Abstract
Context Phillyrea latifolia L. (Oleaceae), commonly found in the Mediterranean region in Turkey, is used as medicinal teas for weight loss and hyperglycaemia in folk medicine.
Objective The study investigated the possible effects of P. latifolia leaves aqueous extract’s on weight loss and biochemical–histological changes in the rats fed a high-energy diet (HED), also isolated and determined the main phenolic compounds.
Materials and methods Twenty-four male Wistar albino rats were divided into four equal groups such as the HED group fed a HED, the PLE group given only the extract of P. latifolia leaves (220 mg/kg), the HED + PLE group administrated with the extract of leaves (220 mg/kg) after being fed with HED and a control group fed with standard pellet diet.
Results PLE administration caused a remarkable decrement of body weight in the HED + PLE group (p < 0.05). PLE showed an improved effect on structural integrity and decreased leukocyte infiltration in liver and small intestinal tissues. The blood glucose (117.3 mmol/L), leptin (5.6 ng/mL), total cholesterol (61.8 mg/dL) and LDL (9.3 mmol/L) levels were significantly increased in the HED group. PLE administration in the HED group decreased these levels. The levels of HDL (26.8 mmol/L) in the HED + PLE group were higher than both control and HED groups. Chemical composition was investigated and luteolin 7-O-glucoside and chlorogenic acid were determined for the first time in Turkish sample from the EtOAc extract of leaves.
Discussion and conclusion Phillyrea latifolia leaves may have beneficial effects on obesity related cellular problems and may become a good source of antidiabetic medication.
Introduction
Phillyrea latifolia L. (Oleaceae) commonly grows in Mediterranean region in Turkey and is known as green olive tree or mock privet, is used for weight loss and hyperglycaemia in folk medicine (Davis Citation1978; Tuzlacı & Bulut Citation2007; Ayranci & Erkan Citation2013). Obesity is one of the most important nutritional disorders in the world. It is the biggest risk factor for some major human diseases such as diabetes, cardiovascular, liver and gastrointestinal diseases, and some types of cancer. According to these, in order to treat these diseases and to lose weight, there have been so many biochemicals, pharmacognosic and histological studies (Bray et al. Citation2002; Estadella et al. Citation2004; Altunkaynak Citation2005; Choi et al. Citation2007; Pang et al. Citation2008). Increased intake of high-caloric (energy and fat) food promotes adiposity, which in turn causes an increment in body fat storages and greater body weight both in humans and in animals.
Members of Phillyrea genus are widely represented in the Mediterranean and Iberian flora, and many of them are being used in traditional medicine. Phillyrea latifolia is an evergreen sclerophyllous shrub with opposite, simple, coriaceous leaves and bluish black berries.
It has been shown that the flavonoids of P. latifolia leaves and fruits have astringent and diuretic effects, which are being used for the treatment of ulcers and oral inflammations (Díaz et al. Citation2000; Janakat & Al-Merie Citation2002). Phenylpropanoids, iridoids and triterpenoid compounds isolated from P. latifolia have anti-inflammatory and antioxidant properties (Díaz et al. Citation2000, Díaz-Lanza et al. Citation2001). Also the flavonoids isolated from methanolic extracts have an anti-inflammatory activity and show significant in vitro complement inhibiting effect (Pieroni et al. Citation2000). Boiled extract has antihepatotoxic effect against liver toxicity (Janakat & Al-Merie Citation2002). Herbal teas from the leaves of this plant are used in passing a kidney stone and infusions of the fruits and the leaves are used for internally reducing blood glucose in Anatolia, and also being utilized for weight loss in and around Istanbul (Tuzlacı & Bulut Citation2007).
This study evaluates the possible effects of aqueous extract of P. latifolia leaves on weight loss and biochemical–histological changes in the rats fed with high-energy diet (HED) and also isolates and determines the phenolic compounds from the P. latifolia leaves.
Materials and methods
Plant material
Fresh leaves of P. latifolia were collected from Ayazaga, Istanbul, on July 2009. These specimens were identified and deposited in the Herbarium of Istanbul University, Faculty of Pharmacy, ISTE No. 93420 by Prof. Dr. Emine Akalin. The leaves were dried at room temperature in shadow and were powdered for phytocemical analysis. Fresh leaves were used for biological activity.
Experimental design of biological activity
Material preparation
The fresh leaves (1.2 g) were weighed and boiled in 200 mL water for 10 min to make the aqueous extract. The extract was cooled and filtered before stored in a dark glass bottle until the day of use.
Preparation of HED
For fatty diet, high-energy chow (2.950 kcal/kg) which consisted of 10% humidity, 22% crude protein, 5.8% crude oil, 1–2% vitamins and minerals in the powder form was mixed with 60% melted cow abdominal and tail fat until it becomes homogeneous in a dough-like consistency. This dough was dried and shaped to a pellet form. It was modified from the studies of Altunkaynak (Citation2005) and others (Choi et al. Citation2007; Reuter Citation2007). Obtained chow blocks were used for feeding the HED group and HED + extract of the P. latifolia leaves (HED + PLE) group over 15 weeks.
Animal studies
Experiments were performed on 150–200 g, 6 week-old male Wistar albino rats from Istanbul University Experimental Medical Research Institute. Animal experiments were reviewed and approved by the Experimental Animal Care and Use Committee of Istanbul University (application date/no. 27.08.2009/114). The rats were housed in plastic cages, subjected to a 12 h dark and 12 h light cycle in a temperature-controlled room (22 ± 3 °C). The animals were divided into four groups each including six rats which were fed with standard pellet diet for 15 weeks as the control group, fed with high-energy diet for 15 weeks as the HED group, fed with standard pellet diet for 10 weeks, then administrated the extract of P. latifolia leaves (220 mg/kg p.o.) for 5 weeks to the P. latifolia group (PLE), and the last group rats were fed with HED for 10 weeks continued with the administration of the extract of P. latifolia leaves (220 mg/kg p.o.) for another 5 weeks (HED + PLE group).
Histological studies
At the end of the 15th week, the rats were anesthetized with pentobarbital sodium (75 mg/kg, i.p.); liver and small intestinal tissue samples were taken for histological evaluations. Samples were fixed for 24 h with 10% neutral formalin. A routine paraffin-embedding method was applied, then 4 μm thick histological sections were prepared. Subsequently, sections of liver and small intestinal tissue were examined by light microscopy after haematoxylin–eosin (HE) staining. Damage of the brush border membranes was also detected by the periodic acid-Schiff (PAS) reaction in small intestinal tissue. Images were obtained by using Kameram 390 CU Software (Mikro Sistem Ltd., Şti, Turkey).
Biochemical studies
At the end of the experiment, blood samples were withdrawn from the heart and centrifuged at 1500 g for 10 min, serums were separated and stored at −80 °C until the biochemical analysis. Serum leptin, total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were measured. Total cholesterol, HDL and LDL levels were determined using commercial kit in Abbott C8000 (Abbott, Lake Bluff, IL) autoanalyzer and the serum leptin levels were examined by enzyme-linked immunsorbent assay (ELISA), using BioVendor kit (Mouse and Rat Leptin, BioVendor, Brno, Czech Republic; Cat no. RD291001200R) and made quantitative analysis. Also blood glucose levels from tail bleeding of each rat were determined using a glucometer (Glucotech α, YD Diagnostics, Gyeonggi-do, Korea).
Statistical analysis
Data were analyzed using GraphPad Prism 5.0 (Version 5, Software Program, GraphPad Prism Inc., San Diego, CA) and presented as mean ± SEM. Results obtained in different groups were compared using one-way analysis of variance for biochemical analysis and two-way analysis of variance for body weights’ changes in all groups during experiment; when appropriate, post hoc analysis was performed with Bonferroni tests. A p < 0.05 was considered to be statistically significant.
Phytochemical study
The dried and powdered leaves (800 g) were extracted exhaustively with 96% ethanol using a Soxhlet apparatus. The extract was concentrated in a rotary evaporator. The dried residue was diluted with water and extracted by petroleum ether, toluene, chloroform and ethyl acetate (Sozer et al. Citation2006). Compound-rich ethyl acetate extract was fractioned using vacuum liquid chromatography (VLC) on silica gel (eluting with PE:CHl3:MeOH mixtures, 1:1:0 > > > > 0:0:2). The compounds were obtained using chromatotron and chromatographic methods (TLC and PTLC) and then purified on Sephadex LH-20 column (Sigma, St. Louis, MO). The isolated compounds were identified by spectroscopic methods and authentic samples (Mericli & Seyhan Citation2006).
Results
Animal studies
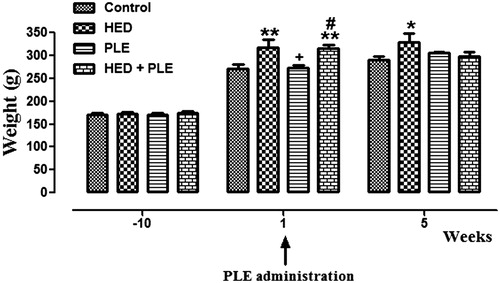
At the beginning of the experiment, body weights of animals were similar in all groups. After 10 weeks, body weights of animals in HED and HED + PLE groups had significantly increased than both controls (p < 0.01) and PLE (p < 0.05) groups. At the end of the experiment, 15th week, while the body weights of control, PLE and HED + PLE groups were close to each other, the body weight of the HED group was significantly higher (p < 0.05). According to these results, we observed that PLE administration caused a decrement of body weight in the HED group, but this decrement was not significant ().
Histological results
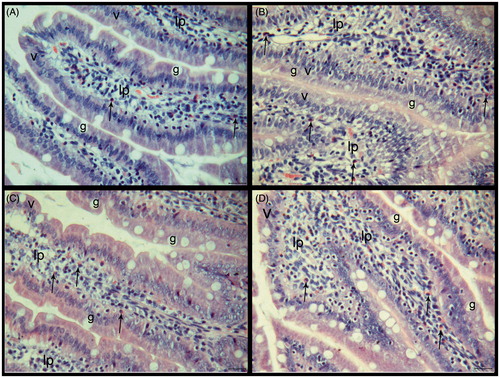
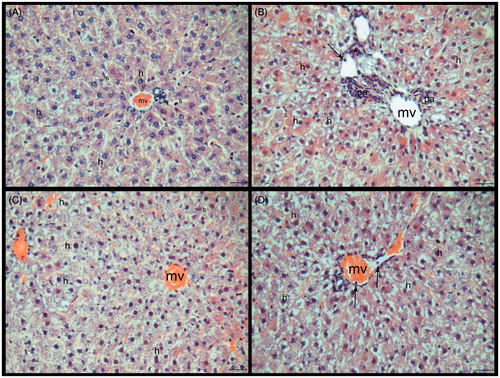
The microscopic appearance of liver tissue from the healthy controls is shown in . The livers from the HED group showed some changes. The sinusoids’ lumens, which were located around the central vein, were enlarged in the HED group at some areas. While hepatocyte cytoplasms were containing unstained zones in some areas, some of them were more stained with eosin. Endothelium of central vein and some other vessels were irregular. The leucocytes were observed in the lumen of vessels and also inflammatuar areas were seen in some sections (). The histological structure of the HED + PLE group was similar to the HED group. Additionally, hepatocytes were irregular and cell boundaries were not marked. Hepatocytes and nuclei were small in this group when compared with control and HED groups (). The endothelium was normal but sinusoids had an irregular formation in the PLE administrated group. While the transparent areas in the cytoplasm were less than the HED group, on the other hand, it was more than the control group ().
Figure 2. (A–D) Histological sections of the liver tissue of the experimental groups. (A) Control, (B) HED group, (C) HED + PLE group, and (D) PLE group. Central veins (mv), sinusoids (s), hepatocytes (h), leukocytes (→), inflammation areas (*), and portal areas (pa). HE, scale bar: 20 μm.
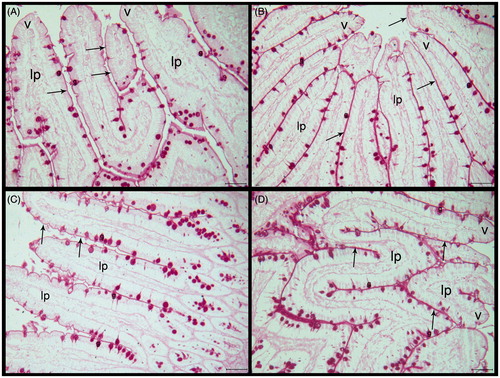
The brush border membranes were regular and continuous in the control group ( and ). The brush borders had protected their continuity and PAS reaction had been similar with the control group in the HED group. There was irregularity in the lamina proprea and there were a number of leucocytes when compared with the control group ( and ). The brush border membranes protected PAS reaction more than control and HED groups in the PLE-treated HED group but there were irregularity and leucocytes in lamina proprea. Also there were numerous cells in villus epithelium and in lamina proprea compared with the control and HED groups ( and ). Administration of PLE alone resulted in increased PAS reaction compared with all groups and the brush borders had protected their continuity ( and ).
Biochemical results
At the end of the experiment, a significant increment was observed in the blood glucose levels (p < 0.05) and leptin levels (p < 0.001) of the HED group. PLE administration in the HED group decreased these levels close to control. The total cholesterol levels were increased in the HED group compared with control (p < 0.01). The total cholesterol levels were significantly lower in PLE (p < 0.05) and HED + PLE (p < 0.01) groups, compared with the HED group. The levels of HDL were also higher in the HED + PLE group than both control (p < 0.001) and HED (p < 0.05) groups. LDL levels were significantly increased in the HED group compared with control (p < 0.05). The LDL levels were decreased after PLE administration, but this decrement was not significant ().
Table 1. Blood glucose, leptin, total cholesterol, HDL and LDL levels of all groups.
Phytochemical results
Luteolin 7-O-glucoside (122 mg) and chlorogenic acid (102 mg) were isolated as the main compounds and determined using the methods given above in phytochemical study part.
Discussion
This study demonstrated that P. latifolia, which is being used for weight loss in folk medicine, is active for losing weight and changes some biochemical and histological parameters.
At the beginning of the experiment, body weights of animals in all groups were similar. After 10 weeks, HED and HED + PLE groups had significantly higher body weights compared with control and PLE groups. At the end of the experiment, the 15th week, while the body weights of control, PLE and HED + PLE groups were close to each other, the HED group was significantly higher. According to these results, we observed that PLE administration caused a significant decrement of body weight in the HED + PLE group.
The increase in the levels of leptin, a hormone synthesized in the fat tissue, and the blood glucose of the rats fed with HED, the fact that in the PLE given groups, values are close to those of the control group, turns out to be the result of high-calorie food intake and over feeding (de Melo et al. Citation2010). Serum leptin levels increase with obesity and are in a positive correlation with body mass index, body fat percentage and fat mass. Rats fed HED become overweight and hyperleptinemic as compared with the controls (Milagro et al. Citation2009). PLE administration to the HED in the HED + PLE group resulted in close values of the increased levels of leptin on the HED group to those of the control group. So, it is thought that PLE may cause weight loss by affecting the leptin levels. Atherosclerosis is an expected outcome for the animals, fed with a high-calorie diet in the LDL, which is about fattening (Tomkin & Owens Citation2012). When the HDL values are taken into consideration, although no statistical significance is valid both in controls and in the HED group, HDL was found to have increased when PLE was given to those fed with HED. In this group, as the HDL increases, LDL decreases, PLE shows a possibility in decreasing the atheosclerosis risk taken from a high-calorie and fat-induced diet (Hennig et al. Citation2001). Serum total cholesterol increased in HED. Free cholesterol increased in both the cases. Tissue total cholesterol was raised in low- and high-fat diets in liver, duodenum, jejunum and ileum. Free cholesterol was raised in duodenum and ileum in both high- and low-fat diets while that of liver and jejunum were increased only in the low-fat group (Pugalendhi & Ramakrishnan Citation1990). Correspondingly, the total cholesterol levels have shown significant changes. The decrease of the total cholesterol in the HED + PLE group, while it increases with high-energy diet, not changing the total cholesterol in the control animal infusion, has shown that PLE has a benefit in this manner too (Janakat & Al-Merie Citation2002). These results suggest that PLE might be used for cholesterol and LDL balance in the blood, as observed.
The expansion of sinusoids on animals fed with a high-energy diet found areas with no cytoplasm eosines, irregularity in the vessel endothels, inflammation areas included leukocytes. These changes with HED and extra animal fat application caused on the vessels and cells by affecting the liver metabolism gave an impression that unmarked areas may show possible fattening. Additionally, it is possible for the leukocytes and inflammation areas to reveal themselves due to the damaged of endothelium and fat (Shoelson et al. Citation2007). Similarly, encountering leukocytes in the animals small intestinal sections showed that an inflammation has also occurred here.
The PAS reaction is applied to the small intestines in order to view the glycocalix. It was similar to the control group as in the HED group. In rats and mice, intestinal peptidase and disaccharidase activities were simultaneously stimulated with increased dietary protein, carbohydrate and fat intake (Šefčíková et al. Citation2008). Several forms of obesity in laboratory rodents were also accompanied with substantially elevated activity of intestinal disaccharidases (Mozes et al. Citation2004). Moreover, some studies revealed that long-lasting exposure of mice and rats to a high-fat diet led to obesity associated with mucosal hypertrophy, i.e. increase in the mucosal protein/DNA ratio (Estornell et al. Citation1995). That is why no effect of high-fat and energy diet on the glycolipids and glycoproteins on the surface of the cells were observed. PLE administration to animals fed with HEDs showed no differences on the cellular changes of the liver and small intestines. The increase of PAS reaction in the small intestines of the animals in this group shows thickening of glycocalix on cell surfaces. The cells which look smaller only on PLE-administrated animals and the fact of a higher PAS reaction of this group with respect to other three groups indicate a possible re-generation capability of this plant. However, further studies regarding this matter needs to be carried out.
No phytochemical investigation on P. latifolia leaves had yet been done in Turkey until now. Parallel to these bioactivity researches, the phytochemical researches also began and luteolin 7-O-glucoside and chlorogenic acid were isolated as major compounds initially. Luteolin 7-O-glucoside is a water-soluble compound.
In light of the foregoing, it is thought that the infusion obtained from the P. latifolia leaves may have a role in the fat metabolism. Researches on the active compounds extracted from the leaves of this plant will need to be improved and deepened for it to be considered as a natural source which gives hope to weight control with its role on the fat metabolism.
Conclusion
In conclusion, aqueous extract of P. latifolia leaves showed an improved effect on structural integrity and decreased leukocyte infiltration in liver and small intestinal tissues. It may have beneficial effects on obesity-related cellular problems and may become a good source of anticholesterol medication and also proliferative, regenerative an anti-inflammatuar drug.
Funding information
This work was supported by The Research Found of the University of Istanbul (Project no. 6387).
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Altunkaynak Z. 2005. Effects of high fat diet induced obesity on female rat livers (a histochemical study). Eur J Gen Med. 2:100–109.
- Ayranci E, Erkan N. 2013. Radical scavenging capacity of methanolic Phillyrea latifolia L. extract: Anthocyanin and phenolic acids composition of fruits. Molecules. 18:1798–1810.
- Bray GA, Lovejoy JC, Smith SR, DeLany JP, Lefevre M, Hwang D, Ryan DH, York DA. 2002. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr. 132:2488–2491.
- Choi H, Eo H, Park K, Jin M, Park EJ, Kim SH, Park JE, Kim S. 2007. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem Biophys Res Commun. 359:419–425.
- Davis PH. (1978). Flora of Turkey and the East Aegean Islands, vol. 6. Edinburgh: Edinburgh University Press. p. 145–158.
- De Melo CL, Queiroz MG, Fonseca SG, Bizerra AM, Lemos TL, Melo TS, Santos FA, Rao VS. 2010. Oleanolic acid, a natural triterpenoid improves blood glucose tolerance in normal mice and ameliorates visceral obesity in mice fed a high-fat diet. Chem Biol Interact. 185:59–65.
- Díaz AM, Abad MJ, Fernandez L, Recuero C, Villaescusa L, Silván AM, Bermejo P. 2000. In vitro anti-inflammatory activity of iridoids and triterpenoid compounds isolated from Phillyrea latifolia L. Biol Pharm Bull. 23:1307–1313.
- Díaz-Lanza AM, Abad-Martinez MJ, Fernández-Matellano L, Recuero Carretero C, Villaescusa Castillo L, Silván Sen AM, Bermejo Benito P. 2001. Lignan and phenylpropanoid glycosides from Phillyrea latifolia and their in vitro anti-inflammatory activity. Planta Med. 67:219–223.
- Estadella D, Oyama LM, Dâmaso AR, Ribeiro EB, Oller Do, Nascimento CM. 2004. Effect of palatable hyperlipidic diet on lipid metabolism of sedentary and exercised rats. Nutrition. 20:218–224.
- Estornell E, Cabo J, Barber T. 1995. Protein synthesis is stimulated in nutritionally obese rats. J Nutr. 125:1309–1315.
- Hennig B, Toborek M, McClain CJ. 2001. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J Am Coll Nutr. 20:97–105.
- Janakat S, Al-Merie H. 2002. Evaluation of hepatoprotective effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca. J Ethnopharmacol. 83:135–138.
- Mericli AH, Seyhan GV. 2006. Constituents of Cynara syriaca leaves. Pharm Biol. 44:643–645.
- Milagro F, Campión J, García-Díaz D, Goyeneche E, Paternain L, Martíne JA. 2009. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 65:1–9.
- Mozes S, Sefcíková Z, Lenhardt L, Racek L. 2004. Obesity and changes of alkaline phosphatase activity in the small intestine of 40- and 80-day-old rats subjected to early postnatal overfeeding or monosodium glutamate. Physiol Res. 53:177–186.
- Pang J, Choi Y, Park T. 2008. Ilex paraguariensis extract ameliorates obesity induced by high-fat diet: potential role of AMPK in the visceral adipose tissue. Arch Biochem Biophys. 476:178–85.
- Pieroni A, Pachaly P, Huang Y, Van Poel B, Vlietinck AJ. 2000. Studies on anti-complementary activity of extracts and isolated flavones from Ligustrum vulgare and Phillyrea latifolia leaves (Oleaceae). J Ethnopharmacol. 70:213–217.
- Pugalendhi KV, Ramakrishnan S. 1990. Cholesterol in serum, liver and small intestine under different dietary compositions. Indian J Exp Biol. 28:895–897.
- Reuter TY. 2007. Diet-induced models for obesity and type 2 diabetes. Drug Discov Today: Dis Models. 4:3–8.
- Šefčíková Z, Hájek T, Lenhardt Ľ, Racek L, Mozes S. 2008. Different functional responsibility of the small intestine to high-fat/high-energy diet determined the expression of obesity-prone and obesity resistant phenotypes in rat. Physiol Res. 57:467–474.
- Shoelson SE, Herrero L, Naaz A. 2007. Obesity, inflammation, and insulin resistance. Gastroenterology. 132:2169–2180.
- Sozer U, Donmez AA, Mericli AH. 2006. Constituents from the leaves of Crataegus davisii Browicz. Sci Pharm. 74:203–208.
- Tomkin GH, Owens D. 2012. LDL as a cause of atherosclerosis. Open Atherosclerosis Thrombosis J. 5:13–21.
- Tuzlacı E, Bulut G. 2007. Turkish folk medicinal plants, Part VII: Ezine (Çanakkale). J Fac Pharm Istanbul. 39:39–47.