Abstract
Context Yellow tea containing the same catechins as other types of tea but in different proportions has been suggested to possess potent anticancer activities.
Objective This study investigates the chemopreventive effect of yellow tea aqueous extract against N-nitrosodiethylamine (NDEA)-induced liver carcinogenesis in rats by employing histological and biochemical methods.
Materials and methods Wistar rats were divided randomly into four groups: control (I), yellow tea (II), NDEA (III), and yellow tea + NDEA (IV). Groups II and IV were exposed via a diet to yellow tea extract in a concentration of 10 g/kg feed; groups III and IV received 0.01% NDEA in drinking water. The experiment lasted for 13 weeks.
Results Daily intake of yellow tea in an average dose of 800 mg/kg b.w. alleviated the carcinogenic effect of NDEA as evidenced by reversed histopathological changes towards normal hepatocellular architecture and decreased lipid peroxidation, protein carbonyl formation, and DNA degradation by 64%, 37% and 15%, respectively, as compared with values obtained in NDEA alone-treated rats. Treatment with yellow tea extract caused protection of superoxide dismutase (SOD) and catalase (CAT); their activity was recovered by 47% and 12%, respectively, as compared with the NDEA-treated rats. Moreover, the extract normalized the NDEA-induced activity of paraoxonase 1 (PON1) and glutathione peroxidase (GPx), while a further increase in the level of reduced glutathione (GSH) was noticed.
Conclusions On the basis of these findings, it can be concluded that treatment with yellow tea partially protected the livers of rats from NDEA-induced hepatocarcinogenesis and that its antioxidant activity contributed to this effect.
Introduction
Tea polyphenols are a class of dietary phytochemicals that have been proven to show chemopreventive and anticancer properties. These effects have been demonstrated in the cancers of many organs and tissues, such as in the bladder, breast, colon, esophagus, head and neck, kidney, lung, pancreas, prostate, stomach, skin, uterus, and liver (Darvesh & Bishayee Citation2013). Tea, an infusion from Camellia sinensis (L.) Kuntze (Theaceae) leaves, is the most widely consumed beverage, after water. On the basis of different manufacturing processes, tea is classified into six categories: green, white, yellow, oolong, black and pu-erh (Hashimoto et al. Citation2007). Yellow tea is processed similar to green tea by heating the leaves to stop fermentation. However, the yellow colour is the result of an additional step called “sealed yellowing”, which involves a slow oxidation process of tea polyphenols (Wang et al. Citation2013). Differences in the tea manufacturing processes result in differences in the composition of these types of tea. The strong chemopreventive and chemotherapeutic effects of green tea polyphenols against cancer have been widely demonstrated (Yiannakopoulou Citation2014). On one hand, catechins are a major group of polyphenols found in tea and their total amount is comparable in green and yellow tea. However, the content of individual catechin components varies, e.g., the amount of (+) catechin and gallocatechin-3-gallate is high in yellow tea but is almost undetected in green tea. On the other hand, the content of (−) epigallocatechin (EGC) and (−) epigallocatechin-3-gallate (EGCG) is over two times higher in green tea than in yellow tea (Gramza-Michałowska Citation2007). Moreover, yellow tea contains 10 times more gallic acid than green tea (Hashimoto et al. Citation2007). In the last decade, much effort has been put into studying the mechanisms involved in the chemopreventive effects of tea polyphenols. As a result, oxidative stress and inflammation have been indicated as key processes in carcinogenesis, which could be affected by phytochemicals including polyphenols. Tea polyphenols have been shown to react with a broad spectrum of reactive oxygen species (ROS) by breaking the free radical chain reaction, chelating metal ions as well as inducing antioxidant enzymes. Our previous in vitro studies have demonstrated that yellow tea aqueous and ethanol extracts show much stronger antioxidant activity than green, white or black tea extracts (Gramza-Michałowska Citation2007); hence, we examined the antioxidant-based chemopreventive potential of yellow tea against dietary carcinogen N-nitrosodiethylamine (NDEA)-induced rat hepatocarcinogenesis.
Primary liver cancer, also called hepatocellular carcinoma (HCC), is the second leading cause of cancer mortality worldwide according to the World Health Organization (WHO) (Stewart & Wild Citation2014). Dietary carcinogens, such as nitrosamines and aflatoxins, have been shown to be implicated in the aetiology of HCC. They contribute to the development of chronic inflammation, oxidative stress and cellular proliferation in response to tissue injury, finally leading to hepatic neoplasia (Darvesh & Bishayee Citation2013). Nitrosamines comprise a wide class of environmental carcinogens found in foodstuffs such as smoked pickled fish, cheese, nitrite-cured meats, dried milk and alcoholic beverages or tobacco smoke. They are also formed in the acidic conditions of the stomach from nitrite precursors and amines which are ingested as food constituents or additives, residues of agricultural chemicals and pharmaceutical drugs (Lijinsky Citation1999; Mittal et al. Citation2006). A representative of this group, N-nitrosodiethylamine (NDEA), undergoes metabolic activation by formation of its active ethyl radical metabolite (CH3CH2+), which covalently bonds with nucleophilic residues in DNA (Bansal et al. Citation2005). The alkylation of DNA bases is mutagenic and promotes carcinogenesis. Furthermore, biotransformation of NDEA mediated by NADPH reductase causes the generation of ROS, thus leading to oxidative stress (Verna et al. Citation1996). The International Agency for Research on Cancer (IARC) categorized NDEA as a “probable carcinogenic to humans” (category A2) (IARC Monographs Citation1987). In rodents, NDEA primarily induces the formation of liver tumours and it has been used as a model hepatic carcinogen in experimental studies of carcinogenesis and chemoprevention (Gopalakrishnan et al. Citation2013).
Because humans are continually exposed to potent environmental carcinogens, modulation of inflammation and oxidative stress by natural substances appears to be highly desirable. Chemoprevention, i.e., strategies that entail the use of naturally occurring compounds derived from fruits, vegetables, and herbs to inhibit, reverse, or suppress carcinogenesis, has been the mainstream in cancer research (Darvesh & Bishayee Citation2013; Steward & Brown Citation2013).
While considerable attention has been paid to the biological activity of green tea polyphenols over the last few decades, only several studies have been devoted exclusively to yellow tea. Hashimoto et al. (Citation2007) reported that yellow tea showed more significant hepatoprotective activity against CCl4-induced hepatic injury in rats in comparison with other types of tea, including green tea. Wang et al. (Citation2013) showed the protective effect of yellow tea against ethanol-induced gastric mucosal injury in rats, which appeared to be related to a decrease in the serum level of pro-inflammatory cytokines IL-6 and TNFα. The present study was designed to evaluate the chemopreventive effect of yellow tea extract on NDEA-induced liver carcinogenesis in rats.
Materials and methods
Materials
The subject of this research was the aqueous extract of yellow tea leaves prepared according to the method presented by Gramza et al. (Citation2006). Camellia sinensis leaves were obtained from a tea company representative (Gemini, Poland). The tea specimen was yellow tea of the Kekecha type originating from certified cultivation in the Guangdong province. Ground tea leaves were dynamically extracted with double-distilled water in triplicate (15 min at 80 °C). Then the collected extracts were filtered, centrifuged and lyophilized. The catechin content in the extract was assayed by the HPLC method according to Khokhar and Magnusdottir (Citation2002); the results are presented in . The yellow tea extract was incorporated into a certified laboratory feed, Labofeed H (ISO 22 000), to obtain a final concentration of 10 g/kg feed.
Table 1. Content of polyphenolic compounds in yellow tea extract (mg/g).
Experimental design
The experimental animals were bred at the Department of Toxicology, Poznan University of Medical Sciences. They were kept (four rats/cage) in polycarbonate cages (Techniplast, Italy) with wood shavings at 22 ± 1 °C, 50% humidity and controlled circulation of air with a 12 h light/dark cycle. A commercial diet (ISO 22000 certified laboratory feed Labofeed H) and water were available ad libitum.
Thirty-two male Wistar rats (250 ± 15 g) were divided into four groups; each group comprised eight animals that were treated for 13 weeks as follows:
Group 1: control rats fed the standard diet.
Group 2: rats exposed via the diet to yellow tea in a concentration of 10 g/kg feed.
Group 3: rats receiving 0.01% NDEA in the drinking water.
Group 4: rats treated with yellow tea via diet (10 g/kg feed) along with the administration of 0.01% NDEA.
On the basis of the feed consumption values and the nominal dietary concentration of yellow tea extract, the calculated mean daily intake of the test substance was 0.80 g/kg b.w./day. At the end of the experiment, the animals were fasted overnight and then sacrificed by exsanguination following deep anaesthesia with ketamine/xylazine (100/7.5 mg/kg b.w., i.p.).
Sample collection
A portion of whole heparinized blood was centrifuged (3000 rpm, 4 °C) for plasma separation. Livers were harvested for a histopathological and biochemical examination. Specimens were prepared as follows: one slice of liver was fixed in 4% paraformaldehyde for more than 24 h for histopathological analysis, and the remaining portion of the liver was rinsed in ice cold 1.15% KCl, portioned and stored at −80 °C for further analyses. One frozen portion was homogenized in buffered Tris/sucrose solution (pH 7.55) for cytosol fraction preparation; other tissue sections were homogenized separately for glutathione, lipid peroxidation and protein carbonyl determination as well as comet assay. Subcellular fractions were prepared by differential centrifugation according to the standard procedure. The protein concentration was determined in each fraction using Folin–Ciocalteu reagent.
The experiment was performed according to the Local Animal Ethics Committee’s guidelines for animal experimentation.
Histopathology
After formalin fixation, the liver specimens were dehydrated in ethanol and embedded in paraffin blocks, which were cut into 4 μm thick slices. The resulting sections were stained by haematoxylin and eosin and then examined using light microscopy.
Biochemical assays
Hepatic enzyme activity (alanine aminotransferase, ALT; aspartate aminotransferase, AST; alkaline phosphatase, ALP, gamma glutamyltransferase, GGT and sorbitol dehydrogenase, SDH) as well as the concentration of total bilirubin were determined in plasma according to the reagent kit’s manufacturer instructions (Pointe Scientific, Warszawa, Poland).
The level of microsomal lipid peroxidation was determined by measuring thiobarbituric acid reactive substances (TBARS) (Sanz et al. Citation1994). The protein carbonyl concentration in the liver was assessed using a commercial ELISA assay kit from BioCell Corporation (BioCell, Papatoetoe, New Zealand) based on the method described by Buss et al. (Citation1997).
Nuclear DNA integrities in the liver were examined by using an alkaline comet assay conducted according to the method of Hartmann et al. (Citation2003). The slides were stained with ethidium bromide after cell lysis, DNA unwinding, electrophoresis and neutralization. Images of comets from a Zeiss fluorescence microscope (magnification 400×) were captured with a digital camera and divided into five groups according to degree of DNA damage (Collins Citation2004). The total damage score for a given slide was derived by multiplying the number of cells assigned to each grade of damage by the numeric value of the grade and summing over all grades.
The reduced glutathione content in the liver was assayed by its reaction with Ellman’s reagent (Sedlak & Lindsay Citation1968). Antioxidant enzyme activities were assayed in the liver cytosol. Superoxide dismutase (SOD) activity was determined on the basis of inhibition of spontaneous epinephrine oxidation (Jodynis-Liebert et al. Citation2000). Catalase (CAT) activity was assayed by measuring the rate of H2O2 decomposition (Jodynis-Liebert et al. Citation2000). Glutathione peroxidase (GPx) activity was determined by the method of Mohandas et al. (Citation1984) using hydrogen peroxide as a substrate. The rate of NADPH disappearance at 340 nm was a measure of the enzyme activity. Glutathione reductase (GR) activity was assayed by measuring NADPH oxidation at 340 nm in the presence of oxidized glutathione (Mohandas et al. Citation1984). Glutathione S-transferase (GST) activity measurement was based on the spectrophotometric determination of the 1-chloro-2,4-dinitrobenzene (CDNB) conjugate formed in a GSH coupled reaction (Mohandas et al. Citation1984). Paraoxonase-1 (PON1) activity in the liver was measured with phenyl acetate as a substrate. The rate of phenol generation was a measure of the enzyme activity (Jurek et al. Citation2006).
Statistical analysis
The data were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparisons test was used. p < 0.05 was considered to be the limit of significance.
Results
Plasma markers of liver function
The alterations in the liver function markers upon yellow tea extract and NDEA treatment are presented in . A significant elevation of activities of ALT, AST, ALP, GGT and SDH (by 102, 103, 105, 253 and 970%, respectively) as well as an increase in the level of serum total bilirubin (by 40%) was observed in NDEA-treated rats as compared with the control group. Supplementation with yellow tea extract resulted in a significant decrease in the activities of ALP, GGT and SDH (14–28%) and in a decrease in the level of bilirubin by 20% when compared with that in animals treated with NDEA alone. The activities of both aminotransferases were not affected by co-treatment with yellow tea extract.
Table 2. Effect of yellow tea extract on plasma clinical chemistry parameters in rats given NDEA.
Microscopic evaluation
presents representative micrographs of H&E staining of hepatic tissue in control and experimental rats. Microscopic examinations of liver sections of the controls and rats treated with yellow tea showed the same normal hepatic structure. The liver tissue of rats treated with NDEA revealed the presence of hepatocellular carcinoma and dysplastic nodules. Combined treatment with yellow tea slightly improved the histological architecture. All identified microscopic liver lesions are summarized in .
Figure 1. Representative images (×100) of liver sections (haematoxylin and eosin staining). I Control rat. Normal liver architecture. Representative images (×100) of liver sections (haematoxylin and eosin staining). II Rat treated with yellow tea alone. Normal liver structure. Representative images (×100) of liver sections (haematoxylin and eosin staining). III Rat treated with NDEA alone. Pathological liver structure: (A) dysplastic nodule or early hepatocellular carcinoma. Representative images (×100) of liver sections (haematoxylin and eosin staining). IV Rat treated with yellow tea + NDEA. Pathological liver structure: (A) dysplastic nodule, (B) intrahepatic cholestasis and © extrahepatic cholestasis.
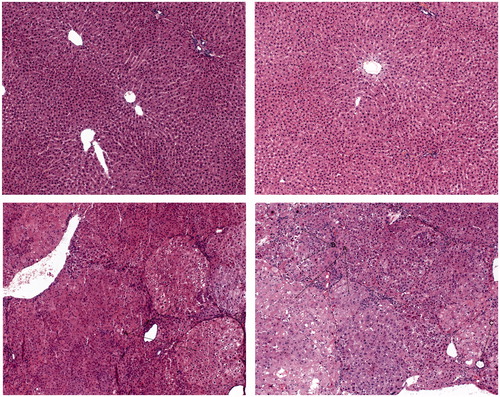
Table 3. Effect of yellow tea extract on histopathological parameters in liver of rats given NDEA.
Oxidative stress evaluation
The effects of yellow tea extract on the biomarkers of oxidative damage to lipids, proteins and DNA are presented in and . Exposure to NDEA significantly increased lipid peroxidation by 166%, protein oxidation by 62% and DNA degradation by 91% in the livers of rats. The images of representative nuclei after electrophoresis () show samples from the control group with normal cell nuclei and the NDEA-treated group with an increased degree of DNA migration. Yellow tea administration to NDEA-treated rats caused a significant decline in levels of hepatic TBARS and protein carbonyls as well as a significant decrease in the comets’ size by 64, 37 and 15%, as compared with those in rats treated with NDEA alone. Yellow tea extract itself insignificantly decreased the content of protein carbonyls by 38%, as compared with the control values.
Figure 2. Fluorescence microscope-derived pictures of DNA damage in the liver of (A) control rats, (B) rats treated with yellow tea alone, (C) rats treated with NDEA alone and (D) rats treated with yellow tea + NDEA.
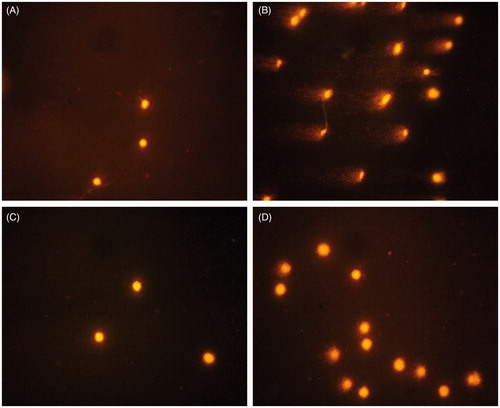
Table 4. Effect of yellow tea extract on lipid peroxidation (LPO), protein carbonyls (PCs) concentration, DNA damage and reduced glutathione (GSH) level in liver of rats given NDEA.
The effects of yellow tea extract on the level of hepatic GSH are presented in . NDEA alone resulted in an increase in glutathione content by 35% as compared with that in the controls. Treatment with yellow tea extract along with the NDEA challenge caused a further significant increase in the GSH level by 61%. Interestingly, yellow tea extract alone insignificantly decreased the concentration of GSH by 27% when compared with the values for the control animals.
The results obtained for the antioxidant enzyme activities are shown in . The responses of antioxidant enzymes both to NDEA alone and to the combined treatment with yellow tea were diversified. NDEA treatment resulted in a decrease in SOD activity by 51% and in an increase in the activities of GPx and GR by 69% and 88%, respectively, as compared with the control values. The CAT and GST activities were not affected by NDEA exposure. Supplementation with yellow tea extract restored NDEA-induced impairment in SOD activity by 47% and caused a further increase in GR activity by 24%. However, a decrease in GPx activity by 20%, as compared with that in NDEA alone-treated rats, was noticed. In rats treated with yellow tea alone, the activities of SOD, GPx and GST were elevated by 15–18% versus the values for the control rats.
Table 5. Effect of yellow tea extract on hepatic antioxidant enzymes activity in rats given NDEA.
NDEA also caused an insignificant increase in hepatic PON1 activity by 23%, which was decreased as a result of yellow tea administration by 50%. Yellow tea itself elevated the activity of this enzyme by 82% when compared with that in the controls.
Discussion
Hepatocarcinogenesis induced by N-nitrosodiethylamine (NDEA), due to histological and biochemical similarities between rodents and human hepatic lesions, is widely used as an experimental model in chemoprevention studies (Paula Santos et al. Citation2014). Several reports have demonstrated a strong relationship between oxidative stress and cancer in the case of NDEA-induced hepatocellular carcinoma (HCC) (Pradeep et al. Citation2007; Santos et al. Citation2012; Paula Santos et al. Citation2014). Since a growing body of evidence has suggested that epicatechins, which are found in large amounts in green tea, display anticancer and chemopreventive properties, we investigated whether catechin-rich yellow tea can provide some protection against NDEA-induced hepatocarcinogenesis in rats.
We assayed some routine clinical chemistry markers to assess the protective influence of yellow tea on the liver. As was expected, exposure of rats to NDEA for 13 weeks caused liver damage, which was evidenced by a rise in the activity of plasma transaminases (ALT and AST), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT) and sorbitol dehydrogenase (SDH), as well as by an increase in the level of total bilirubin (TBIL). The combined treatment with yellow tea extract moderately protected the livers since the activities of ALP, GGT and SDH as well as the level of TBIL were decreased. However, the activities of aminotransferases remained unchanged.
A microscopic examination revealed hepatocellular carcinomas and dysplastic nodules in the livers of NDEA-treated animals as well as cirrhosis, dilatation of bile ducts and ballooning degeneration. The effects of NDEA on the liver, as noticed in this study, are similar to those previously reported by other authors (Arul & Subramanian Citation2013; Ali et al. Citation2014). The small pre-neoplastic focal lesions have been demonstrated to lead into malignant transformation, with the formation of neoplastic nodules and, ultimately, HCC (Shen et al. Citation2014). Yellow tea supplementation reduced altered hepatocyte foci formation and reversed histopathological changes towards normal cellular architecture. Green tea polyphenols have also been shown to moderately improve the hepatocellular structure damaged as a result of NDEA/phenobarbital treatment (Shen et al. Citation2014). These results suggest that yellow tea may protect the liver against damage induced by NDEA. The hepatoprotective effect of yellow tea in NDEA-challenged rats might be related to its high antioxidant activity, which is in agreement with current knowledge concerning NDEA biotransformation that requires oxygen-dependent metabolic activation by CYP2E1 and the consequent accumulation of ROS and DNA damage (Kang et al. Citation2007; Bakiri & Wagner Citation2013). It has been demonstrated that the reactive metabolites of NDEA and oxygen radical by-products of CYP2E1-catalyzedmetabolic activation induce oxidative stress which leads to carcinogenesis (Arul & Subramanian Citation2013). We previously reported that yellow tea extracts, both aqueous and ethanol, demonstrated the highest in vitro antioxidant activity, as compared with green, white and black tea extracts (Gramza-Michałowska Citation2007). While the antioxidant activity of green tea has been extensively investigated, only scarce information can be found regarding the antioxidant effects in vivo of yellow tea. We measured the hepatic levels of TBARS, protein carbonyl and DNA degradation reflecting the extent of oxidative damages in the liver in order to evaluate the antioxidant effects of yellow tea extract. In the present experiment, NDEA caused severe damages to hepatic macromolecules. The administration of yellow tea ameliorated these injuries, which can be interpreted as a result of suppression of NDEA-induced oxidative stress. Yellow tea alone decreased even the basal level of protein carbonyls, as compared with the control values. These results strongly confirm the antioxidant action of the phenolic compounds of yellow tea in the in vivo model. Yellow tea, as compared with other types of tea, contains the highest amount of gallic acid and catechin derivatives (Hashimoto et al. Citation2007). Gallic acid has been reported to possess strong antioxidant, anti-inflammatory, antimutagenic and anticancer properties (Vijaya Padma et al. Citation2011). Hepatoprotective effects coupled with the ability to inhibit lipid peroxidation have been demonstrated for gallic acid in carbon tetrachloride and lindane-induced liver injury (Jadon et al. Citation2007; Vijaya Padma et al. Citation2011). Similar effects have been demonstrated for catechin and catechin-rich extracts in carbon tetrachloride-induced liver injury (Liu et al. Citation2015), thioacetamide-induced liver fibrosis (Esmat et al. Citation2013) and in 7,12-dimethylbenz(a)anthracene (DMBA)-induced hepatocellular carcinoma in rodents (Monga et al. Citation2012).
It is well known that the carcinogenic effect of NDEA is also due to oxidative damage to DNA (Ferk et al. Citation2011). In the present study, the prevention of the formation of preneoplastic lesions correlated well with the decrease in DNA damage level in the livers of rats given combined treatment with NDEA and yellow tea. It has been demonstrated that gallic acid found in large amounts in yellow tea had a protective effect on DNA in mice exposed to gamma radiation as well as in in vitro experiments with human and rat lymphocytes treated with ROS (Ferk et al. Citation2011; Sevgi et al. Citation2015). Furthermore, since gallic acid has been reported to decrease hepatic CYP2E1 protein expression in CCl4-treated rats (Tung et al. Citation2009), its protective effect on DNA may also be attributed to the inhibition of CYP2E1-mediated bioactivation of procarcinogens such as NDEA and the reduced formation of covalent adducts between nucleophilic residues in DNA and active metabolites as a consequence (Vijaya Padma et al. Citation2011).
Glutathione is an important non-protein thiol molecule involved in many cellular functions, including protection against oxidative stress and detoxification of xenobiotics. The GSH steady-state level is a result of the balance between its production and consumption (Reed Citation1990). In the experiment presented here, we observed that the administration of NDEA induced an increase in the hepatic GSH concentration in rats. Similar findings have been reported by other authors (Jeena et al. Citation1999; Rajeshkumar & Kuttan Citation2000; Anis et al. Citation2001; Rajkapoor et al. Citation2005). Marinho et al. (Citation1997) reported that chronic exposure to this carcinogen caused an increase in the activities of γ-glutamyl transferase and γ-glutamylcysteine synthetase that correlated well with the increase in GSH content. Thus, on the basis of this report, our results can be interpreted as a possible adaptation mechanism. Administration of yellow tea extract enhanced NDEA-induced overcompensation of glutathione, while treatment with the extract alone caused a decrease in the hepatic glutathione pool. These diverse effects of the extract on the hepatic glutathione pool may reflect the dual antioxidant and pro-oxidant activities of polyphenols. While physiological doses of exogenous antioxidants are required to maintain or re-establish redox homeostasis, their high doses may disrupt the redox balance (Bouayed & Bohn Citation2010). Hu (Citation2011) reported that pro-oxidant activity can be demonstrated by small phenolics, such as gallic acid, which are easily oxidized and thereby mobilize cellular copper ions or decrease cellular-reduced glutathione. Gallic acid has been identified as both a pro-oxidant and an antioxidant that could cause a dose-dependent reduction of the intracellular GSH level (Verma et al. Citation2013; Chang et al. Citation2015). Furthermore, as most xenobiotics, polyphenols also undergo phase I and II metabolism. Since some polyphenols can be converted to quinones or epoxides able to form adducts with GSH (Barnes et al. Citation2011), it is possible that in the absence of pre-existing oxidative stress, unexploited tea polyphenols are metabolized using GSH. On the contrary, the increase in hepatic GSH by yellow tea in rats challenged with NDEA might be correlated with its antioxidant and free radical scavenger activity. An increase in this parameter, even above the control level, has also been reported in the literature as a response to polyphenol treatment. Gallic acid supplementation of rats treated with CCl4 caused more than a three-fold increase in hepatic GSH as compared with the control values (Tung et al. Citation2009). Similarly, green tea polyphenols have elevated glutathione levels by 26% above the control level in the livers of rats exposed to azathioprine (El-Beshbishy et al. Citation2011).
In the present study, the activities of antioxidant enzymes varied extensively in response to NDEA and/or yellow tea treatment. NDEA caused a decrease in SOD activity. This effect was corroborated by the findings of other studies that revealed the inhibitory effect of chronic administration of NDEA on hepatic antioxidant enzymes, including SOD (Zhang et al. Citation2012; Arul & Subramanian Citation2013). The decrease in SOD activity was probably due to intensive generation of ROS during NDEA metabolism, which was reflected by the high levels of TBARS, protein carbonyls and DNA fragmentation. Combined treatment of rats with yellow tea containing polyphenols with free radical scavenging properties prevented, to some extent, the decrease in SOD activity as evoked by NDEA. With regard to glutathione-dependent enzymes, we found increases in the activity of GPx and GR in the livers of rats exposed to NDEA that correlated well with the elevated content of hepatic GSH. Similar effects of chronic administration of NDEA on the activity of GR and GSH content were reported by Santos et al. (Citation2012). In our experiment, yellow tea ameliorated the NDEA-induced increase in GPx activity, which might be interpreted as a result of suppression of oxidative stress. However, we observed a further increase in GR activity in response to yellow tea administration to NDEA-challenged rats. Since GR contributes to the maintenance of the GSH pool, the positive correlation of GSH overcompensation and increased GR activity could be interpreted as a beneficial effect of yellow tea in protecting hepatic GSH homeostasis against NDEA-induced oxidative stress. Supporting this idea, Rahman (Citation2008) reported that naturally occurring dietary polyphenols can, beyond their free radical scavenging activity, also modulate signalling pathways mediated via NF-κB and MAP kinase, and upregulate genes involved in glutathione production via Nrf2 activation.
Paraoxonase (PON1) is a high-density lipoprotein (HDL)-associated enzyme synthesized in the liver which also exhibits antioxidant activity. Since the activity of PON1 is related to the provision of hepatic protection against oxidative stress (Ferré et al. Citation2002), we also studied its response to yellow tea treatment. We found that NDEA caused an increase in the activity of this enzyme. As PON1 has been reported to remove and prevent lipid peroxidation (Shen et al. Citation2012), this effect may be explained by an adaptive response of the hepatocytes to enhanced oxidative stress caused by NDEA. A similar effect has been found in rats exposed to 3,3′,4,4′,5 (PCB)-pentachlorobiphenyl inducing oxidative stress in the liver (Shen et al. Citation2012). PCB treatment caused up-regulation of PON1 gene expression and its activity in the liver (Shen et al. Citation2012). In the present experiment, the administration of yellow tea suppressed oxidative stress, as evidenced by the decreased level of lipid peroxidation, and probably resulted in the preservation of PON1 activity. Yellow tea alone significantly induced PON1 activity, and its response was similar to that of other antioxidant enzymes. However, PON1 expression is not regulated by the Nrf2-signalling pathway which is involved in the regulation of other antioxidant enzymes but rather by the arylhydrocarbon receptor (AhR) pathway. Polyphenols, including catechins, have been shown to interact directly with AhR and, therefore, to up-regulate PON1 gene expression (Noll et al. Citation2011).
Summing up, we noticed a positive correlation between the oxidative macromolecular damages and the activities of GPx, GR and PON1 as well as the GSH content that could be interpreted as a hypercompensation response to oxidative stress evoked by NDEA. Administration of yellow tea to NDEA-treated rats normalized, to some extent, the activity of these enzymes and the level of GSH. This effect could be due to the free radical scavenging activity of polyphenols which protect PON1 and GPx, and in addition preserves the GSH pool.
In conclusion, the results of both the histological and biochemical findings from the present study demonstrate that treatment with yellow tea partially protected the livers of rats from NDEA-induced injury, probably due to the tea’s antioxidant activity. Furthermore, it could be suggested that long-term multiple administration of yellow tea may provide protection against hepatocarcinogenesis caused by NDEA exposure.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Ali F, Rahul F, Naz F, Jyoti S, Siddique YH. 2014. Protective effect of apigenin against N-nitrosodiethylamine (NDEA)-induced hepatotoxicity in albino rats. Mutat Res Genet Toxicol Environ Mutagen. 767:13–20.
- Anis KV, Rajeshkumar NV, Kuttan R. 2001. Inhibition of chemical carcinogenesis by berberine in rats and mice. J Pharm Pharmacol. 53:763–768.
- Arul D, Subramanian P. 2013. Inhibitory effect of naringenin (citrus flavonone) on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Biochem Biophys Res Commun. 434:203–209.
- Bakiri L, Wagner EF. 2013. Mouse models for liver cancer. Mol Oncol. 7:206–223.
- Bansal AK, Bansal M, Soni G, Bhatnagar D. 2005. Modulation of N-nitrosodiethylamine (NDEA) induced oxidative stress by vitamin E in rat erythrocytes. Hum Exp Toxicol. 24:297–302.
- Barnes S, Prasain J, D'Alessandro T, Arabshahi A, Botting N, Lila MA, Jackson G, Janle EM, Weaver CM. 2011. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2:235–244.
- Bouayed J, Bohn T. 2010. Exogenous antioxidants – double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev. 3:228–237.
- Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. 1997. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol. Med. 23:361–366.
- Chang YJ, Hsu SL, Liu YT, Lin YH, Lin MH, Huang SJ, Ho JA, Wu LC. 2015. Gallic acid induces necroptosis via TNF-α signaling pathway in activated hepatic stellate cells. PLoS One. 10:e0120713
- Collins AR. 2004. The comet assay for DNA damage and repair: principles, applications and limitations. Mol Biotechnol. 26:249–261.
- Darvesh AS, Bishayee A. 2013. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 65:329–344.
- El-Beshbishy HA, Tork OM, El-Bab MF, Autifi MA. 2011. Antioxidant and antiapoptotic effects of green tea polyphenols against azathioprine-induced liver injury in rats. Pathophysiology. 18:125–35.
- Esmat AY, Said MM, Soliman AA, El-Masry KS, Badiea EA. 2013. Bioactive compounds, antioxidant potential, and hepatoprotective activity of sea cucumber (Holothuria atra) against thioacetamide intoxication in rats. Nutrition. 29:258–267.
- Ferk F, Chakraborty A, Jäger W, Kundi M, Bichler J, Mišík M, Wagner KH, Grasl-Kraupp B, Sagmeister S, Haidinger G, et al. 2011. Potent protection of gallic acid against DNA oxidation: results of human and animal experiments. Mutat Res. 715:61–71.
- Ferré N, Camps J, Prats E, Vilella E, Paul A, Figuera L, Joven J. 2002. Serum paraoxonase activity: a new additional test for the improved evaluation of chronic liver damage. Clin Chem. 48:261–268.
- Gopalakrishnan R, Sundaram J, Sattu K, Pandi A, Thiruvengadam D. 2013. Dietary supplementation of silymarin is associated with decreased cell proliferation, increased apoptosis, and activation of detoxification system in hepatocellular carcinoma. Mol Cell Biochem. 377:163–176.
- Gramza A, Khokhar S, Yoko S, Gliszczynska-Swiglo A, Hes M, Korczak J. 2006. Antioxidant activity of tea extracts in lipids and correlation with polyphenol content. Eur J Lipid Sci Tech. 108:351–362.
- Gramza-Michałowska A. 2007. Antioxidant potential and radical scavenging activity of different fermentation degree tea leaves extracts. Int J Tea Sci. 6:15–28.
- Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, et al. 2003. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis. 18:45–51.
- Hashimoto T, Goto M, Sakakibara H, Oi N, Okamoto M, Kanazawa K. 2007. Yellow tea is more potent than other types of tea in suppressing liver toxicity induced by carbon tetrachloride in rats. Phytother Res. 21:668–670.
- Hu ML. 2011. Dietary polyphenols as antioxidants and anticancer agents: more questions than answers. Chang Gung Med J. 34:449–460.
- IARC. 1987. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France: IARC, Vol. 17 (suppl. 7).
- Jadon A, Bhadauria M, Shukla S. 2007. Protective effect of Terminalia belerica Roxb. and gallic acid against carbon tetrachloride induced damage in albino rats. J Ethnopharmacol. 109:214–218.
- Jeena KJ, Joy KL, Kuttan R. 1999. Effect of Emblica officinalis, Phyllanthus amarus and Picrorrhiza kurroa on N-nitrosodiethylamine induced hepatocarcinogenesis. Cancer Lett. 136:11–6.
- Jodynis-Liebert J, Murias M, Błoszyk E. 2000. Effect of sesquiterpene lactones on antioxidant enzymes and some drug-metabolizing enzymes in rat liver and kidney. Planta Med. 66:199–205.
- Jurek A, Turyna B, Kubit P, Klein A. 2006. LDL susceptibility to oxidation and HDL antioxidant capacity in patients with renal failure. Clin Biochem. 39:19–27.
- Kang JS, Wanibuchi H, Morimura K, Gonzalez FJ, Fukushima S. 2007. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Res. 67:11141–11146.
- Khokhar S, Magnusdottir SG. 2002. Total phenol, catechin and caffeine contents of teas commonly consumed in the United Kingdom. J Agric Food Chem. 50:565–570.
- Lijinsky W. 1999. N-Nitroso compounds in the diet. Mutat Res. 443:129–138.
- Liu J, Lu JF, Wen XY, Kan J, Jin CH. 2015. Antioxidant and protective effect of inulin and catechin grafted inulin against CCl4-induced liver injury. Int J Biol Macromol. 72:1479–1484.
- Marinho HS, Baptista M, Pinto RE. 1997. Glutathione metabolism in hepatomous liver of rats treated with diethylnitrosamine. Biochim Biophys Acta. 1360:157–168.
- Mittal G, Brar AP, Soni G. 2006. Impact of hypercholesterolemia on toxicity of N-nitrosodiethylamine: biochemical and histopathological effects. Pharmacol Rep. 58:413–419.
- Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. 1984. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 44:5086–5091.
- Monga J, Chauhan CS, Sharma M. 2012. Chemopreventive efficacy of (+)-catechin-rich aqueous extract of Acacia catechu Willd. heartwood against 7,12-dimethylbenz[a]anthracene-induced hepatocarcinoma in Balb/c mice. J Environ Pathol Toxicol Oncol. 31:313–323.
- Noll C, Dairou J, Ripoll C, Paul JL, Dupret JM, Delabar JM, Rodrigues-Lima F, Janel N. 2011. Effect of red wine polyphenol dietary supplementation on two phase II enzymes in liver of hyperhomocysteinemic mice. Food Chem Toxicol. 49:1764–1769.
- Paula Santos N, Colaço A, Gil da Costa RM, Manuel Oliveira M, Peixoto F, Alexandra Oliveira P. 2014. N-Diethylnitrosamine mouse hepatotoxicity: time-related effects on histology and oxidative stress. Exp Toxicol Pathol. 66:429–436.
- Pradeep K, Mohan CV, Gobianand K, Karthikeyan S. 2007. Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol. 560:110–116.
- Rahman I. 2008. Dietary polyphenols mediated regulation of oxidative stress and chromatin remodeling in inflammation. Nutr Rev. 66:S42–S45.
- Rajeshkumar NV, Kuttan R. 2000. Phyllanthus amarus extract administration increases the life span of rats with hepatocellular carcinoma. J Ethnopharmacol. 73:215–219.
- Rajkapoor B, Murugesh N, Chodon D, Sakthisekaran D. 2005. Chemoprevention of N-nitrosodiethylamine induced phenobarbitol promoted liver tumors in rat by extract of Indigofera aspalathoides. Biol Pharm Bull. 28:364–366.
- Reed DJ. 1990. Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol. 30:603–631.
- Santos NP, Pereira IC, Pires MJ, Lopes C, Andrade R, Oliveira MM, Colaço A, Peixoto F, Oliveira PA. 2012. Histology, bioenergetics and oxidative stress in mouse liver exposed to N-diethylnitrosamine. In Vivo. 26:921–929.
- Sanz MJ, Ferrandiz ML, Cejudo M, Terencio MC, Gil B, Bustos G, Ubeda A, Gunasegaran R, Alcaraz MJ. 1994. Influence of a series of natural flavonoids on free radical generating systems and oxidative stress. Xenobiotica. 24:689–699.
- Sedlak J, Lindsay RH. 1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 25:192–205.
- Sevgi K, Tepe B, Sarikurkcu C. 2015. Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem Toxicol. 77:12–21.
- Shen H, Robertson LW, Ludewig G. 2012. Regulation of paraoxonase 1 (PON1) in PCB 126-exposed male Sprague Dawley rats. Toxicol Lett. 209:291–298.
- Shen T, Khor SC, Zhou F, Duan T, Xu YY, Zheng YF, Hsu S, DE Stefano J, Yang J, Xu LH. 2014. Chemoprevention by lipid-soluble tea polyphenols in diethylnitrosamine/phenobarbital-induced hepatic pre-cancerous lesions. Anticancer Res. 34:683–693.
- Steward WP, Brown K. 2013. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 109:1–7.
- Stewart B, Wild CP. (2014). World cancer report 2014. Lyon, France: International Agency for Research on Cancer.
- Tung YT, Wu JH, Huang CC, Peng HC, Chen YL, Yang SC, Chang ST. 2009. Protective effect of Acacia confusa bark extract and its active compound gallic acid against carbon tetrachloride-induced chronic liver injury in rats. Food Chem Toxicol. 47:1385–1392.
- Verma S, Singh A, Mishra A. 2013. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 35:473–485.
- Verna L, Whysner J, Williams GM. 1996. N-Nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 71:57–81.
- Vijaya Padma V, Sowmya P, Arun Felix T, Baskaran R, Poornima P. 2011. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem Toxicol. 49:991–998.
- Wang Q, Zhao X, Qian Y, Wang R. 2013. In vitro antioxidative activity of yellow tea and its in vivo preventive effect on gastric injury. Exp Ther Med. 6:423–426.
- Yiannakopoulou ECh. 2014. Green tea catechins: proposed mechanisms of action in breast cancer focusing on the interplay between survival and apoptosis. Anticancer Agents Med Chem. 14:290–295.
- Zhang CL, Zeng T, Zhao XL, Yu LH, Zhu ZP, Xie KQ. 2012. Protective effects of garlic oil on hepatocarcinoma induced by N-nitrosodiethylamine in rats. Int J Biol Sci. 8:363–374.
