure in the substance. Therefore, a compound with low total phenolic and flavonoid content might also possess an antioxidant effect due to the presence of other bioactive compounds. Substances with such properties might be included to prevent skin aging associated with oxidative damage.
MMPs are a group of zinc-containing proteinases. MMP-1 or interstitial collagenase initiates the breakdown mostly of type I, II and III collagens which are the most abundant interstitial collagens in dermis while MMP-2 is responsible for breakdown of type I–III, IV and VII collagens in which the latter two are most abundant in the dermal–epidermal junction. In addition to MMPs, elastase is an enzyme that digests another interstitial fibre in the skin, called elastin. Depletion of both structural fibres in skin results in the lack of skin integrity and elasticity contributing to wrinkle formation and aging skin. Amla extract was previously reported to inhibit collagenase activity, determined by using the EnzChek® gelatinase/collagenase assay kit (Chanvorachote et al. Citation2009) and which conformed to the results of this experiment. It significantly inhibited MMP-1 and MMP-2 with activity comparable with that of sapota. The inhibition effect of amla extract might involve several mechanisms. Hydroxyl groups of polyphenol could interact with the backbone or other functional group side chain of collagenase. In addition, hydrophobic interaction between the benzene ring of polyphenol and collagenase could also result in the conformational changes leading to unfunctioned enzyme (Madhan et al. Citation2007). Another mechanism involves the Zn ion active site on collagenase. Collagenase contains a structural Zn ion at its active site which plays a major role in facilitating interaction with an inhibitor (Bigg et al. Citation1994). Phyllanthus emblica fruit contains polyphenol compounds including gallic acid and hydrolyzable tannin which are known to be metal chelators and, thus, may bind to a Zn ion active site and prevent the substrate from enzyme digestion (McDonald et al. Citation1996). In addition to the polyphenol, flavonoid also chelated Zn metal by its 3-hydroxyflavon structure (Malesev & Kuntic Citation2007). An ability of silymarin for collagenase inhibition might also be caused by binding to a Zn active site. Significant inhibition on MMP-1 and MMP-2 of amla and sapota suggested their ability to delay breakdown of collagen fibre, hence, maintaining integrity of skin layer.
Sapota and silymarin exhibited significant inhibition in elastase activity. They showed a potent anti-elastase property that was nearly three-fold superior to EGCG, while amla extract exhibited weak elastase inhibition. The data are consistent with previous reports from several groups where certain phenolic compounds and flavonoid possess anti-elastase activity in dose dependency. Phenols, such as epicatechin, catechin, resveratrol and procyanidin B2 (Hrenn et al. Citation2006; Wittenauer et al. Citation2015), and flavonoid such as kaempferol, quercetin and myricetin (Kanashiro et al. Citation2007) significantly inhibited elastase activity. In agreement with Wittenauer et al. (Citation2015), amla extract containing gallic acid as major component possessed poor inhibitory property toward elastase enzyme. Surprisingly, sapota showed significant collagenase inhibition comparable with amla, and was significantly higher in elastase inhibition although it contains lower total phenolic and undetectable flavonoid content. Sapota, thus, might contain a different type of phenolic compound or other bioactive components which perform other mechanism of inhibition. Compounds with anti-proteinase activity could, therefore, be included in anti-aging formulations in order to delay the breakdown of skin fibres.
Skin aging involves many complex pathways. The current study suggests that TPC and/or TFC might refer to antioxidant properties to some extent but are not well correlated with anti-proteinase activity. The single test parameter of TPC, TFC, DPPH or ABTS free radical scavenging assay frequently used to screen antioxidant properties might not provide enough information to determine the potential anti-aging agent. Amla exhibited the potent antioxidant effect, but it was not a good source of elastase inhibitor. Although sapota had undetected flavonoid content and possessed the moderate antioxidant activity, it showed excellent collagenase and elastase inhibition among test extracts. Often, extracts might be ignored when the results indicate poor antioxidant activity. However, those extracts might present other benefits as seen with sapota. Thus, collagenase and elastase inhibitions should be additionally investigated for screening of anti-aging agents.
Conclusions
Among the test samples, ethanol amla extract contained high phenolic content and showed the most potent antioxidant with moderate collagenase and poor elastase inhibition. Sapota showed the highest collagenase and elastase inhibition with a slightly antioxidant effect. Silymarin showed high flavonoid content and inhibited the elastase comparable with sapota but was poor in anti-collagenase activity compared with others. All test extracts presented anti-aging property with different mechanisms. There is hardly an “all in one” component that exhibits total anti-aging effects on each of the skin-aging pathways. Thus, extracts might be added as a mixture to gain the overall effect in cosmetic products. Purification of each extract and investigation into the effect of different combinations shall be further studied.
Funding information
This research was financially supported by a research grant from the Faculty of Pharmaceutical Sciences, Chulalongkorn University, Thailand.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Amir M, Khan A, Mujeeb M, Ahmad MA, Siddiqui NA. 2011. Phytochemical screening and in vitro antioxidant activity of Jawarish amla – a poly herbal formulation. PHCOG J. 3:54–60.
- Assaf H, Adly MA, Hussein MR. 2010. Aging and intrinsic aging: pathogenesis and manifestations. In: Farage MA, Miller KW, Maibach HI, editors. Textbook of aging skin. Heidelberg: Springer. p. 129–138.
- Bigg HF, Clark IM, Cawston TE. 1994. Fragments of human fibroblast collagenase: interaction with metalloproteinase inhibitors and substrates. Biochim Biophys Acta. 1208:157–165.
- Cai XL, Li DN, Qiao JQ, Lian HZ, Wang SK. 2009. Determination of silymarin flavonoids by HPLC and LC-MS and investigation of extraction rate of silymarin in Silybum marianum fruits by boiling water. Asian J Chem. 21:63–74.
- Chang CC, Yang MH, Wen HM, Chern JC. 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 10:178–182.
- Chanvorachote P, Pongrakhananon V, Luanpitpong S, Chanvorachote B, Wannachaiyasit S, Nimmannit U. 2009. Type I pro-collagen promoting and anti-collagenase activities of Phyllanthus emblica extract in mouse fibroblasts. J Cosmet Sci. 60:395–403.
- Demeule M, Brossard M, Page M, Gingras D, Beliveau R. 2000. Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta. 1478:51–60.
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. 2002. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 138:1462–1470.
- Floegel A, Kim DO, Chung SJ, Koo SI, Chun OK. 2011. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compost Anal. 24:1043–1048.
- Fujii T, Wakaizumi M, Ikami T, Saito M. 2008. Amla (Emblica officinalis Gaertn.) extract promotes procollagen production and inhibits matrix metalloproteinase-1 in human skin fibroblasts. J Ethnopharmacol. 119:53–57.
- Gomathy K, Baskar R, Kumaresan K. 2013. Comparison of antioxidant potential in pulp and peel extracts of Manilkara zapota (L.) P. Royen. Afr J Biotechnol. 12:4936–4943.
- Hrenn A, Steinbrecher T, Labahn A, Schwager J, Schempp CM, Merfort I. 2006. Plant phenolics inhibit neutrophil elastase. Planta Med. 72:1127–1131.
- Jain DP, Pancholi SS, Patel R. 2011. Synergistic antioxidant activity of green tea with some herbs. J Adv Pharm Technol Res. 2:177–183.
- Joseph N, Rao MPB, Geevarughese NM, Pallaty PL, Baliga S. 2013. Amla (Emblica officinalis Gaertn.) the Indian indigenous berry in skin care. In: Watson RR, Zibadi S, editors. Bioactive dietary factors and plant extracts in dermatology nutrition and health. New York: Springer Science. p. 113–123.
- Kanashiro A, Souza JG, Kabeya LM, Azzolini AECS, Lucisano-Valim YM. 2007. Elastase release by stimulated neutrophils inhibited by flavonoids: importance of the catechol group. Z Naturforsch C: J Biosci. 62:357–361.
- Karadag A, Ozcelik B, Saner S. 2009. Review of methods to determine antioxidant capacities. Food Anal Method. 2:41–60.
- Katiyar SK, Meleth S, Sharma SD. 2008. Silymarin, a flavonoid from milk thistle (Silybum marianum L.), inhibits UV-induced oxidative stress through targeting infiltrating CD11b + cells in mouse skin. Photochem Photobiol. 84:266–271.
- Kim JH, Yokozawa T, Kim HY, Tohda C, Rao TP, Juneja LR. 2005. Influence of amla (Emblica officinalis Gaertn.) on hypercholesterolemia and lipid peroxidation in cholesterol-fed rats. J Nutr Sci Vitaminol. 51:413–418.
- Lee KJ, Jung PM, Oh YC, Song NY, Kim T, Ma JY. 2014. Extraction and bioactivity analysis of major flavones compounds from Scutellaria baicalensis using in vitro assay and online screening HPLC-ABTS system. J Anal Methods Chem. 2014:1–9.
- Ma J, Luo XD, Protiva P, Yang H, Ma C, Basile MJ, Weinstein IB, Kennelly EJ. 2003. Bioactive novel polyphenols from the fruit of Manilkara zapota (Sapodilla). J Nat Prod. 66:983–986.
- Madhan B, Krishnamoorthy G, Rao JR, Nair BU. 2007. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int J Biol Macromol. 41:16–22.
- Majeed M, Bhat B, Jadhav AN, Srivastava JS, Nagadhushanam K. 2009. Ascorbic acid and tannins from Emblica officinalis Gaertn. Fruits-a revisit. J Agric Food Chem. 57:220–225.
- Malesev D, Kuntic V. 2007. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J Serb Chem Soc. 72:921–939.
- Marinova G, Batchvarov V. 2011. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg J Agric Sci. 17:11–24.
- Mc Donald M, Mila I, Scalbert A. 1996. Precipitaion of metal ions by plant polyphenols: optimal conditions and origin of precipitation. J Agric Food Chem. 44:599–606.
- Miliauskas G, Venskutonis PR, van Beek TA. 2004. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 85:231–237.
- Pallab K, Tapan KB, Tapas KP, Ramen K. 2013. Estimation of total flavonoids content (TFC) and anti oxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J Drug Deliv Ther. 3:33–37.
- Sim GS, Lee BC, Cho HS, Lee JW, Kim JH, Lee DH, Kim JH, Pyo HB, Moon DC, Oh KW, et al. 2007. Structure activity relationship of antioxidative property of flavonoids and inhibitory effect on matrix metalloproteinase activity in UVA-irradiated human dermal fibroblast. Arch Pharmacol Res. 30:290–298.
- Spiridon I, Bodirlau R, Teaca CA. 2011. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent Eur J Biol. 6:388–396.
- Thring TS, Hili P, Naughton DP. 2009. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement Altern Med. 9:1–27.
- Wittenauer J, Mackle S, Sussmann D, Schweiggert-Weisz U, Carle R. 2015. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 101:179–187.
- Yokozawa T, Kim HY, Kim HJ, Tanaka T, Sugino H, Okubo T, Chu DC, Juneja LR. 2007. Amla (Emblica officinalis Gaertn.) attenuates age-related renal dysfunction by oxidative stress. J Agric Food Chem. 55:7744–7752.
