Abstract
Context Despite several pharmacological studies of volatile oils of Angelica sinensis (Oliv.) Diels (Umbelliferae) (VOAS), its anti-inflammatory mechanism remains unknown.
Objective The study investigates the effects of VOAS on the lipopolysaccharide (LPS)-induced acute inflammation rat model and analyzes its possible anti-inflammatory mechanisms.
Materials and methods Fourty rats were randomly divided into the control, model, VOAS and dexamethasone (Dex) groups. The VOAS and Dex groups were given VOAS (0.176 mL/kg) and Dex (40 μg/kg), respectively. Rats in all groups except the control group were intraperitoneally injected with LPS (100 μg/kg), their exterior behaviour and liver pathological changes were observed, and the level of white blood cell (WBC), the number of neutrophils (NE)%, glutamic oxalacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), alkaline phosphatase (ALP), tumour necrosis factor (TNF-α), interleukin (IL)-1β, IL-6, IL-10, histamine (HIS), 5-hydroxytryptamine (5-HT), nitric oxide (NO), prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) were detected.
Results Compared with the model group, VOAS and Dex significantly accelerated the recovery of the exterior behaviour, the liver pathological changes of rats, and increased the level of IL-10, but decreased the level of WBC, NE%, GOT, GPT, ALP, TNF-α, IL-1β, IL-6, HIS, 5-HT, NO, PGE2, iNOS and COX-2 (p < 0.05).
Conclusion VOAS exhibits anti-inflammatory and liver protection effects by inhibiting the secretion of the pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), the inflammatory mediators (HIS, 5-HT, PGE2 and NO), the inflammation-related enzymes (iNOS and COX-2), as well as promoting the production of the anti-inflammatory cytokines IL-10.
Introduction
Inflammation reaction, symptom or complication of many diseases, is a complex biological response to harmful stimuli that requires removal of an offensive agent and initiation of healing process (Bauer et al. Citation2011). It is mainly induced by germ, virus and probably physic stimulation, chemic stimulation and trauma (Lin et al. Citation2008). And its essence is the imbalance of anti-inflammatory and pro-inflammatory factors (Bone Citation1996). Inflammation reaction possesses highly complex regulating system and signalling pathways. Most cytokines, inflammatory mediators and inflammation-related enzymes are involved in these pathways (Wang et al. Citation2013). The stimulation and the damage of inflammatory factors promote the synthesis and the secretion of cytokines, inflammatory mediators and inflammation-related enzymes, and then the inflammation reaction will be activated to kill the invader. When the invader is killed, the organism will limit the continuous or excessive inflammatory reaction by anti-inflammatory cytokines or inhibitory factors of some specific cytokines, then restore to a stable state (Ferreira et al. Citation2013). Studies revealed that several anti-inflammatory drugs carried out their anti-inflammatory functions by affecting the levels, functions and relationships of cytokines, inflammatory mediators and inflammation-related enzymes (Sakurada et al. Citation1996; Wang et al. Citation2013). Therefore, analysis of these effects, levels, functions and relationships would help people to enlarge their knowledge on inflammatory reaction and drug intervention mechanisms, and consequently find effective prevention and control measures.
Recently, more attention has been focused on studying some traditional Chinese medicines and their anti-inflammatory efficacy. They exhibit multi-component features, including the ability to affect multiple targets, different levels of signalling pathways and multiple mechanisms of mitigating inflammation (Drayton et al. Citation2006). Thus, these traditional Chinese medicines are expected to be another popular category of anti-inflammatory drug sequence to non-steroidal and glucocorticoid anti-inflammatory drugs (Li et al. Citation2010). Angelica sinensis (Oliv.) Diels (Umbelliferae) (AS), also known as Dang-gui in China, has high value in medical and hygienical use, such as enriching the blood, regulating painful menstruation and relaxing bowels (Deng et al. Citation2005). At the same time, with the advantage of little or no toxicity, AS has also been used in treating cancer patients and showed clinical efficacy (Cai and Luo, Citation2003). The volatile oils of Angelica sinensis (VOAS), an important constituent of AS, contain many chemical compounds, including ligustilide, monoterpene, n-butylidene phthalide and sesquiterpene (Li et al. Citation2006; Yeh et al. Citation2012). Pharmacological studies have revealed that VOAS could dual-directionally regulate the contraction of the uterine smooth muscle, heal dysmenorrhoea, protect focal cerebral ischemia, improve myocardial ischemia, relieve asthma and improve the immune system of the body. Moreover, VOAS also has good anti-inflammation effects (Liu et al. Citation2002; Ni et al. Citation2007).
Lipopolysaccharide (LPS), a component of cytoderm of the Gram-negative bacteria, is a classic pathogen-associated molecular pattern that causes inflammation by activating the nuclear factor κB (NF-κB) and activator protein-1 (AP-1) signalling pathways. These signalling pathways induce the expression of various inflammatory genes, including tumour necrosis factor (TNF-α), nitric oxide (NO), prostaglandin E2 (PGE2), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) and among others (Buchanan et al. Citation2010). To date, it has been a widely used modelling method for acute inflammation in which enterocoelia or vein is injected with a dose of LPS (Ferrero et al. Citation1993; Kotlyarov et al. Citation1999).
Dexamethasone (Dex), a potent synthetic member of the glucocorticoid family, has many functions, such as anti-inflammation, anti-allergy, anti-shock and anti-endotoxin (Wang et al. Citation2013). Dex has been widely used to treat inflammatory and autoimmune diseases, including severe sepsis, multiple sclerosis, rheumatoid arthritis, asthma and systemic lupus erythematosus. It could affect the activities of signal-dependent transcription factors, including members of the AP-1 and NF-κB families (Ray & Prefontaine Citation1994). Moreover, the effects of Dex on the cytokines, inflammatory mediators and inflammation-related enzymes have been deeply explored (Visser et al. Citation1998; Shivkar & Kumar Citation2004; Connor et al. Citation2005).
Thus, the acute inflammation model was induced by LPS. Using Dex (positive drug) and VOAS (tested drug) to treat acute inflammation, the changes and relationships among cytokines, inflammatory mediators and inflammation-related enzymes during the process of inflammation development and VOAS intervention were investigated in this study. And then the possible anti-inflammation mechanisms of VOAS were evaluated.
Materials and methods
Chemicals and reagents
LPS (serotype 055: B5) was purchased from Sigma-Aldrich (St. Louis, MO). Dexamethasone sodium phosphate injection (specification 1 mL: 2 mg; batch no. 1402201) was obtained from Tianjin Pharmaceutical Group Xinzheng Co., Ltd (Xinzheng, China). Interleukin (IL)-1β, TNF-α, IL-6, IL-10, COX-2, histamine (HIS) and 5-hydroxytryptamine (5-HT) rat test kits were obtained from Qisong Biological Technology Co., Ltd (Beijing, China). iNOS and NO rat test kits were purchased from Nanjing Jiancheng Biological Engineering Institute (Nanjing, China). Other reagents were of analytically pure grade.
Animals
Forty SD male rats (weighing 180–210 g) were supplied by the Experimental Animal Center of Lanzhou University (Lanzhou, China). The qualified number is SCXK (Gan) 2011-0001.
Preparation of VOAS
AS were purchased from Minxian County, Gansu Province, China, and were authenticated by Dr. Yan-ming Wei from the College of Veterinary Medicine, Gansu Agricultural University, Lanzhou, China. VOAS was prepared according to the Pharmacopoeia Commission of the People’s Republic of China and Gansu Processing Standard of TCM. A total of 200 g of AS was ground into powder, passed through a 40-mesh sieve, mixed with 1000 mL of distilled water and soaked for 1 d. Afterward, the mixture was placed in an extracting device and subjected to hydro-distillation for 8 h to obtain volatile oil. Almost 0.6 mL of canary clear oil-like volatile oil was obtained. Anhydrous sodium sulphate was used to remove water from the volatile oil, which was then stored in amber laboratory bottle at 4 °C for analysis (Cui et al. Citation2006). VOAS was dissolved in Tween 80 (2%, v/v) before use.
In vivo experimental protocol
SD male rats were kept at an ambient temperature of 22 ± 2 °C under a 12 h normal phase light–dark cycle and had free access to food and water. What’s more, the rats were allowed to acclimate for 4 d prior to the start of the experiment. Afterward, the rats were randomly divided into four groups, with 10 rats in each group: (A) control group, (B) model group, (C) VOAS group, and (D) Dex group. The specific experimental steps and the processing method are shown in .
Table 1. Grouping and processing of the experiment.
The experiment was completed 8 h after all the processes. Animal welfare and experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006) and were approved by the Animal Ethics Committee of Gansu Agricultural University.
Sample collection and preparation
After the experiment was completed, the rats were intraperitoneally anesthetized with 1% pentobarbital sodium (35 mg/kg), and blood was then drawn from the heart. One part of the blood was used for the detection of white blood cell (WBC) and the number of neutrophils (NE)%, while the other part was added to the Eppendorf vial, and then centrifuged (3000 rpm) for 10 min at 4 °C. The serum sample was collected and stored at −80 °C for the detection of blood biochemical indexes, cytokines, inflammatory mediators and inflammation-related enzymes. Besides, one part of the left lobe of the liver tissue was fixed in 10% phosphate-buffered formalin for observations of pathological changes.
Blood routine and biochemical indexes analyses
Blood routine analysis was carried out using Mindray BC-5300Vet Auto Hematology Analyzer (Mindray Corporation, Shenzhen, China). Glutamic oxalacetic transaminase (GOT), glutamic pyruvic transaminase (GPT) and alkaline phosphatase (ALP) analyses were carried out using Mindray BS-200 Auto Chemistry Analyzer (Mindray Corporation, Shenzhen, China). TNF-α, IL-1β, IL-6, IL-10, HIS, 5-HT, NO, iNOS and COX-2 analyses were carried out according to the directions of their own test kits and BIO-RAD 680 microplate reader (BIO-RAD Corporation, Hercules, CA). PGE2 analysis was carried out using U5100 ultraviolet spectrophotometer (Beijing Purkinje General Instrument Co., Ltd., Beijing, China).
Pathological changes observation
After 72 h of being fixed in 10% phosphate-buffered formalin, the liver blocks were embedded in paraffin, cut into 5 μm sections and stained with haematoxylin and eosin (H&E staining).
Data analysis
All data were expressed as mean ± SD. SPSS 17.0 software (SPSS Inc., Chicago, IL) was used for one-way analysis of variance. p < 0.05 was considered statistically significant.
Results
General behaviour observation
The rats in the control group showed good mental state, lively behaviour and normal diet consumption after being injected in enterocoelia with normal saline, while the rats in the model, VOAS and Dex groups exhibited some signs of depression, sluggishness, chills, decrease in diet consumption and unresponsiveness to external stimuli (such as noise and vibration) after intraperitoneal injection of LPS. After 3 h, all the rats in the model, VOAS and Dex groups which were administrated with LPS started on activity, and the recovery rate of rats in the VOAS and Dex groups was significantly better than that in the model group. However, their activities were not as good as the control group at last. At 8 h after LPS injection, all the rats were alive with a survival rate of 100%.
Influence of VOAS on blood routine, GOT, GPT and ALP in rats with acute inflammation
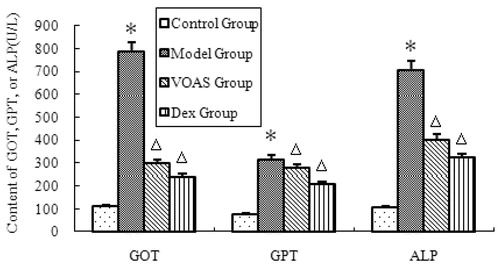
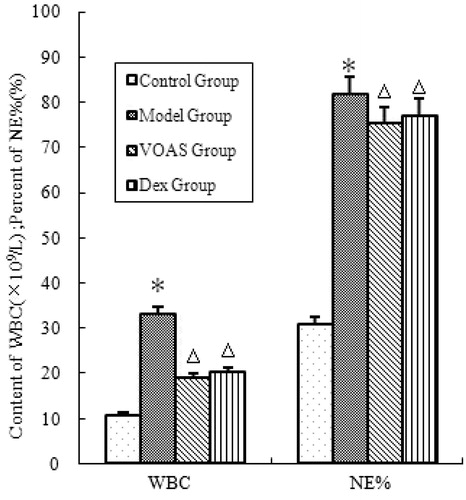
As shown in , the degree of WBC and NE% in the model group significantly increased (p < 0.05) compared with that in the control group. However, that in the VOAS and Dex groups decreased significantly (p < 0.05) compared with the model group.
Figure 1. The influence of VOAS on blood routine of rats. Note: Compared with the control group, *represents significant increase (p < 0.05); compared with the model group, delta represents significant decrease (p < 0.05).
As shown in , the degree of GOT, GPT and ALP in the model group significantly increased (p < 0.05) compared with that in the control group. However, that in the VOAS and Dex groups decreased significantly (p < 0.05) compared with the model group.
Influence of VOAS on cytokines in rats with acute inflammation
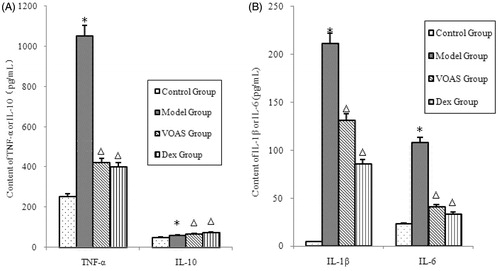
As shown in , the degree of TNF-α, IL-1β and IL-6 in the model group significantly increased (p < 0.05) compared with that in the control group. However, that in the VOAS and Dex groups decreased significantly (p < 0.05) compared with the model group. The degree of IL-10 in the model group significantly increased (p < 0.05) compared with that in the control group. The VOAS and Dex groups had higher level of IL-10 compared with the model group (p < 0.05).
Figure 3. (A) The influence of VOAS on TNF-α and IL-10 with serum. Note: Compared with the control group, *represents significant increase (p < 0.05); compared with the model group, delta represents significant decrease (p < 0.05). (B) The influence of VOAS on IL-1β and IL-6 with serum. Note: Compared with the control group, *represents significant increase (p < 0.05); compared with the model group, delta represents significant decrease (p < 0.05).
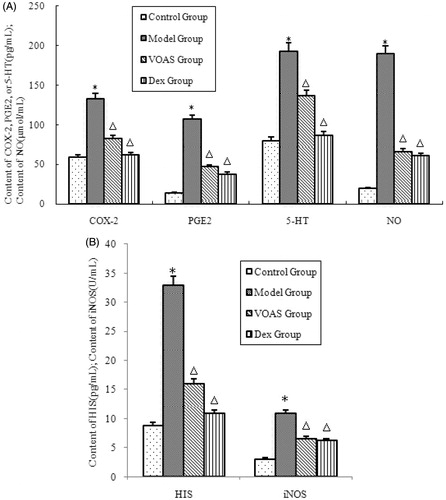
Influence of VOAS on inflammatory mediators and inflammation-related enzymes in rats with acute inflammation
As shown in , the degree of inflammatory mediators (HIS, 5-HT, PGE2 and NO) and inflammation-related enzymes (COX-2 and iNOS) in the model group significantly increased (p < 0.05) compared with that in the control group. However, that in the VOAS and Dex groups decreased significantly (p < 0.05) compared with the model group.
Figure 4. (A) The influence of VOAS on COX-2, PGE2, 5-HT and NO with serum. Note: Compared with the control group, *represents significant increase (p < 0.05); compared with the model group, delta represents significant decrease (p < 0.05). (B) The influence of HIS and iNOS with serum. Note: Compared with the control group, *represents significant increase (p < 0.05); compared with the model group, delta represents significant decrease (p < 0.05).
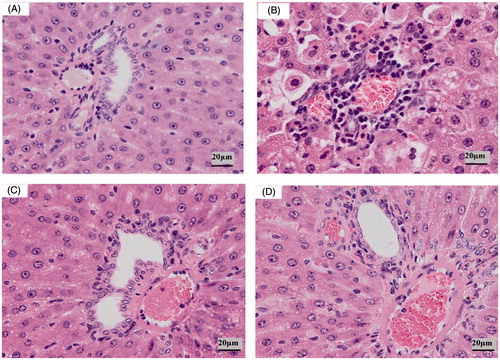
Pathological changes observation
As shown in , in the control group, hepatocyte was normal, nuclear structure was clear, hepatic cords were visible clearly and perisinusoidal space was also obvious (). In the model group, hepatocyte had a large area with hydropic degeneration, hepatic cords were disorganized, perisinusoidal space disappeared and inflammatory cell infiltration was present (). In the VOAS and Dex groups, the injury and damage basically recovered, hepatocyte was mainly normal, hydropic degeneration was absent and hepatic cords were clear ().
Discussion
Determination of VOAS and Dex doses
An acute toxicity test for VOAS was conducted in view of the pharmacological research needs. The results showed that the LD50 value was 1.76 mL/kg and that 95% of the confidence limit was 2.177–1.425 mL/kg. In order to obtain the best dosage, 1/5, 1/10 and 1/20 of LD50 values were selected to perform the dose selection experiment for VOAS. Then we found that the anti-inflammatory effect reached the highest when the dose was 1/10 of LD50 value. Xue-mei Mo found that Dex (40 μg/kg) could effectively intervene the acute inflammation of mice caused by LPS (Mo Citation2012). Then, the dose of Dex for the rats was set to 30 μg/kg according to the surface area formula. To make the Dex dose more accurate, in the preliminary experiment, we separately used 10, 20, 30, 40 and 50 μg/kg of Dex to determine the proper dose for the rat acute inflammation model. We found that the anti-inflammatory effect reached the highest when the dose of Dex was 40 μg/kg. Thus, we, respectively, selected 40 μg/kg and 0.176 mL/kg as the therapeutic dose of Dex and VOAS in this study.
Determination of experimental protocol
Numerous studies have shown that when studying the intervention effects of VOAS or Dex on the inflammation or inflammation-related disease model, VOAS and Dex with no model group often need not be designed (Jia et al. Citation2007; Chao Citation2010; Zhang et al. Citation2014). Our previous study and the preliminary experiment of this study also showed that VOAS and Dex caused no interference with LPS in the model of acute inflammation (Li et al. Citation2014). In the VOAS group, because VOAS was emulsified by Tween 80, the control, model and Dex groups were all orally administered with Tween 80 (2%, v/v) to rule out the influence of Tween 80. VOAS given for several days and Dex given for only 1 d all have been proved in many studies in which they were used in the inflammation or inflammation-related diseases model (Xie et al. Citation2004; Xu et al. Citation2007; Zhang et al. Citation2014; Yang et al. Citation2015), and the design that the tested drug is orally administered for several days while the positive drug Dex is intraperitoneally injected for only 1 d in the inflammation model induced by LPS has also been reported in many studies (Liu et al. Citation2010; Wang et al. Citation2013; Xie et al. Citation2013). Besides, the preliminary experiment of this study proved that VOAS produced the best effects on acute-inflammation induced by LPS when it was used continuously for 4 d. Therefore, according to all the above discussion, the experiments protocol is designed as in this study.
Effects of VOAS on blood routine, GOT, GPT, ALP and liver pathological changes in rats with LPS-induced acute inflammation
LPS can cause acute inflammatory symptoms, including mental haziness, sluggishness, loss of appetite and chills (Chen et al. Citation2009), as well as cause inflammation in multiple body organs. In these organs, liver is the most vulnerable one because it is the main place for endotoxin removal. Therefore, it is meaningful to evaluate the degree of inflammation caused by LPS according to the extent of liver damage (Guo et al. Citation2007). WBC and NE% in blood routine are indexes for valuing inflammation in common use. The level of GOT, GPT and ALP, which belong to blood biochemical indexes, are closely related to liver damage (Dubinsky et al. Citation2003). In this study, the rats in the model group showed the worst recovery. The degree of WBC, NE%, GOT, GPT and ALP in the model group significantly increased compared with that in the control group, and the liver of the model group rats showed the corresponding pathological changes (such as hydropic degeneration, disorganized hepatic cords and inflammatory cell infiltration). These suggested that the rats in the model group exhibited acute inflammatory reaction, and their liver were significantly damaged. Thus, LPS could successfully reproduce acute inflammation model in rats.
Compared with the model group, the exterior behaviour and the liver of the VOAS and Dex groups significantly recovered, the WBC, NE%, ALP, GOT and GPT of the VOAS and Dex groups were significantly decreased. These results demonstrated that VOAS and Dex could reduce inflammation reaction and protect the liver of the rats to some extent.
Effects of VOAS on cytokines, inflammatory mediators and inflammation-related enzymes in rats with LPS-induced acute inflammation
Cytokines, inflammatory mediators and inflammation-related enzymes play an important role in the occurrence and development of inflammation (Stenvinkel et al. Citation2005). In this study, cytokines (TNF-α, IL-1β, IL-6 and IL-10), inflammatory mediators (HIS, 5-HT, NO and PGE2) and inflammation-related enzymes (iNOS and COX-2) were detected to determine the effects of VOAS on inflammation and investigate the anti-inflammatory mechanism of VOAS. Combined with the research achievements from previous studies, the metabolic pathways of VOAS affecting the changes of cytokines, inflammatory mediators and inflammation-related enzymes on LPS-induced acute inflammation rat model are drawn as .
Figure 6. Metabolic pathways of VOAS affecting the changes of cytokines, inflammatory mediators, and inflammation-related enzymes on LPS-induced acute inflammation rat model.
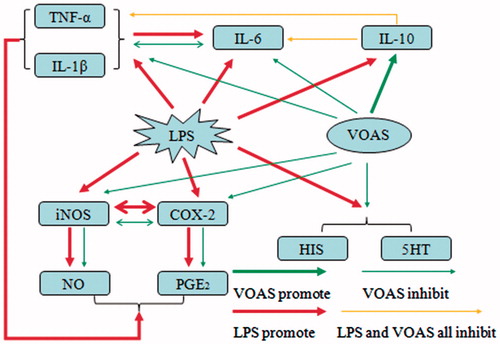
TNF-α and IL-1β are the most important pro-inflammatory cytokines involved in acute inflammation, they could work together to activate initial inflammation, rapidly produce subsequent pro-inflammatory cytokines (e.g., IL-6), and then cause a sequence of pathological processes (Shao et al. Citation2011). IL-6 has both pro- and anti-inflammatory effects (Scheller et al. Citation2011), which are related to the level of IL-6 in the tissues. Normal level of IL-6 is good for organism, but high level causes a series of inflammatory damage (Lin et al. Citation2008). This study showed that TNF-α, IL-1β and IL-6 in the rat inflammation model significantly enhanced. It was similar to the previous studies, which suggest that LPS could prompt the high level of TNF-α, IL-1β and IL-6 mRNA, and then increase the production of TNF-α, IL-1β and IL-6. The increase of TNF-α and IL-1β could also lead to the increase of IL-6 (Guha & Mackman Citation2001; Shao et al. Citation2011).
Compared with the model group, the TNF-α, IL-1β and IL-6 of the rats in the VOAS group were significantly decreased. Shao-meng found that ligustilide, a major component of VOAS, could control the release of TNF-α and IL-1β (Shao et al. Citation2011). Jia-min found that VOAS had good effect on healing septicopyemia, and thought that the effect had something to do with decreasing IL-6 and relaxing inflammation damage (Jia et al. Citation2007). Whats more, Li-yan Lin summarized the research of predecessors and found that the normal level of IL-6 could protect alveolar cell in the experiment of LPS-induced lung injury by inhibiting IL-1β and TNF-α (Lin et al. Citation2008). These results were in accordance with our conclusion that VOAS could inhibit the production of TNF-α, IL-1β and IL-6. Further, we infer that the inhibitory effects of VOAS in the LPS-induced high level of TNF-α, IL-1β and IL-6 of rats may be, at least in part, due to the following reasons. First, VOAS could directly inhibit the production of TNF-α, IL-1β and IL-6. Second, the decrease in TNF-α and IL-1β affects the initiation of inflammation and rapid production of subsequent pro-inflammatory cytokine IL-6. Finally, when IL-6 recovers to the relatively normal level, it could inversely inhibit the production of IL-1β and TNF-α.
Previous study has shown that LPS could prompt high-level mRNA of IL-10 (de Waal Malefyt et al. Citation1991). Furthermore, the production of TNF-α, IL-1β and IL-6 is usually accompanied by the secretion of IL-10 (Oberholzer et al. Citation2001). These data were consistent with our conclusion that the level of IL-10 was significantly enhanced after LPS injection. IL-10 could inhibit the production of pro-inflammatory cytokines and play a crucial role in restraining excessive inflammatory reaction and preventing self-damage (Hasegawa et al. Citation1997). Strengthening the production of IL-10 is also an effective means of healing septic shock in clinics (Zheng et al. Citation2012). Dex could induce the synthesis of IL-10 to inhibit the secretion of pro-inflammatory factors, such as TNF-α, IL-1β and IL-6 (Visser et al. Citation1998). Compared with the model group, the IL-10 of the Dex and VOAS groups increased significantly, whereas TNF-α, IL-1β and IL-6 significantly decreased. Therefore, we conclude that VOAS could significantly promote the secretion of IL-10, which consequently affects the secretion of pro-inflammatory factors TNF-α, IL-6 and IL-1β.
HIS has similar functions with 5-HT in systolic and the increase of local blood vessel permeability, which results in plasma extravasation and oedema formation in inflammatory reaction (Medzhitov Citation2008). Study has demonstrated that LPS could cause the rapid production of several inflammatory mediators such as histamine, serotonin and bradykinin (Campos et al. Citation1996). These results were consistent with the variation trend of HIS and 5-HT in the model group in this study. Wen-quan Zhang found that VOAS could significantly alleviate the foot swelling degree of carrageen-induced foot swelling inflammation in rats and decrease the level of HIS and 5-HT in the inflammatory leach liquor (Zhang et al. Citation2014). This study also found VOAS could significantly decrease HIS and 5-HT, suggesting that VOAS could be used against inflammation by inhibiting the generation of HIS and 5-HT.
A significant relationship exists between inflammatory mediators (NO and PGE2) and inflammation-related enzymes (iNOS and COX-2). NO is a potent vasodilator, the involvement of which during inflammation reaction may be related to its ability to increase vascular permeability and oedema by changing the local blood flow (Moncada & Higgs Citation1993). PGE2 could also promote changes in vascular tonus and blood flow. Furthermore, PGE2 could attract neutrophile granulocyte and result in pain and fever (Dos Santos et al. Citation2006). COX-2 and iNOS are pro-inflammatory enzymes that mediate PGE2 and NO production (Li et al. Citation2006). Studies have shown that several animal models, including the LPS-induced inflammatory rat model, in which activated factors could induce the increased expression level of COX-2 and iNOS, and resulted in the increased level of PGE2 and NO (Surh et al. Citation2001; Hwang et al. Citation2011). At the same time, COX-2 and iNOS could activate each other and further promote the production of PGE2 and NO (Corbett et al. Citation1993; Salvemini et al. Citation1993; Blanco & Lotz Citation1995; Hajjar et al. Citation1995; Salvemini et al. Citation1996; Tetsuka et al. Citation1996). Moreover, the increased production of cytokines, such as IL-1β and TNF-α, could also stimulate PGE2 and NO production (Kozuka et al. Citation2005; Yu et al. Citation2010). The change trend of PGE2, NO, COX-2 and iNOS in this study was similar to these previous studies. Jian-fen Shen found that the angelica active site A3 extract from VOAS could suppress the expression level of mRNA and protein of COX-2 in the LPS-induced isolated uterus (Shen et al. Citation2006). Guang-Yi Zhang discovered that angelica lactone, an extract component of VOAS, had significant protective function on local cerebral ischemic injury of rats, and thought that the mechanism might be related to the decrease of iNOS, which resulted in the reduction of NO and affected the inflammation mediated by NO (Zhang et al. Citation2006). In this study, compared with the model group, the levels of COX-2, PGE2, iNOS and NO in the VOAS group significantly decreased, which demonstrated that VOAS could significantly inhibit the production of COX-2, PGE2, iNOS and NO. Moreover, COX-2 and iNOS could activate each other. The mechanism is presumed that VOAS could inhibit the expression of COX-2 and iNOS, then result in the decrease of PGE2 and NO, and the weak activated function of COX-2 and iNOS could weaken the production of PGE2 and NO.
Conclusion
VOAS has good effects on inflammation and protecting the rat liver, and could significantly influence the cytokines, inflammatory mediators and inflammation-related enzymes in acute inflammation rat model. The anti-inflammatory mechanism may be attributed to directly or indirectly inhibit the secretion of the pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), the inflammatory mediators (HIS, 5-HT, PGE2 and NO) and the inflammation-related enzymes (iNOS and COX-2), as well as promote the production of the anti-inflammatory cytokines IL-10.
Funding information
This study was financially supported by the National Natural Science Foundation of China (No. 31272600).
Acknowledgements
The authors are grateful to all other staff in the Institute of Traditional Chinese Veterinary Medicine of Gansu Agricultural University for their assistance in the experiments.
Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Bauer J, Koeberle A, Dehm F, Pollastro F, Appendino G, Northoff H, Rossi A, Sautebin L, Werz O. 2011. Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem Pharmacol. 81:259–268.
- Blanco FJ, Lotz M. 1995. IL-1-induced nitric oxide inhibits chondrocyte proliferation via PGE2. Exp Cell Res. 218:319–325.
- Bone RC. 1996. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 24:1125–1128.
- Buchanan MM, Hutchinson M, Watkins LR, Yin H. 2010. Toll-like receptor 4 in CNS pathologies. J Neurochem. 114:13–27.
- Cai HB, Luo RC. 2003. Prevention and therapy of radiation-induced pulmonary injury with traditional Chinese medicine. Acad J First Med Coll PLA. 23:958–960.
- Campos MM, Souza GE, Calixto JB. 1996. Upregulation of B1 receptor mediating des-Arg9-BK-induced rat paw oedema by systemic treatment with bacterial endotoxin. Br J Pharmacol. 117:793–798.
- Chao WW. 2010. Inhibitory effects of Angelica sinensis ethyl acetate extract and major compounds on NF-kappaB trans-activation activity and LPS-induced inflammation. J Ethnopharmacol. 129:244–249.
- Chen J, Jiang H, Zhu YS. 2009. Effect of Xue-bi Jing on TNF-α of cardiac muscle in endotoxin shock rats. Chinese J Med Guide. 11:1020.
- Connor TJ, Brewer C, Kelly JP, Harkin A. 2005. Acute stress suppresses pro-inflammatory cytokines TNF-alpha and IL-1 beta independent of a catecholamine-driven increase in IL-10 production. J Neuroimmunol. 159:119–128.
- Corbett JA, Kwon G, Turk J, McDaniel ML. 1993. IL-1, beta, induces the coexpression of both nitric oxide synthase and cyclooxygenase by Islets of Langerhans: activation of cyclooxygenase by nitric oxide. Biochemistry. 32:13767–13770.
- Cui F, Feng L, Hu J. 2006. Factors affecting stability of Z-ligustilide in the volatile oil of radix Angelicae sinensis and Ligusticum chuanxiong and its stability prediction. Drug Dev Ind Pharm. 32:747–755.
- Deng C, Ji J, Wang X, Zhang X. 2005. Development of pressurized hot water extraction followed by headspace solid-phase microextraction and gas chromatography–mass spectrometry for determination of ligustilides in Ligusticum chuanxiong and Angelica sinensis. J Sep Sci. 28:1237–1243.
- de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, De Vries JE. 1991. Interleukin10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 174:1209–1220.
- Dos Santos MD, Almeida MC, Lopes NP, De Souza GEP. 2006. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. 29:2236–2240.
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. 2006. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 7:344–353.
- Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, Vierling JM, Geller SA, Targan SR, et al. 2003. 6-Thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 125:298–303.
- Ferreira LC, Grabe-Guimarães A, de Paula CA, Michel MC, Guimarães RG, Rezende SA, de Souza Filho JD, Saúde-Guimarães DA. 2013. Anti-inflammatory and antinociceptive activities of Campomanesia adamantium. J Ethnopharmacol. 145:100–108.
- Ferrero E, Jiao D, Tsuberi BZ, Tesio L, Rong GW, Haziot A, Goyert SM. 1993. Transgenic mice expressing human CD14 are hypersensitive to lipopolysaccharide. Proc Natl Acad Sci USA. 90:2380–2384.
- Guha M, Mackman N. 2001. LPS induction of gene expression in human monocytes. Cell Signal. 13:85–94.
- Guo J, Shuai Y, Peng SQ, Zhang LS, Dong YS, Chen LJ. 2007. Protective effects of metallothionein against lipopolysaccharide-induced acute liver injury. J Toxicol. 21:172–175.
- Hajjar DP, Lander HM, Pearce SFA, Upmacis R, Pomerantz KB. 1995. Nitric oxide enhances prostaglandin-H synthase-1 activity by a heme-independent mechanism: evidence implicating nitrosothiols. J Am Chem Soc. 117:3340–3346.
- Hasegawa M, Fujimoto M, Kikuchi K, Takehara K. 1997. Elevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosis. J Rheumatol. 24:328–332.
- Hwang YP, Choi JH, Yun HJ, Han EH, Kim HG, Kim JY, Park BH, Khanal T, Choi JM, Chung YC, et al. 2011. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem Toxicol. 49:93–99.
- Jia M, Yang TH, Pan F, Luo XX. 2007. Effects of essential oil from Radix Angelicae Sinensis in mice with sepsis. Chinese J Clin Pharmacol Ther. 12:1364–1366.
- Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. 1999. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1:94–97.
- Kozuka N, Itofusa R, Kudo Y, Morita M. 2005. Lipopolysaccharide and proinflammatory cytokines require different astrocyte states to induce nitric oxide production. J Neurosci Res. 82:717–728.
- Li P, Li SP, Lao SC, Fu CM, Kan KK, Wang YT. 2006. Optimization of pressurized liquid extraction for Z-ligustilide, Z-butylidenephthalide and ferulic acid in Angelica sinensis. J Pharm Biomed Anal. 40:1073–1079.
- Li J, Yu X, Ma YX, Li XR. 2010. Research progress of anti-inflammatory mechanism of traditional Chinese medicine and its effective components. Acta Chinese Med Pharmacol. 38:134–137.
- Li JX, Zhang M, Sun LB, Zhang L, Zhang WQ, Zhao HF, Li PL, Hua YL, Ji P, Wei YM. 2014. Comparative metabonomics study on urine in rat treated by Angelica sinensis volatile oil. China J Chinese Mater Med. 39:1293–1299.
- Lin W, Wu RT, Wu T, Khor TO, Wang H, Kong AN. 2008. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 76:967–973.
- Lin LY, Zhang HY, He SF. 2008. Advance in the research of correlation of IL-6 and its receptors with inflammation diseases. China Trop Med. 8:680–682.
- Liu L, Xiong H, Ping J, Ju Y, Zhang X. 2010. Taraxacum officinale protects against lipopolysaccharide-induced acute lung injury in mice. J Ethnopharmacol. 130:392–397.
- Liu LN, Jia M, Mei QB, Yang T, Wang Q, Cheng J. 2002. Anti-inflammatory and analgesic actions of essential oil extracted from radix Angelica sinensis by ethanol. China Pharm. 13:526–527.
- Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature. 454:428–435.
- Moncada S, Higgs A. 1993. The L-arginine-nitric oxide pathway. N Engl J Med. 329:2002–2012.
- Mo XM. 2012. The effects and mechanisms of CXCR4 antagonist-N15P peptide on LPS-induced inflammation [Doctoral dissertation]. Jinan University.
- Ni ZN, Lu GY, Lou ZH, Wu RJ. 2007. Research progress of chemical composition and pharmacological activity of Angelica sinensis volatile oil. Chin J Inform Tradit Chinese Med. 14:93–95.
- Oberholzer A, Oberholzer C, Moldawer LL. 2001. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 16:83–96.
- Ray A, Prefontaine KE. 1994. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 91:752–756.
- Sakurada S, Kato T, Okamoto T. 1996. Induction of cytokines and ICAM-1 by proinflammatory cytokines in primary rheumatoid synovial fibroblasts and inhibition by N-acetyl-L-cysteine and aspirin. Int Immunol. 8:1483–1493.
- Salvemini D, Currie MG, Mollace V. 1996. Nitric oxide-mediated cyclooxygenase activation. A key event in the antiplatelet effects of nitrovasodilators. J Clin Invest. 97:2562–2568.
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. 1993. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 90:7240–7244.
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. 2011. The pro-and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 1813:878–888.
- Shao M, Qu K, Liu K, Zhang Y, Zhang L, Lian Z, Chen T, Liu J, Wu A, Tang Y, et al. 2011. Effects of ligustilide on lipopolysaccharide-induced endotoxic shock in rabbits. Planta Med. 77:809–816.
- Shen JF, Xiao JH, Wang JL. 2006. Effect of angelica A∼3 active fraction on anti-inflammation and cyclooxygenase-2 expression of isolated rat uterus. Chinese Tradit Herb Drugs. 37:1371.
- Shivkar YM, Kumar VL. 2004. Effect of anti-inflammatory drugs on pleurisy induced by latex of Calotropis procera in rats. Pharmacol Res. 50:335–340.
- Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimburger O, Cederholm T, Girndt M. 2005. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia – the good, the bad, and the ugly. Kidney Int. 67:1216–1233.
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. 2001. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res-Fund Mol M. 480:243–268.
- Tetsuka T, Daphna-Iken D, Miller BW, Guan Z, Baier LD, Morrison AR. 1996. Nitric oxide amplifies interleukin 1-induced cyclooxygenase-2 expression in rat mesangial cells. J Clin Invest. 97:2051.
- Visser J, van Boxel-Dezaire A, Methorst D, Brunt T, De Kloet ER, Nagelkerken L. 1998. Differential regulation of interleukin-10 (IL-10) and IL-12 by glucocorticoids in vitro. Blood. 91:4255–4264.
- Wang Q, Kuang H, Su Y, Sun Y, Feng J, Guo R, Chan K. 2013. Naturally derived anti-inflammatory compounds from Chinese medicinal plants. J Ethnopharmacol. 146:9–39.
- Wang Y, Li Y, Xie J, Zhang Y, Wang J, Sun X, Zhang H. 2013. Protective effects of probiotic Lactobacillus casei Zhang againstendotoxin-and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol. 15:30–37.
- Wang ZH, Liang YB, Tang H, Yun XL, Gong P, Liu JR, Zhao SM, Kang HB, Zhang HP. 2013. Dexamethasone down-regulates the expression of microRNA-155 in the livers of septic mice. PloS One. 8:e80547.
- Xie JM, Wang YZ, Zhang W, et al. 2013. Protective effects of probiotic Lactobacillus casei Zhang against acute liver injury in rats and its effect on the tlr4-erk-ppar-γ signaling pathway. Chinese J Immunol. 29:910–913.
- Xie KM, Yong M, Xie P, Gu YP. 2004. Inhibitory effects of Angelicae sinensis and sodium ferulate on acute inflammatory liver injury and expression of icam-1 and e-selectin in mice. Chinese J Pathophysiol. 12:038.
- Xu SJ, Shen YJ, Xie YH. 2007. Experimental study on the anti-inflammation effect of volatile oil in ramulus cinnamomi. Tradit Chinese Drug Res Clin Pharmacol. 3:005.
- Yang H, Li Y, Huo P, Li XO, Kong D, Mu W, Fang W, Li L, Liu N, Fang L, et al. 2015. Protective effect of Jolkinolide B on LPS-induced mouse acute lung injury. Int Immunopharmacol. 26:119–124.
- Yeh JC, Garrard IJ, Cho CWC, Bligh SA, Lu GH, Fan TP, Fisher D. 2012. Bioactivity-guided fractionation of the volatile oil of Angelica sinensis radix designed to preserve the synergistic effects of the mixture followed by identification of the active principles. J Chromatogr A. 1236:132–138.
- Yu Y, Zhang ZH, Wei SG, Serrats J, Weiss RM, Felder RB. 2010. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 55:652–659.
- Zhang GY, Du JR, Kuang X, Yao Y, Liu YX, Wang CY, Qian ZM. 2006. Therapeutic effects and its mechanism of angelica lactone on focal cerebral ischemia in rats. W China J Pharm Sci. 21:114.
- Zhang WQ, Hua YL, Zhang M, Ji P, Li JX, Zhang L, Li PL, Wei YM. 2014. Metabonomic analysis of the anti-inflammatory effects of volatile oils of Angelica sinensis on rat model of acute inflammation. Biomed Chromatogr. 29:902–910.
- Zheng Y, Guo Z, He W, Yang Y, Li Y, Zheng A, Li P, Zhang Y, Ma J, Wen M, et al. 2012. Ephedrine hydrochloride protects mice from LPS challenge by promoting IL-10 secretion and inhibiting proinflammatory cytokines. Int Immunopharmacol. 13:46–53.