Abstract
Context Coriandrum sativum L. (Apiaceae) (coriander) is an herb grown throughout the world as a culinary, medicinal or essential crop. In traditional medicine, it is used for the relief of anxiety and insomnia. Systemic hydro-alcoholic and aqueous extract from aerial parts and seeds had anxiolytic and sedative action in rodents, but little is known about its central effect in chicks.
Objective To study the effects of intracerebroventricular administration of essential oil from coriander seeds and its major component linalool on locomotor activity and emotionality of neonatal chicks.
Materials and methods The chemical composition of coriander essential oil was determined by a gas-chromatographic analysis (> 80% linalool). Behavioural effects of central administration of coriander oil and linalool (both at doses of 0.86, 8.6 and 86 μg/chick) versus saline and a sedative diazepam dose (17.5 μg/chick, standard drug) in an open field test for 10 min were observed.
Results Doses of 8.6 and 86 μg from coriander oil and linalool significantly decreased (p < 0.05) squares crossed number, attempted escapes, defecation number and distress calls, and significantly increased (p < 0.05) the sleeping posture on an open field compared with saline and were similar to the diazepam group.
Discussion and conclusion The results indicate that intracerebroventricular injection of essential oil from Coriandrum sativum seeds induced a sedative effect at 8.6 and 86 μg doses. This effect may be due to monoterpene linalool, which also induced a similar sedative effect, and, therefore, could be considered as a potential therapeutic agent similar to diazepam.
Introduction
Coriander [Coriandrum sativum L. (Apiceae)] is an herbal plant grown throughout the world as a culinary, medicinal or essential oil crop. All parts of this herb are in use as flavouring agents and/or as traditional remedies for the treatment of different disorders in the folk medicine systems (Sahib et al. Citation2013; Zheljazkov et al. Citation2014). In Persian traditional medicine, it has been used for the relief of anxiety and insomnia, the powdered dried seeds of coriander are given in a formulation to the treatment of children with frightening during sleep and awakening (Ahmad Citation2013). Similar uses of coriander seed have been indicated in other folk medicines as well (Duke et al. Citation2002).
Coriander essential oil (CEO), a very complex natural mixture of concentrated volatile compounds from aromatic plants, is extracted from coriander seeds (fruits), although the whole plant also contains other volatile oils (Zheljazkov et al. Citation2014). It is widely used in baked foods, condiments, and as a fundamental ingredient in curry mixes, creams, detergents, lotions and perfumes (Burdock & Carabin Citation2009; Sahib et al. Citation2013). It has also been demonstrated that coriander leaves and seeds, their extracts and essential oils show antimicrobial, antioxidant and anti-diabetic activities (Sahib et al. Citation2013). Besides, coriander and its oil have a long history in dietary use, with no record of harm caused by consumption of these ingredients. Therefore, the use of coriander oil is considered safe (Burdock & Carabin Citation2009).
Pharmacological studies have demonstrated that hydro-alcoholic extract prepared from coriander aerial parts significantly prolonged sleep duration without major neurotoxic effect (Rakhshandeh et al. Citation2012) and exerted an anti-anxiety effect observed in an elevated plus maze and open field (OF) test in mice (Harsha & Anilakumar Citation2012). Furthermore, different doses from aqueous extract of coriander seed systemically administered had anxiolytic action in mice (Pathan et al. Citation2011; Ravindran et al. Citation2014), and caused a dose-dependent reduction on spontaneous activity, indicating that it may have a sedative effect. Additionally, Emamghoreishi and Heidari-Hamedani (Citation2006) observed that intraperitoneal administration (i.p.) of the aqueous and hydro-alcoholic extracts from seed and CEO increased pentobarbital-induced sleeping time showing a sedative hypnotic activity in mice.
On one hand, it has been reported that the major component of CEO is the monoterpene linalool (Zheljazkov et al. Citation2014). Psychopharmacological evaluations of linalool inhalation (Linck et al. Citation2009) or intraperitoneal and intracerebroventricular (i.c.v.) administration (Elisabetsky et al. Citation1999) showed sedative and anticonvulsant properties in glutamate-related seizure models. On the other hand, it has been observed that linalool i.p. did not produced anxiolysis but modulated motor movements and locomotion (Cline et al. Citation2008). Thus, pharmacological effects of CEO may be due to the phytocomponent linalool.
In chicks, there are no published reports about the effect of coriander oil or linalool on anxiety-like behaviour, but when were included as dietary supplements it enhanced the performance and health status of broiler (Abou-Elkhair et al. Citation2014; Beier et al. Citation2014). Furthermore, little is known about central action of CEO and linalool. In the present study, we investigated the effects of i.c.v. administration of CEO, on locomotor activity and emotionality of neonatal chicks on an OF test. Additionally, we evaluated whether these behaviours are caused by the major component of the essential oil, the monoterpene linalool.
Materials and methods
Plant material
Mature seeds of Coriandrum sativum L. (Apiaceae) (coriander) were provided in December 2013 by a local producer from a commercial source in Córdoba, Argentina. They were stored in a plastic bag in a cool, dry area and protected from direct light until use. The plant material was identified and authenticated by Mr. G. M. Ruiz (Agr.), Director of Marcelino Sayago Herbarium of Facultad de Ciencias Agropecuarias, Universidad Católica de Córdoba, Córdoba, Argentina. A voucher specimen with reference number UCCOR 422 was deposited in the institutional herbarium.
Obtainment of the coriander essential oil
Mature seeds of coriander were weighed and each 150 g of the plant material was ground in a coffee grinder and immediately later were hydrodistilled for 3 h using a Clevenger-like apparatus. The CEO layer was separated from the aqueous distillate, dried over anhydrous MgSO4 and filtered to obtain 3.0 mL of pure essential oil (a yield of 2.0% v/w). The CEO was stored at −20 °C until analyzed.
Determination of the chemical composition of the coriander essential oil
Determination of chemical composition of CEO was carried out using established procedures (NIST National Institute of Standards and Technology, 2012; Pherobase, 2012). CEO (50 μL) was dissolved in 1 mL of chloroform and 1 μL of this solution was analyzed by GC-FID and GC-MS. The analyses were performed using a gas chromatograph HP 5890 Series II equipped with a manual injection port operating in a splitless mode and coupled to an HP 5970 Mass Detector. The column used was an HP-5 capillary column (30 m, 0.25 mm ID and 0.25 μm coating thickness). The analytical conditions were the following: injector: 225 °C, initial temperature: 40 °C; final temperature: 130 °C (5 min); heating rate: 2 °C/min; interface: 230 °C, gas carrier: He 99.99%; head pressure: 5 psi. The mass spectrometer was operated at 70 eV and the spectra were recorded in the range of m/z 50–550 amu in the acquisition mode “scan-full.” The data processing system used was the HP-MS ChemStation (Agilent Technologies, Santa Clara, CA) including database Wiley 275 and NIST. The volatile components were identified by comparing their mass spectra with library data (match ≥ 90) and by the determination of the respective Kovat's retention indices, (alkanes standards provided by Sigma-Aldrich SA, Buenos Aires, Argentina). The retention indices were compared with those reported in the NIST 2012 database and Pherobase 2012 (Agilent Technologies, Santa Clara, CA). The identity of the major component (Linalool) was additionally confirmed by co-elution with a standard sample (Sigma-Aldrich SA, Buenos Aires, Argentina). For the quantification of individual components, the CEO was analyzed using a Shimadzu GC-14B gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector (GC-FID), and a capillary column ZB-5 (30 m, 0.32 mm i.d. and 0.25 μm thick coat) was used for the separation of individual components. Nitrogen was employed as the carrier gas with a head pressure of 25 kPa. The temperature program was 40–130 °C at 2 °C/min, and a final hold time of 5 min, with the injector and detector being maintained at 225 °C. The sample (1 μL) was injected with a 1:100 split ratio and the quantitative composition was obtained by peak area normalization, with the response factor for each component being considered to equal one.
Animals
Day old meat-type chicks (Cobb) of both sexes were obtained immediately after hatching from a commercial hatchery INDACOR (Argentina) when they were only a few hours old. They were housed in a white wooden box (90 × 40 × 60 cm) before performing the OF test. This box was illuminated with an incandescent lamp hanging just above it and kept in a small room (3 × 3 m) at controlled temperature (30–32 °C) in a 12–12 h dark–light cycle (lights on at 7 am). Tap water and food were freely available. Daily food replenishment (Cargill, broiler BB, and 20% minimum crude protein 12.34 MJ/kg) and maintenance were performed at 13 pm.
All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and efforts were made to minimize animal suffering and to reduce the number of animals used.
Drugs and injections
Coriander oil and linalool (Sigma, St. Louis, MO) were diluted in 0.85% saline with 2% Tween 80 (v/v) (Sigma, St. Louis, MO) at doses of 0.86, 8.6 and 86 μg/chick. The different doses of coriander oil and linalool were prepared at the test day and immediately later were injected. These doses used produced no death of the birds. The control group received 0.85% saline with 2% Tween 80 (v/v) at the same volume as the treated groups. Diazepam (Sigma Chemical Co, St. Louis, MO), GABAA/benzodiazepine agonist, was used as a standard drug (positive control) and was also dissolved in 0.85% saline with 2% Tween 80 (v/v) and administered at a sedative dose of 17.5 μg/chick. The diazepam dose used in this study was determined in a separated experiment (data not shown). Intracerebroventricular injections were given freehand at a volume of 10 μl using a Hamilton syringe (Andrew Citation1991; Johnston et al. Citation1999). The depth of the injection was 2.5 mm, controlled by using a plastic sleeve on a 27-gauge needle (Kuenzel & Masson Citation1988). The needle was left inside during a period of 5 s in order to avoid reflux of the solution as well as any possible bleeding through the drilling of the epithelium and meningeal. As the chicks have soft unossified skulls, this procedure did not require an anaesthetic and could be routinely performed without any administration of analgesics (Andrew Citation1991). The i.c.v. administration have the advantages that allow dissociation of CNS effects from potentially confounding effects of systemically produced metabolites and is an ideal investigational route for in vivo brain effects, both desired and undesired (Kuo & Smith Citation2014).
Experimental design
Chicks of 4–6 d old were used in the experiments. In the experiment 1, chicks were individually gently captured and placed in a cardboard box before being taken to a separate room where injected i.c.v. with saline (n = 15), diazepam (n = 14) and CEO at doses of 0.86 μg (n = 16), 8.6 μg (n = 14) and 86 μg (n = 16), immediately then were exposed to OF during 10 min. In the experiment 2, chicks were individually gently captured and placed in a cardboard box before being taken to a separate room where injected i.c.v. with saline (n = 15), diazepam (n = 14) and linalool at doses of 0.86 μg (n = 15), 8.6 μg (n = 14) and 86 μg (n = 13), immediately then were exposed to OF during 10 min.
Open field (OF) test
The OF test has been extensively validated in the detection of anxiolytic and sedative agents such as benzodiazepines (Marin et al. Citation1997; Salvatierra & Arce Citation2001). Immediately after treatments, chicks were placed in the centre of a 60 × 60 cm OF apparatus with sides 30 cm high. This OF was made of white wood and the floor was marked off into 25 squares of 12 × 12 cm each, illuminated by a 100 W overhead bulb (Gallup & Suarez Citation1980). Chicks were individually monitored for 10 min and the following behavioural parameters were registered: number of squares crossed and attempt to escape (both indexes of ambulatory activity), number of defecations, time spent in sitting motionless with head drooped (sleeping posture) and number of vocalizations (distress calls) (Gallup & Suarez Citation1980; van Luijtelaar et al. Citation1987). A digital camera suspended 1.5 m above the centre of the apparatus recorded spontaneous activity. Distress calls were simultaneously recorded for 10 min and were counted using a computer with Audacity software (Audacity, San Antonio, TX). Immediately later, the birds were decapitated and their brains were removed and inspected in order to control the accuracy of the placement of the injection. After testing, the floor of the OF apparatus was cleaned with towels wetted with 70% ethanol. The forebrain hemispheres, such as the telencephalic structures, are neurochemically and functionally comparable with the mammalian neocortex, claustrum and pallial amygdala, in addition to other pallial areas such as the hippocampus (Reiner et al. Citation2004).
Statistical analysis
Data from OF behaviours assumed a non-normal distribution and were analyzed using the Kruskal–Wallis non-parametric tests. Whenever the test indicated significant effects (p < 0.05), a pairwise comparison (Dunńs pos hoc test) was carried out. The experimental data were expressed as the median (interquartile range).
Results
Determination of the chemical composition of coriander essential oil
The analysis by GC-FID and GC-MS showed that linalool is the major component present in the CEO and this compound represents more than 81% of total. All other compounds were found at appreciable amounts (> 1%), for example: γ-terpinene (5.7%), α-pinene (5.5%) and camphor (3.1%). The rest of the components were present at amounts ranging from 0.79% (o-cimene) to 0.18% (dodecane). These results are summarized in and .
Figure 1. Chromatogram of coriander seed essential oil. Peak number indicates different compounds (Table 1).
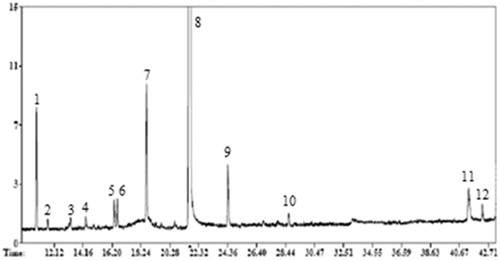
Table 1. Composition of coriander seed essential oil (CEO).
Effects of coriander essential oil intracerebroventricular administration on the open field behaviour
The Kruskal–Wallis test showed a significant effect of CEO on the number of squares crossed (H = 44.08, p < 0.0001), attempt to escape (H = 44.95, p < 0.0001), number of defecations (H = 27.3, p < 0.001) and sleeping posture (H = 44.24, p < 0.0001). Dunn’s post hoc test revealed that CEO at doses of 8.6 and 86 μg significantly decreased the squares crossed number (p < 0.001), attempt to escape (p < 0.001) and the number of defecations (p < 0.05) with respect to the saline group, and was similar to the diazepam group. Also, Dunn’s post hoc test revealed that CEO at dose of 86 μg significantly increased the sleeping posture with respect to saline (p < 0.001), and was similar to sedative diazepam dose (). The Kruskal–Wallis test also showed a significant effect of CEO doses on the distress calls (H = 40.18, p < 0.0001). Dunn’s post hoc test revealed that larger CEO doses (8.6 and 86 μg) significantly decreased the number of vocalizations (p < 0.001) with respect to saline, and were similar to sedative diazepam dose (). All the above suggest that CEO induced a sedative behaviour similar to induced it by sedative diazepam dose.
Figure 2. Effect of i.c.v. administration of CEO on the number of vocalizations on OF in 4–6-d-old chicks. Bars represent median (interquartile range). *p < 0.05 compared with saline (Dunn's post hoc test).
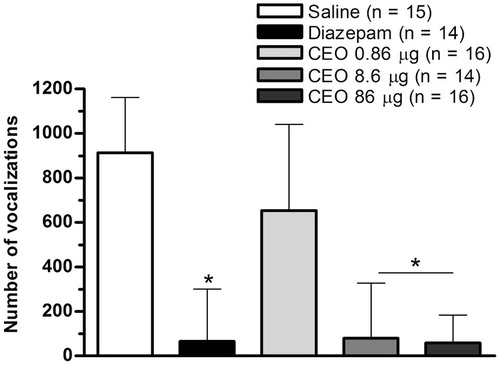
Table 2. Effect of i.c.v. CEO injection on the behavioural patterns of chicks exposed to OF test for 10 min.
Effects of linalool intracerebroventricular administration on the open field behaviour
The Kruskal–Wallis test showed a significant effect of linalool on the number of squares crossed (H = 40.44, p < 0.0001), attempt to escape (H = 43.23, p < 0.0001), number of defecations (H = 27.52, p < 0.01) and sleeping posture (H = 34.58, p < 0.0001). Dunn’s post hoc test revealed that linalool at doses of 8.6 and 86 μg significantly decreased the squares crossed number (p < 0.001), attempt to escape (p < 0.001) and number of defecations (p < 0.01) with respect to the saline group and it had similar values to the diazepam group. Furthermore, these doses significantly increased the sleeping posture (p < 0.01) with respect to the saline group and it had similar values to sedative diazepam dose (). The Kruskal–Wallis test also showed a significant effect of linalool on the distress calls (H = 37.65, p < 0.0001). Dunn’s post hoc test revealed that linalool at doses of 8.6 and 86 μg significantly decreased the number of vocalizations (p < 0.001) with respect to the saline group and it had similar values to sedative diazepam dose (). All the above suggest that linalool induced a sedative behaviour similar to induced it by sedative diazepam dose.
Figure 3. Effect of i.c.v. administration of linalool on the number of vocalizations on OF in 4–6-d-old chicks. Bars represent median (interquartile range). *p < 0.05 compared with saline (Dunn's post hoc test).
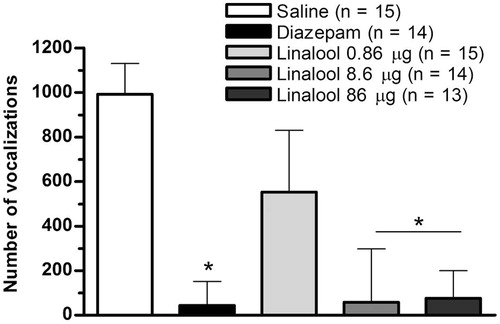
Table 3. Effect of i.c.v. linalool injection on behavioural patterns of chicks exposed to OF test for 10 min.
Discussion
In the present study, we showed for the first time that i.c.v. administration of the essential oil from Coriandrum sativum seeds induced a sedative behaviour in neonatal chicks, and this effect may be due to its major constituent, linalool.
In chicks, social separation produces a behavioural pattern associated with isolation stress, which induces an increase in movements and distress vocalizations for re-establishing social contact (Gallup & Suarez Citation1980). Thus, a higher number of squares crossed and a higher number of escape attempts can be regarded as socially motivated behaviour patterns that increase the likelihood of the isolated chick reinstating social contact (Gallup & Suarez Citation1980). It has been suggested that the suppression of the exploratory behaviour would indicate a CNS depressant activity (File & Wardill Citation1975). In relation to this, van Luijtelaar (Citation1987) observed that an increase in sitting motionless with head-dipping (sleeping posture) is related to an increased state of sedation in hens.
In addition, defecation may be used as an index of emotionality in animals; a rise in the number of defecations is related to a higher emotionality and the administration of anxiolytic drugs can reduce it (Angrini et al. Citation1998). Furthermore, it has been observed that several anxiolytic benzodiazepines reduced distress vocalizations (Watson et al. Citation1999). According to this, it has been shown that systemic administration of high doses of diazepam decreased the locomotor activity and the number of defecations (Marin et al. Citation1997) also increased the sleeping posture and decreased the distress calls, suggesting a sedative effect (Mousa & Mohammad Citation2012). In this study, we observed that central administration of diazepam at dose of 17.5 μg significantly decreased the number of squares crossed, the escape attempts, the number of defecations and the distress calls and induced a significant rise on the time spent in sleeping posture with respect to the saline group, validating use of diazepam as a standard sedative drug.
In the present work, CEO centrally injected showed sedative effects similar to diazepam. Only at 8.6 and 86 μg, doses significantly decreased the number of squares crossed, the escape attempts and the number of defecations, and significantly increased the sleeping posture with respect to the saline group. In addition, these doses induced a significant decrease on the number of vocalizations, suggesting that CEO exerted a sedative action in neonatal chicks. There are no reports about a central effect of CEO. However, some studies revealed that i.p. or oral administration of aqueous or alcoholic coriander extracts and CEO have anxiolytic effect and may have potential sedative effect in mice exposed to elevated plus-maze or dark/light arena (Emamghoreishi et al. Citation2005; Emamghoreishi & Heidari-Hamedani Citation2006; Pathan et al. Citation2011; Ravindran et al. Citation2014). Interaction among the various substances of an essential oil can modify the pharmacodynamic and pharmacokinetic properties of each one; nevertheless, studying the isolated components is helpful for a comprehensive understanding of the basis of essential oil physiological and psychopharmacological effects (Linck et al. Citation2009). In the present study, according to GC-FIS and GC-MS analyses of CEO, we identified that the major component was the monoterpene linalool (81.7%), addition to various constituents in minor quantity as γ-terpinene, α-pinene and camphor (3–6%), among others. These results were consistent with similar values reported by Gil et al. (Citation2002) for CEO obtained from seeds of varieties grown in our country. The yield of coriander seed essential oil obtained by hydrodistillation is 0.1–5.2% being mostly linalool (65–83%). The main difference reported among coriander oils was due to the presence and amount of the minor components, which may be affected by several environmental factors such as the climatic conditions and the geographic position of the growth region (Zheljazkov et al. Citation2008; Msaada et al. Citation2009; Orav et al. Citation2011). These factors also may affect the yield of coriander seed essential oil (Laribi et al. Citation2015).
After evaluating the CEO action, we focused on the investigation of the effect of central administration of linalool. Linalool at 8.6 and 86 μg doses significantly decreased the number of squares crossed, the escape attempts and the number of defecations, and induced a significant increase the sleeping posture with respect to the saline group. The number of distress vocalizations elicited by 8.6 and 86 μg doses of linalool was significantly lower than the saline group. These results suggest that linalool had a dose-dependent sedative effect in neonatal chicks as observed for CEO (8.6 and 86 μg) and diazepam (17.5 μg). It has been reported that i.p administration or inhalation of linalool had a sedative effect in rodents and humans (Kuroda et al. Citation2005; Linck et al. Citation2009), however, until now, there are no reports with similar behavioural effects in avian species.
On one hand, the mechanism of CEO and linalool sedation has not been extensively studied. Cline et al. (Citation2008) reported that linalool may modulate motor movements and locomotion but not via γ-aminobutyric acid type A (GABAA) receptor. On the other hand, in vitro assays demonstrated that linalool acts as a competitive antagonist of ionotropic receptors of the type N-methyl-d-aspartate (NMDA) and inhibits glutamate release (Elisabetsky et al. Citation1999), compatible with and relevant to the sedative effects observed, whether inhaled or otherwise administered (Linck et al. Citation2009). Additional studies should be performed to determine the site of central action of CEO and linalool, and understand the molecular mechanisms involved for future clinical applications.
Conclusion
In summary, our results show that i.c.v. administration of the essential oil of Coriandrum sativum seeds induced a sedative effect at doses of 8.6 and 86 μg in neonatal chicks. Pharmacological and behavioural responses of CEO may be due to its major component, the linalool, in it that also induced a similar sedative effect. Therefore, both could be used in clinical therapy similar to diazepam, although further studies should be carried out to determine the mechanism of sedation of linalool and other constituents of CEO.
Funding information
This work was supported by grants from CONICET (PIP 112-201001-00495) and SECyT-Universidad Nacional de Córdoba (Argentina) (PIP 307-201101-00911CB). Ana M. Vazquez gratefully acknowledges financial support from the Universidad Católica de Córdoba.
Acknowledgements
Nancy A. Salvatierra, Mariana P. Cid and Laura I. Rossi are members of CONICET. M. Soledad Gaston holds a research fellowship from CONICET.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Abou-Elkhair R, Ahmed HA, Selim S. 2014. Effects of black pepper (Piper nigrum), turmeric powder (Curcuma longa) and coriander seeds (Coriandrum sativum) and their combinations as feed additives on growth performance, carcass traits, some blood parameters and humoral immune response of broiler chickens. Asian-Australas. J Anim Sci. 27:847–854.
- Ahmad A. 2013. A study of a Persian manuscript ‘Ilajul Atfal’ written by a Deccan physician ‘Syed Fazl Ali Shifai Khan’ and its translation into English. JISHIM. 1011:68–71.
- Angrini M, Leslie JC, Shephard RA. 1998. Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol Biochem Behav. 59:387–397.
- Andrew RJ. 1991. The chick in experiment: techniques and tests. In: Andrew RJ, editor. Neural and behavioral plasticity: the use of the domestic chick as a model. Oxford: Oxford University Press. pp. 34–35.
- Beier RC, Byrd II JA, Kubena LF, Hume ME, McReynolds JL, Anderson RC, Nisbet DJ. 2014. Evaluation of linalool, a natural antimicrobial and insecticidal essential oil from basil: effects on poultry. Poult Sci. 93:267–272.
- Burdock GA, Carabin IG. 2009. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem Toxicol. 47:22–34.
- Cline M, Taylor JE, Flores J, Bracken S, McCall S, Ceremuga TE. 2008. Investigation of the anxiolytic effects of linalool, a lavender extract, in the male Sprague-Dawley rat. AANA J. 76:47–52.
- Davis JL, Masuoka DT, Gerbrandt LK, Cherkin A. 1979. Autoradiographic distribution of l-proline in chicks after intracerebral injection. Physiol Behav. 22:693–695.
- Duke JA, Bogenschutz-Godwin M, du Cellier J, Duke PK. 2002. Handbook of medicinal herbs. 2nd ed. Florida, USA: CRC Press LLC.
- Elisabetsky E, Brum LF, Souza DO. 1999. Anticonvulsant properties of linalool in glutamate-related seizure models. Phytomedicine. 6:107–113.
- Emamghoreishi M, Khasaki M, Aazam MF. 2005. Coriandrum sativum: evaluation of its anxiolytic effect in the elevated plus-maze. J Ethnopharmacol. 96:365–370.
- Emamghoreishi M, Heidari-Hamedani G. 2006. Sedative-hypnotic activity of extracts and essential oil of coriander seeds. Iran J Med Sci. 31:22–27.
- File SE, Wardill AG. 1975. Validity of head dipping as a measure of exploration in a modified hole-board. Psychopharmacologia. 44:53–59.
- Gallup GG Jr, Suarez SD. 1980. An ethological analysis of open-field behaviour in chickens. Anim Behav. 8:368–378.
- Gil A, de la Fuente EB, Lenardis AE, López Pereira M, Suárez SA, Bandoni A, Van Baren C, Di Leo Lira P, Ghersa CM. 2002. Coriander essential oil composition from two genotypes grown in different environmental conditions. J Agric Food Chem. 50:2870–2877.
- Harsha SN, Anilakumar KR. 2012. Effects of Coriandrum sativum extract on exploratory behaviour pattern and locomotor activity in mice: an experimental study. Int J Green Pharm. 6:157–162.
- Johnston ANB, Clements MP, Rose SPR. 1999. Role of brain-derived neurotrophic factor and presynaptic proteins in passive avoidance learning in day-old domestic chicks. Neuroscience. 88:1033–1042.
- Kuenzel WJ, Masson MA. 1988. A stereotaxic atlas of the brain of the chick (Gallus domesticus). Baltimore: Johns Hopkins University Press.
- Kuo A, Smith MT. 2014. Theoretical and practical applications of the intracerebroventricular route for CSF sampling and drug administration in CNS drug discovery research: a mini review. J Neurosci Methods. 233:166–171.
- Kuroda K, Inoue N, Ito Y, Kubota K, Sugimoto A, Kakuda T, Fushiki T. 2005. Sedative effects of the jasmine tea odor and (R)-(-)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur J Appl Physiol. 95:107–114.
- Laribi B, Kouki K, M'Hamdi M, Bettaieb T. 2015. Coriander (Coriandrum sativum L.) and its bioactive constituents. Fitoterapia. 103:9–26.
- Linck VM, da Silva AL, Figueiró M, Piato AL, Herrmann AP, Dupont Birck F, Caramão EB, Nunes DS, Moreno PR, Elisabetsky E. 2009. Inhaled linalool induced sedation in mice. Phytomedicine. 16:303–307.
- Marin RH, Martijena ID, Arce A. 1997. Effect of diazepam and a beta-carboline on open-field and T-maze behaviors in 2-day-old chicks. Pharmacol Biochem Behav. 58:915–921.
- Mousa YJ, Mohammad FK. 2012. Effects of hydrogen peroxide on diazepam and xylazine sedation in chicks. Interdiscip Toxicol. 5:179–183.
- Msaada K, Hosni K, Ben Taarit M, Ouchikh O, Marzouk B. 2009. Variations in essential oil composition during maturation of coriander (Coriandrum sativum l.) fruits. J Food Biochem. 33:603–612.
- Pathan AR, Kothawade KA, Logade MN. 2011. Anxiolytic and analgesic effect of seeds of Coriandrum sativum Linn. Int J Res Pharm Chem. 1:1087–1099.
- Orav A, Arak E, Raal A. 2011. Essential oil composition of Coriandrum sativum L. fruits from different countries. JEOBP. 14:118–123.
- Rakhshandeh H, Sadeghnia HR, Ghorbani A. 2012. Sleep-prolonging effect of Coriandrum sativum hydro-alcoholic extract in mice. Nat Prod Res. 26: 2095–2098.
- Ravindran A, Rai M, Manohar VR, Raveendran N, Vernon D’Souza F. 2014. Evaluation of anxiolytic activity of aqueous extract of Coriandrum sativum seeds on sub-acute administration using dark/light arena in Swiss albino mice. Int J Res Ayur Pharm. 5:123–125.
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, et al. 2004. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 473:377–414.
- Sahib NG, Anwar F, Gilani AH, Hamid AA, Saari N, Alkharfy KM. 2013. Coriander (Coriandrum sativum L.): a potential source of high-value components for functional foods and nutraceuticals – a review. Phytother Res. 27:1439–1456.
- Salvatierra NA, Arce A. 2001. Day-old chicks categorised on latency to peck, exhibit a stable fear pattern until 15 days of age. Appl Anim Behav Sci. 73:103–116.
- van Luijtelaar ELJM, van Der Grinten CPM, Blokhuis HJ, Coenen AML. 1987. Sleep in the domestic hen (Gallus domesticus). Physiol Behav. 41:409–414.
- Watson GS, Roach JT, Sufka KJ. 1999. Benzodiazepine receptor function in the chick social separation-stress procedure. Exp Clin Psychopharmacol. 7:83–89.
- Zheljazkov VD, Pickett KM, Caldwell CD, Pincock JA, Roberts JC, Mapplebeck L. 2008. Cultivar and sowing date effects on seed yield and oil composition of coriander in Atlantic Canada. Ind Crop Prod. 28:88–94.
- Zheljazkov VD, Astatkie T, Schlegel V. 2014. Hydrodistillation extraction time effect on essential oil yield, composition, and bioactivity of coriander oil. J Oleo Sci. 63:857–865.
