Abstract
Context Cyclophosphamide (CTX) is used to treat different cancer types, although it causes severe hepatotoxicity due to its oxidative stress effect. Rosmarinus officinalis, L. (Lamiaceae) has a therapeutic potential against hepatotoxicity due to its antioxidant activity.
Objective The objective of this study is to investigate the phytochemical analysis of the methanol extract of Rosmarinus officianalis leaves (MEROL) and its efficacy against CTX-induced hepatotoxicity.
Materials and methods The phytochemical analyses were assessed spectrophotometericaly. To assess the MEROL efficacy, 72 Swiss albino mice were divided into six groups. Group 1 was control, groups 2 and 3 included mice which were injected intraperitoneally (i.p.) with 100 or 200 mg/kg of MEROL at days 1, 4, 7, 10, 13 and 16; group 4 was injected (i.p.) with CTX (200 mg/kg) at day 17, groups 5 and 6 were injected (i.p.) with MEROL as groups 3 and 4 followed by 200 mg/kg CTX at day 17, respectively. At day 22, six mice from each group were sacrificed and the others were sacrificed at day 37.
Results MEROL has a high content of total phenolics, saponins, total antioxidant capacity and DPPH radical scavenging activity. The median lethal dose (LD50) value of MEROL was 4.125 g/kg b.w. The inhibitory concentration 50 (IC50) value for DPPH radical scavenging was 55 μg/mL. Pretreatment with 100 mg/kg MEROL for 16 d ameliorated CTX-induced hepatotoxicity represented in lowering the levels of the aspartate aminotransferase (AST) and lipid profile and minimizing the histological damage.
Conclusions Pretreatment with 100 mg/kg b.w. MEROL mitigated CTX-induced hepatotoxicity due to its antioxidant activity.
Keywords:
Introduction
Cyclophosphamide (CTX) is not only used as anticancer drug against a variety of cancer types but also used to enhance immunotherapy and gene therapy for cancer treatment in pre-clinical studies (Berd & Mastrangelo Citation1988; Hermans et al. Citation2003; Mitrus et al. Citation2006; Salem et al. Citation2009; Young et al. Citation2013; Denies et al. Citation2014). However, earlier and recent studies have shown that the therapeutic dose of CTX could cause liver toxicity (Snover et al. Citation1989; El-Naggar et al. Citation2014). Ludeman (Citation1999) and Selvakumar et al. (2005) reported that due to the metabolic activation by cytochrome P450 mixed functional oxidase system, CTX produced two different metabolites, phosphoramide mustard and acrolein. These metabolites are responsible to induce the oxidative stress which is considered the main cause of CTX hepatotoxicity. Therefore, several studies have been performed to overcome this toxic effect through decreasing CTX-induced oxidative stress by antioxidant agents, preferably natural ones (Selvakumar et al. Citation2005; Alenzi et al. Citation2010; El-Naggar et al. Citation2015).
The use of plant extracts with high level of antioxidant constitutes has been proposed as an effective therapeutic approach against CTX toxicity. For instance, administration of Phyllanthus amarus Schum & Thonn (Euphorbiaceae), Vernonia cinerea L. Less. (Asteraceae) and Cardiospermum halicacabumcan L. (Sapindaceae) were significantly reduced the toxic side effects of CTX (Kumar & Kuttan Citation2005; Pratheeshkumar & Kuttan Citation2010). Recently, it has reported that treatment with the aqueous extract of the roots of Decalepis hamiltonii Wight & Arn (Apocynaceae) mitigated CTX-induced oxidative stress (Zarei & Shivanandappa Citation2013).
Rosemary [Rosmarinus officinalis, L. (Lamiaceae)] is a native Mediterranean small green shrub with small pale-blue flowers that blooms in late winter and early spring. Rosemary leaves contain many constituents such as volatile oil, diterpenes, triterpenes, phenolic acids, flavonoids, alkaloids, tannins, saponins, glycolic acid, glyceric acid, vitamin C, vitamin B and choline (Anadón et al. Citation2008; WHO Citation2009). Therefore, this plant has been known to have a therapeutic potential in the prevention and/or treatment of several diseases such as bronchial asthma (Inoue et al. Citation2006), peptic ulcers (Amaral et al. Citation2013), hepatotoxicity (Válenzuela et al. Citation2004), atherosclerosis (Sinkovic et al. Citation2011), inflammation diseases (Mengoni et al. Citation2011) and cancer (Válenzuela et al. Citation2004). It was also used as antibacterial agent (Wang et al. Citation2012). Moreover, rosemary leaves extract was found to contain high antioxidant activity (Inatani et al. Citation1983; Aruoma et al. Citation1992; Ahmed & Abdella Citation2010). Thus, this study was undertaken to elucidate the possible protective effect of the methanolic extract of Rosmarinus officianalis leaves (MEROL) against CTX-induced liver toxicity in male mice.
Materials and methods
Plant collection and extraction
Rosmarinus officinalis leaves were collected from the Ranger and Camel Centre 30 km from Sakaka City, Aljouf, Saudi Arabia. The plant materials were identified and authenticated by taxonomist at Camel and Range Research Center, Sakaka, Aljouf, KSA. Leaves were separated, air dried at room temperature and then grounded into powder using electrical mixer. The ground leaves (100 g) were extracted in 4 mL of 80% methanol in water bath at 60 °C for 1 h. Samples were centrifuged and the supernatant was used for the determination of secondary metabolites.
Spectrophotometrical analysis
Total concentration of phenolics in the extracts was determined using the Folin–Ciocalteu reagent with gallic acid as a standard and expressed (mg) as gallic acid equivalents per gram of extract according to Singelton et al. (Citation1999). Total flavonoids content was determined using the aluminium chloride colorimetric method with quercetin as a standard and expressed (mg) as quercetin equivalent per gram of extract according to Zhishen et al. (Citation1999). Saponins content was determined using vanillin solution and expressed (mg) as saponins equivalents per gram of extract according to Ebrahimzadah and Niknam (Citation1998). Total antioxidant capacity (TAC) was determined using phosphomolybednum method according to Prieto and Pineda (Citation1999). The antioxidant capacity was expressed as ascorbic acid equivalent. Free radical scavenging activity of the sample extracts was determined spectrophotometrically using the method of Blois (Citation1958) after obtaining crude extracts from the samples through evaporation of the solvent. The scavenging activity on the DPPH radical was expressed as inhibition percentage using the following equation:
Where Ac = Absorbance of negative control at 517 nm and As = Absorbance of sample at 517 nm (Wang & Mazza, Citation2002).
The anthocyanins content of the plant was determined according to the modified method of Padmavati et al. (Citation1997). Plant materials (100 mg) were dissolved in acidified methanol in well-closed tubes covered with aluminium foils and incubated in a refrigerator for 24 h. The absorbance was read at 530 and 657 nm. The concentration was calculated using the following equation: anthocyanin concentration (μmol/g) = ([A530 − 0.33 × A657]/31.6) × (volume [mL]/weight [g]), where A is the absorbance.
Experimental animals
A hundred male Swiss albino mice were obtained from the animal colony that was maintained at the College of Pharmacy, Aljouf University. The animals were kept at room temperature (25 ± 2 °C), controlled cycle of 12 h light/dark, standard laboratory animal feed and water were provided ad libitum. The guidelines of the animal ethics committee at Aljouf University were followed for the animal experiments. Animals were acclimatized to the experimental conditions for a period of 1 week before starting the experiment.
Determination of the LD50 of MEROL
A total number of 28 adult male mice of eight weeks old (25–27 g) were divided into seven groups of four mice each. These groups were injected with a single dose of 1, 2, 3, 4, 5, 6, and 7 g/kg of MEROL. Mice were noticed for 24 h to assess the acute toxicity of (MEROL). LD50 value of the extract was calculated using the arithmetic method of Karber as modified by Aliu and Nwude (Citation1982). The formula isWhere: LDy = Highest dose (LD100), N = Number of animals per group, Dd = Dose difference, Md = Mean dead.
MEROL and CTX doses
A total number of 72 adult male Swiss albino mice of eight weeks old (25–27 g) were divided into six groups of 12 mice each. Based on the LD50 calculation, two doses of MEROL as 100 and 200 mg/kg equal to 1/40 and 1/20 of LD50 values were selected to be injected i.p. every 3 d for 16 d, respectively. The dose of CTX was selected based on our earlier studies as 200 mg/kg b.w. (El-Naggar et al. Citation2015).
Experimental groupings
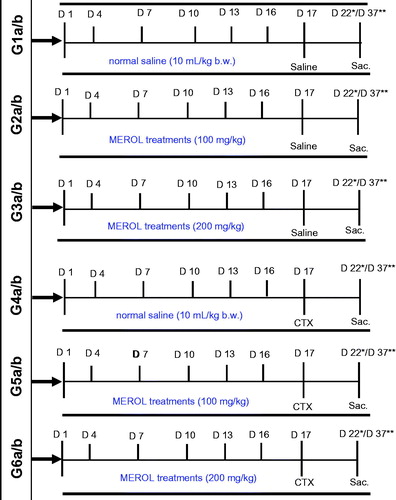
Group 1 (G1): control mice which were injected with 10 mL/kg b.w. normal saline at days 1, 4, 7, 10, 13, 16 and 17; Groups 2 and 3 (G2 & 3): mice that were injected every 3 d with 100 and 200 mg/kg b.w. MEROL for 16 d (i.e. at days 1, 4, 7, 10, 13 and 16) then followed with 10 mL/kg b.w. normal saline at day 17. Group 4 (G4): mice which were injected every 3 d with 10 mL/kg b.w. normal saline for 16 d followed with a single dose of 200 mg/kg b.w. CTX diluted in saline at day 17. Groups 5 and 6 (G5 & 6): mice that were injected every 3 d with 100 and 200 mg/kg b.w. MEROL for 16 d followed by a single dose of 200 mg/kg CTX at day 17, respectively. At day 22, half of the mice from each group (Gs 1a–6a) were sacrificed and at day 37, the other halves (Gs 1b–6b) were sacrificed to analyze the biochemical and the histopathological alterations ().
Figure 1. Diagram represents the design of the experiment. Group 1 (G1): control mice which were injected with saline, group 2 (G2): mice that were injected with 100 mg/kg b.w. MEROL, group 3 (G3): mice that were injected with 200 mg/kg b.w. MEROL, group 4 (G4): mice which were injected with saline followed with a single dose of 200 mg/kg b.w. CTX, group 5 (G4): mice that were injected with 100 mg/kg b.w. MEROL followed by a single dose of 200 mg/kg CTX, group 6 (G6): mice that were injected with 200 mg/kg b.w. MEROL followed by a single dose of 200 mg/kg CTX. *(Gs 1a–6a): groups of mice that were sacrificed at day (d22) from the starting of the experiment at (d1). **(Gs 1b–6b): groups of mice that were sacrificed at day (d37) from the starting of the experiment at (d1).
Determination of relative liver weights
Livers were dissected and dried by blotting paper and weighted on digital balance and expressed as liver weight/body weight × 100.
Estimation of biochemical parameters
For biochemical parameters, blood samples were collected from the orbital plexus in heparinized glass tubes. Serum was separated by centrifugation at 3000 rpm for 15 min. The enzymatic activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the serum samples were measured by commercial kits (Reitman & Frankel Citation1957). Total triglycerides (TG) and total cholesterol (TC) levels were assessed according to the commercial kits.
Histological investigation
Livers were immediately removed from the mice, sliced and fixed in bouin fixative for 24 h. The specimens were then dehydrated, cleared and embedded in paraffin. Serial sections of 5 μm thick were cut by a rotary microtome and processed for haematoxylin and eosin staining (Bancroft & Cook Citation1994).
Statistical analysis
Numerical data obtained from each experiment were expressed as mean ± S.D. Statistical differences between the experimental groups were assessed using one-way ANOVA. p Values less than 0.05 were considered to indicate statistical significance. Pearson's correlation was used to evaluate the correlation between TAC and DPPH radical scavenging activity from one side and the correlation between the detected metabolites and both TAC and DPPH radical scavenging activity from another side. All statistical analyses were performed using SPSS statistical version 16 software package (SPSS® Inc., Chicago, IL) and Minitab version 12.21, Minitab, Pennsylvania, PA).
Results
Phytochemical analysis of R. officinalis using spectrophotometer
Total phenolics, total flavonoids, total saponins, anthocyanins, total antioxidant capacity and DPPH radical scavenging activity were determined in MEROL. The extract showed high content of total phenolics (121 mg gallic acid equivalent/g extract), flavonoids (14 mg quercetin equivalent/g extract), saponins (53 mg saponin equivalent/g extract) and anthocyanin (0.1 μmole/g extract). MEROL showed very high DPPH free radical scavenging activities. The level of DPPH free radical scavenging activity at 100 μg/mL was 91%. The inhibitory concentration 50 (IC50) value for DPPH radical scavenging for MEROL was 55 μg/mL ().
Table 1. Phytochemical analysis, DPPH radical scavenging activities, IC50 value, and total antioxidant capacity (TAC) of R. officinalis leaves.
Correlation between DPPH radical scavenging activity, total antioxidant capacity and detected metabolites. Pearson's correlation was carried out to assess the correlation between DPPH radical scavenging activity, total antioxidant capacity and the detected secondary metabolites in MEROL. Total antioxidant capacity was found to be positively correlated with DPPH radical scavenging activity and with all detected secondary metabolites (phenolics, flavonoids, saponin and anthocyanin). DPPH free radical scavenging activity was correlated with all detected secondary metabolites except total phenolics. MEROL showed very strong DPPH radical scavenging activity and total antioxidant capacity ().
Table 2. Correlation coefficient (r) between total antioxidant capacity (TAC) and DPPH radical scavenging activity and the determined metabolites by spectrophotometer.
LD50 of the MEROL
To determine the lethal concentration of MEROL that killed 50% of mice, seven groups (four mice each) were injected with 1, 2, 3, 4, 5, 6 and 7 g/kg of MEROL. Using the arithmetic method of Karber as modified by Aliu and Nwude (Citation1982), the LD50 value was 4.125 g/kg of MEROL ().
Table 3. Determination of LD50 of MEROL on albino Swiss mice.
Pretreatment with MEROL protect the CTX-injected mice from losing weight and restore the relative liver weights into their normal values
The results showed that at either day 22 or day 37, the body weight and the relative body weight of the mice that were treated with 100 or 200 mg/kg alone for six times in 16 d (G2a, b and G3a, b), showed a non-significant change (p ≥ 0.05) compared with the mice in the control group (G1a, b), respectively. Not surprisingly, at day 22, the body weight of the mice which were injected with CTX alone (G4a) had a non-significant change (p ≥ 0.05) compared with the mice in the control group (G1a). However, at day 37, the body weight of those mice (G4b) was significantly decreased (p ≤ 0.05) compared with the mice of the control group (G1b). Interestingly, either at day 22 or day 37, the body weight of the mice that were pretreated with 100 or 200 mg/kg six times in 16 d then injected with CTX (G5a, b and G6a, b) showed a non-significant change (p ≥ 0.05) compared with the mice in the control groups (G1a, b), respectively ().
Table 4. Effect of MEROL treatment prior CTX injection on the total body and relative liver weights.
Table 5. Effect of MEROL treatment prior CTX injection on the liver function.
The results showed that at day 22, the relative liver weight of the mice that were treated with CTX (200 mg/kg) (G4a), showed a significant change (p ≤ 0.05) compared with the mice in the control group (G1a). At day 37, the relative liver weight of the mice which were injected with CTX alone (G4b) had a non-significant change (p ≥ 0.05) compared with the mice in the control group (G1b). Interestingly, either at day 22 or day 37, the body weight of the mice that were pretreated with 100 or 200 mg/kg six times in 16 d then injected with CTX (G5a, b and G6a, b) showed a non-significant change in the relative liver weights (p≥ 0.05) compared with the mice in the control groups (G1a, b), respectively ().
Treatment with 100 mg/kg of MEROL ameliorates the liver toxicity induced by CTX
The animals that were treated with 100 mg/kg MEROL only at either day 22 or day 37 (G2a, b) showed a non-significant change (p ≥ 0.05) in ALT, AST, cholesterol and triglycerides levels compared with the control group (G1a, b). Remarkably, the mice that were treated with 200 mg/kg MEROL only at day 22 (G3a) showed a significant increase (p ≤ 0.05) in ALT, cholesterol and triglycerides levels, while at day 37, those mice (G3b) showed a significant increase in AST, ALT and triglycerides levels compared with the animals in the control group (G1a, b). As expected, the levels of AST, ALT, cholesterol and triglycerides in CTX injected animals either at day 22 or day 37 (G4a, b) were significantly increased (p ≤ 0.05) compared with the control group (G1a, b).
At day 22, the animals which were pretreated with 100 mg/kg MEROL for six times in 16 d before CTX injection (G5a) showed a non-significant change (p ≥ 0.05) in AST and triglycerides levels while showed a significant increase (p ≤ 0.05) in the levels of ALT and cholesterol levels compared with the animals in the control group (G1a). Interestingly, at day 37, the animals which were pretreated with 100 mg/kg MEROL for six times in 16 d before CTX injection (G5b) showed a non-significant change (p ≥ 0.05) in the AST, cholesterol and triglycerides levels while only the ALT level showed a significant increase (p ≤ 0.05) compared with the animals in the control group (G1b).
As not expected, at days 22 and 37, the animals which were pretreated with 200 mg/kg MEROL before CTX injection (G6a, b) showed a significant increase (p ≤ 0.05) in ALT, cholesterol and triglycerides levels compared with control groups (G1a, b). At day 22, only AST level of those animals (G6a) showed a non-significant change while at day 37, the animals of (G6b) showed a significant increase (p ≤ 0.05) in its level compared with control groups (G1a, b).
Histological examination of the liver sections
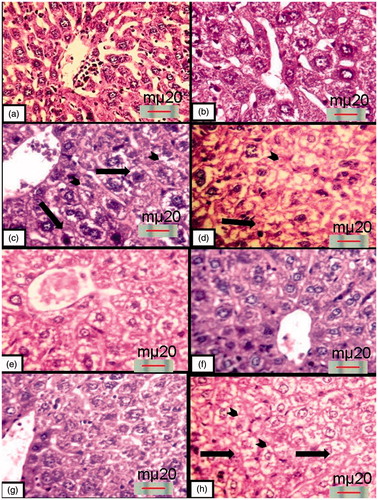
Histological examination of the liver sections of the mice that were treated with either saline, 100 or 200 mg/kg MEROL at either day 22 or 37 (G1a, b, G2a, b and G3a, b), respectively, showed normal hepatic architecture with radial arrangement of polygonal hepatocytes that contain pronounced nuclei and obvious nucleoli (). Examination of the liver sections of the mice that were treated with 200 mg/kg CTX at day 22 (G4a) showed vacuolated hepatocytes that had either pyknotic or swollen nuclei. Dilated congested central veins and sinusoidal spaces were also observed. Observation of the liver sections of the mice that were treated with 200 mg/kg CTX at day 37 (G4b) showed clearly hepatocytes arrangement disorder. It showed also wide vacuolar-degenerated hepatocytes and massive necrotic areas (). Observation of the liver sections of the mice that were treated with 100 mg/kg MEROL before CTX injection at day 22 (G5a) showed centrilobular pattern of degeneration and necrosis. Moreover, vacuolar degeneration of hepatocytes was observed. Interestingly, minimal tissue degeneration was observed in the liver sections of the mice that were treated with 100 mg/kg MEROL before CTX injection at day 37 (G5b). The histological appearance of the hepatocytes was nearly similar to that of the control groups and the tissue damage and the necrosis were of less extent (). However, examination of the liver sections of the mice that were treated with 200 mg/kg MEROL before CTX injection at day 22 (G6a) showed partial improvement in the liver architecture. Degenerative changes and vacuolar degeneration were sometimes noticed. As not expected, observation of the liver sections of the mice that were treated with 200 mg/kg MEROL before CTX injection at day 37(G6b) showed a sever centrilobular pattern of degeneration and necrosis. Moreover, wide vacuolar degeneration of hepatocytes was observed ().
Figure 2. Haematoxylin and eosin-stained liver sections: (a) and (b) control mice at days 22 and 37, respectively, showing normal hepatic architecture; (c) and (d) mice that were injected with 200 mg/kg CTX at days 22 and 37, respectively, showing vacuolar-degenerated hepatocytes with piknotic nuclei (arrows). Also, notice the coagulative necrosis of many hepatocyte (arrowheads); (e) and (f) mice that were pretreated with 100 mg/kg MEROL before CTX injection at days 22 and 37, respectively, showing that hepatocytes has partial improvement at day 22 and regained its normal architecture at day 37; (g) and (h) mice that were pretreated with 200 mg/kg MEROL before CTX injection at days 22 and 37, respectively, showing partial improvement in the hepatic architecture at 22 d while showing a hepatic degeneration at day 37 representing in the detection sever centrilobular pattern of degeneration and necrosis (arrowheads) with wide vacuolar degeneration (arrows) (haematoxylin- and eosin-stained paraffin sections; H& E ×400).
Discussion
Earlier and recent studies have reported that the therapeutic dose of CTX could cause liver toxicity (Snover et al. Citation1989; El-Naggar et al. Citation2015). In this study, we investigated the efficacy of MEROL against CTX-induced liver toxicity. Our results reported the presence of total phenolics, total flavonoids, total saponins, anthocyanins, total antioxidant capacity and DPPH radical scavenging activity in MEROL. In agreement with our findings, rosmarinic acid, diterpenoids, carotenoids and alpha-tocopherol have been documented as principal antioxidant constituents of water rosemary extract (Munne-Bosch et al. Citation1999).
Correlation of TAC with flavonoids content was in line with Hamouz et al. (Citation2011) and Abdel-Farid et al. (2014). The correlation of TAC and saponins is in accordance with many previous reports such as Lee et al. (Citation2011) and Vu et al. (Citation2013). The positive correlation between DPPH radical scavenging activity and the content of total saponins and total flavonoids was in line with our previous work on the antioxidant capacity and DPPH radical scavenging activity of some Acacia species (Abdel-Farid et al. Citation2014).
The correlation between TAC and DPPH radical scavenging activity with some detected secondary metabolites such as phenolics, flavonoids and saponins may shed the light on the importance of these compounds in antioxidant potentiality and radical scavenging activity of a plant extract. Our finding clearly revealed that MEROL has a potent antioxidant activity as previously showed by Inatani et al. (Citation1983) and Ahmed and Abdella (2010).
The study was extended to assess the dose of MEROL that killed 50% of Swiss mice after 24 h (LD50). The results showed that the acute toxicity of MEROL was > 4.5 g/kg and this finding indicates that there was no significant toxicity in albino Swiss mice due to the treatment with MEROL.
In this study, we found that the administration of CTX caused damages in mice livers due to its oxidative stress, and this finding was consistent with previous reports by Selvakumar et al. (Citation2005) and El-Naggar et al. (Citation2015). The results revealed that CTX induced marked increase in relative liver weight, serum levels of ALT and AST which is considered a marker of liver damage by CTX, this finding was in agreement with El Deib et al. (Citation2011).
To examine the biological effect of MEROL, we tested the effect of pretreatment of two doses of MEROL, 100 and 200 mg/kg b.w. on the liver toxicity induced by CTX injection. The results showed that either 5 or 20 d post-CTX injection (days 22 and 37); there was a significant decrease in the mice body weight, dramatic changes in AST, ALT, cholesterol and triglycerides levels together with a histopathological change in the liver pictures were observed. These changes due the conversion of the microsomal enzymes in the liver by CTX induction to produce two metabolites called phosphoramide mustard and acrolein (Ludeman Citation1999). These metabolites are responsible for induction of the oxidative stress (Sladek Citation1971). Our findings were in agreement with the previous studies by Rzymowska (Citation1999), Alenzi et al. (Citation2010) and El-Naggar et al. (Citation2015).
There is evidence which indicated that antioxidants could protect normal cells against CTX toxicity (Sheeja & Kuttan Citation2006; Tripathi & Jena Citation2010). Rosemary leaves extract was found to contain high antioxidant activity (Inatani et al. Citation1983; Ahmed & Abdella Citation2010). Interestingly, pretreatment of the mice with either 100 or 200 mg/kg MEROL before CTX injection could protect the mice from losing their weight. Moreover, the animals that were pretreated with 100 mg/kg MEROL before CTX injection at either day 22 or day 37 showed an improvement in AST and triglycerides levels while showed an increase in the ALT and cholesterol levels at day 22 and remarkably, only a change in ALT level at day 37. The animals that were pretreated with 200 mg/kg MEROL before CTX injection at either day 22 or day 37 showed a significant increase in ALT, cholesterol and triglycerides levels. Only AST level showed an improvement at day 22, while a significant change in its level was recorded at day 37. A previous study showed that the pretreatment of mice for 7 d with essential oil of R. officinalis followed by i.p. injection with CTX induced potential hepatoprotective activity. In agreement with this finding, Fuhrman et al. (Citation2000) reported that rosmarinic acid (derived from rosemary) inhibited LDL oxidation in a dose-dependent manner. Furthermore, in recent study, Labban et al. (Citation2014) reported that 10 g of rosmary leaves powder for 4 weeks significantly enhanced profiles in human lipid.
The histopathological pictures of the liver were matched with the previous biochemical investigation. The liver sections of the mice that were treated with 100 mg/kg MEROL before CTX injection at day 22 showed vacuolar degeneration of hepatocytes. Minimal tissue degeneration was observed in the mice that were pretreated with 100 mg/kg MEROL before CTX injection day 37 and the histological appearance of the hepatocytes was nearly similar to that of the control group. However, examination of the liver sections of the mice that were pretreated with 200 mg/kg MEROL before CTX injection at day 22 showed a partial improvement in the liver architecture while showed a sever degeneration of the hepatocytes at day 37.
Conclusions
The treatment with the 100 mg/kg b.w. MEROL every 3 d for 16 d mitigated CTX-induced liver toxicity. Therefore, it could be taken as a safe medicinal supplement during CTX chemotherapy
Funding information
This article was supported by the Aljouf University, Project no. 34/218.
Acknowledgements
The authors wish to thank Biology Department, Faculty of Science, Aljouf University, for supporting and providing the facilities to accomplish the practical work of the study.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Abdel-Farid IB, Sheded MG, Mohamed EA. 2014. Metabolomic profiling and antioxidant activity of some Acacia species. Saudi J Biol Sci. 21:400–408.
- Ahmed RR, Abdella EM. 2010. Modulatory effects of rosemary leaves aqueous extract on doxorubicin-induced histological lesions, apoptosis and oxidative stress in mice. Iran J Cancer Prev. 3:1–21.
- Alenzi FQ, El-bolkiny YE, Salem ML. 2010. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br J Biomed Sci. 67:20–28.
- Aliu YO, Nwude N. 1982. Veterinary pharmacology and toxicology experiments. Zaria: A.B.U. Press. p. 104–110.
- Amaral GP, Nelson RC, Rôulo PB. 2013. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem Toxicol. 55:48–55.
- Anadón A, Martínez-Larrañaga MR, Martìnez MA, Ares I, Garcia-Risco MR, Senorans FJ, Reglero G. 2008. Acute oral safety study of rosemary extracts in rats. J Food Prot. 71:790–795.
- Aruoma O, Haliwell B, Aeschbach R, Loligers J. 1992. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica 22:257–268.
- Bancroft JD, Cook HC. 1994. Manual of histological techniques and their diagnostic application. Edinburgh: Churchill Livingstone.
- Berd D, Mastrangelo MJ. 1988. Active immunotherapy of human melanoma exploiting the immunopotentiating effects of cyclophosphamide. Cancer Invest. 6:337–349.
- Blois MS. 1958. Antioxidant determinations by the use of astable free radical. Nature 181:1199–1200.
- Denies S, Cicchelero L, Van Audenhove I, Sanders N. 2014. Combination of interleukin-12 gene therapy, metronomic cyclophosphamide and DNA cancer vaccination directs all arms of the immune system towards tumor eradication. J Control Release. 187:175–182.
- Ebrahimzadeh H, Niknam V. 1998. A revised spectrophotometric method for determination of triterpenoid saponins. Ind Drugs. 35:379–381.
- El Deib KM, Ahmed MM, Ahmed NZ. 2011. Biochemical evaluation of the protective impact of silymarin against cyclophosphamide induced hepatotoxicity in rats. Egypt J Biochem Mol Biol. 29:291–310.
- El-Naggar SA, Alm-Eldeen AA, Germoush MO, El-Boray KF, Elgebaly HA. 2015. Ameliorative effect of propolis against cyclophosphamide induced toxicity in mice. Pharm Biol. 53:235–241.
- Fuhrman B, Volkova N, Rosenblat M, Aviram M. 2000. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid Redox Signal. 2:491–506.
- Hamouz K, Lachman J, Pazderů K, Tomasek J, Hejtmankova K, Pivec V. 2011. Differences in anthocyanin content and antioxidant activity of potato tubers with different flesh color. Plant Soil Environ. 57:478–485.
- Hermans IF, Chong TW, Palmowski MJ, Harris AL, Cerundolo V. 2003. Synergistic effect of metronomic dosing of cyclophosphamide combined with specific antitumor immunotherapy in a murine melanoma model. Cancer Res. 63:8408–8413.
- Inatani R, Nakatani N, Fuwa H. 1983. Antioxidative effect of the constituents of rosemary (Rosmarinus officinalis) and their derivatives. J Agric Biol Chem. 47:521–528.
- Inoue EI, Takano H, Shiga A, Fujita Y, Makino H, Yanagisawa R, Kato Y, Yoshikawa T. 2006. Effects of volatile constituents of rosemary extract on lung inflammation induced by diesel exhaust particles. Basic Clin Pharmacol Toxicol. 99:52–57.
- Kumar KB, Kuttan R. 2005. Chemoprotective activity of an extract of Phyllanthus amarus against cyclophosphamide induced toxicity in mice. Phytomedicine 12:494–500.
- Labban L, Mustafa U, Ibrahim YM. 2014. The effect of rosmary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid peroxidation. Int J Clinic Med. 5:297–304.
- Lee JH, Jeon JK, Kim SG, Kim SH, Chun T, Imm JY. 2011. Comparative analyses of total phenols, flavonoids, saponins and antioxidant activity in yellow soy beans and mung beans. Int J Food Sci Technol. 46:2513–2519.
- Ludeman SM. 1999. The chemistry of the metabolites of cyclophosphamide. Curr Pharm Des. 5:627–643.
- Mengoni ES, Vichera G, Rigano LA, Rodriguez-Puebla ML, Galliano SR, Cafferata EE, Pivetta OH, Moreno S, Vojnov AA. 2011. Suppression of COX-2, IL-1β and TNF-α expression and leukocyte infiltration in inflamed skin by bioactive compounds from Rosmarinus officinalis L. Fitoterapia 82:414–421.
- Mitrus I, Delić K, Wróbel N, Missol-Kolka E, Szala S. 2006. Combination of IL-12 gene therapy and CTX chemotherapy inhibits growth of primary B16(F10) melanoma tumors in mice. Acta Biochim Pol. 53:357–360.
- Munne-Bosch S, Schwarz K, Alegre L. 1999. Enhanced formation of alpha-tocopherol and highly oxidized abietane diterpenes in water-stressed rosemary plants. Plant Physiol. 121:1047–1052.
- Padmavati M, Sakthivel N, Thara TV, Reddy AR. 1997. Differential sensitivity of rice pathogens to growth inhibition by flavonoids. Phytochemistry 46:449–502.
- Pratheeshkumar P, Kuttan G. 2010. Cardiospermum halicacabum inhibits cyclophosphamide induced immunosupression and oxidative stress in mice and also regulates iNOS and COX-2 gene expression in LPS stimulated macrophages. Asian Pac J Cancer Prev. 11:1245–1252.
- Prieto P, Pineda M. 1999. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 269:337–341.
- Reitman S, Frankel S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63.
- Rzymowska J. 1999. Effect of cytotoxic chemotherapy on serum lipid levels in breast cancer patients. Pathobiology 67:129–132.
- Salem ML, Díaz-Montero CM, AL-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, Cole DJ. 2009. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist Poly(I:C). J Immunol. 182:2030–2040.
- Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P. 2005. Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by DL-alphalipoic acid. Mol Cell Biochem. 272:179–185.
- Sheeja K, Kuttan G. 2006. Ameliorating effects of Andrographis paniculata extract against cyclophosphamide-induced toxicity in mice. Asian Pac J Cancer Prev. 7:609–614.
- Singelton VR, Orthifer R, Lamuela-Raventos RM. 1999. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 299:152–178.
- Sinkovic A, Suran D, Lokar L, Fliser E, Skerget M, Novak Z, Knez Z. 2011. Rosemary extracts improve flow-mediated dilatation of the brachial artery and plasma PAI-1 activity in healthy young volunteers. Phytother Res. 25:402–407.
- Sladek N. 1971. Metabolism of cyclophosphamide by rat hepatic microsomes. Cancer Res. 1:901–908.
- Snover DC, Weisdorf S, Bloomer J, McGlave P, Weisdorf D. 1989. Nodular regenerative hyperplasia of the liver following bone marrow transplantation. Hepatology 9:443–448.
- Tripathi DN, Jena GB. 2010. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res. 696:69–80.
- Válenzuela A, Sanhueza J, Alonso P, Corbari A, Nieto S. 2004. Inhibitory action of conventional food-grade natural antioxidants and of natural antioxidants of new development on the thermal-induced oxidation of cholesterol. Int J Food Sci Nutr. 55:155–162.
- Vu OH, Hang TM, Yaguchi S, Ono Y, Phuong-Pharn TM, Yamauchi N, Shigyo M. 2013. Assessment of biochemical and antioxidant diversities in a shallot germplasm collection from Vietnam and its surrounding countries. Gen Res Crop Evol. 60:297–1312.
- Wang J, Mazza G. 2002. Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem. 50:4183–4189.
- Wang W, Li N, Luo M, Zu Y, Efferth T. 2012. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 17:2704–2713.
- World Health Organization (WHO). 2009. Monographs on selected medicinal plants. Geneva, Switzerland: WHO Press.
- Young BA, Spencer JF, Ying B, Tollefson AE, Toth K, Wold WSM. 2013. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Therapy. 20:521–530.
- Zarei M, Shivanandappa T. 2013. Amelioration of cyclophosphamide-induced hepatotoxicity by the root extract of Decalepis hamiltonii in mice. Food Chem Toxicol. 57:179–184.
- Zhishen J, Mengcheng T, Jianming W. 1999. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 64:555–559.
