Abstract
Context Landolphia owariensis P. Beauv. (Apocyanaceae) leaf is used in southeast Nigeria to treat malaria.
Objective This study evaluated the antiplasmodial activity of L. owariensis leaf extract and fractions, also the phytoconstituents were standardized and analyzed.
Methods The effects of daily, oral administrations of 200, 400 and 800 mg/kg of L. owariensis leaf extract (LOE), its hexane (LOHF), ethyl acetate (LOEF) and methanol (LOMF) fractions on early, established and residual infections in Plasmodium berghei-infected albino mice were evaluated in vivo. The extract and fractions were subjected to phytochemical analysis and HPLC fingerprinting, and the acute toxicity of LOE was evaluated.
Results The extract and fractions elicited 29–86, 18–95 and 75–96% significant (p < 0.001) suppression of parasitemia in early, established and residual infections, respectively. The ED50 values for suppressive activity of LOE, LOHF, LOEF and LOMF were 266.56, 514.93, 392.95 and 165.70 mg/kg, respectively. The post-day 30-survival index was 16.7–50, 16.7, 16.7–66.7 and 50–83.3% for LOE, LOHF, LOEF, and LOMF, respectively. Extract-treated mice significantly (p < 0.001) gained weight and had reduced mortality compared with negative control (untreated) mice. An oral LD50 value >5000 mg/kg in mice was established for LOE. The LOMF showed the greatest antiplasmodial activity in all the models, suggesting that the antimalarial activity of the plant may be attributed to alkaloids, flavonoids, saponins and tannins present in the fraction.
Conclusion Results demonstrate the antiplasmodial activity of L. owariensis leaf, and provide a pharmacological rationale for its ethnomedicinal use as an antimalarial agent.
Introduction
Malaria continues to be a threat in Africa and other regions of the world; in 2013, there were estimated 198 million cases of malaria and 584 000 deaths globally (WHO Citation2014). The burden is heaviest in Africa, where an estimated 90% of malaria results in death and in children below 5 years who account for 78% of malaria deaths (WHO Citation2014).
The search for new antimalarial drugs has become inevitable, largely due to the parasite’s resistance to current drugs including artemisinin-based combination therapy (ACT) (WHO Citation2012; Held et al. Citation2013; WHO Citation2014) and development of resistance to insecticides by the disease vector (WHO Citation2014). Researchers are currently focusing on other alternatives, including investigation of herbal remedies used to manage malaria. Antimalarial drugs such as quinine and artemisinin were derived from medicinal plants; hence exploration of medicinal plants as source of novel antimalarial drugs and lead compounds is a promising approach.
About 75% of Nigerians rely on herbal medicines to treat malaria, and one of such remedies is Landolphia owariensis P. Beauv. (Apocyanaceae) leaf. L. owariensis, commonly known as white rubber vine or vine rubber, is widely used for the treatment of many ailments. The leaf decoction is used to treat malaria and as a purgative (Burkill Citation1985). Other parts of the plants are variously used to treat fever, pains, gonorrhoea and as vermifuge (Lewis & Elvin-Lewis Citation1977; Gill Citation1992). Landolphia owariensis is also used to make native beer and beverages (Dalziel Citation1937), and serves as a natural preservative (Anthony Citation1995).
The antimicrobial (Ebi & Ofoefule Citation1997; Nwaogu et al. Citation2007), anti-inflammatory and analgesic (Owoyele et al. Citation2001), hepatoprotective (Okonkwo & Osadebe Citation2010), antioxidant (Oke & Hamburger Citation2002; Okonkwo & Osadebe Citation2013), antiulcer and gastric antisecretory (Olaleye et al. Citation2008) activities of L. owariensis have been reported.
This study was designed to evaluate the antiplasmodial activity of L. owariensis leaf using rodent models of malaria.
Materials and methods
Chemicals, reagents and drugs
All the solvents used for extraction, fractionation and high-performance liquid chromatography (HPLC) analysis were purchased from Sigma Aldrich, Darmstadt, Germany. Chloroquine sulphate was sourced from May & Baker Nigeria PLC, Ikeja, Nigeria.
Animals
Conventional grade UN-FERH:NS outbred strain of albino mice (19–29 g) of either gender bred in the Laboratory Animal Facility of the Department of Pharmacology and Toxicology, University of Nigeria, Nsukka, were used for the study. The animals were maintained ad libitum on standard pellets and water. All animal experiments were in compliance with National Institute of Health Guide for Care and Use of Laboratory Animals (Pub no. 85-23, revised 1985) and with prior permission from the National Health Research Ethics Committee (NHREC) of the University of Nigeria, with protocol ethical clearance number NHREC/05/01/2012A.
Preparation of extract
Fresh leaves of Landolphia owariensis were collected from Nsukka, Nigeria, in March. The plant was identified and authenticated by Mr. Alfred Ozioko, a taxonomist at the International Centre for Ethnomedicine and Drug Development (InterCEDD), Nsukka, where a voucher specimen was deposited (specimen no. InterCEDD/067). The leaves were cleaned, dried under shade for 7 d and pulverized to coarse powder using a milling machine. The powdered plant material (6 kg) was extracted by maceration in methanol at room temperature (28 ± 1 °C) for 48 h, and the mixture was filtered. The plant material was repeatedly washed with fresh solvent until the filtrate became clear. The filtrate was concentrated using a rotary vacuum evaporator under reduced pressure at 40 °C to obtain 220 g of the methanol extract (LOE; 3.67% w/w).
Solvent-guided fractionation of LOE
The LOE (215 g) was subjected to solvent-guided fractionation in a silica gel (70–230 mesh size) column successively eluted with n-hexane, ethyl acetate and methanol in order of increasing polarity, the eluents were concentrated using a rotary evaporator under reduced pressure at 40 °C to yield hexane (LOHF; 69.47 g; 32.31% w/w), ethyl acetate (LOEF; 45.79 g; 21.30% w/w) and methanol (LOMF; 41.09 g; 19.11% w/w) fractions, respectively. The extract and fractions were subjected to phytochemical analysis using standard procedures (Harborne Citation1973; Iwu Citation1978; Trease & Evans Citation1983).
HPLC fingerprinting
The HPLC fingerprinting of LOE, and the two most active fractions LOEF and LOMF were performed on a Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan) consisting of Ultra-Fast LC-20AB prominence equipped with a SIL-20AC auto sampler, DGU-20A3 degasser, SPD-M20AB UV diode array detector, column oven CTO-20AC, system controller CBM-20Alite (Shimadzu Corporation, Kyoto, Japan). The column used was VP-ODS 5 μm (Shimadzu Corporation, Kyoto, Japan), with a dimension of 150 × 4.6 mm. The chromatographic conditions included mobile phase: solvent A: 0.2% v/v formic acid solution; solvent B: acetonitrile. The separation was achieved with solvent A: 80% and solvent B: 20%, the column oven temperature was maintained at 40 °C, and the total run time was 35 min. The mode was isocratic with a flow rate of 0.6 mL/min; an injection volume 2 μL of 100 μg/mL solution of extract and 5 μL of 100 μg/mL solution of fraction in the mobile phase. Detection was done at UV 254 nm. Data were collected and analyzed using a Windows LC solution software (Shimadzu Corporation, Kyoto, Japan).
Acute toxicity and lethality (LD50) test
The acute toxicity and lethality (LD50) of LOE was estimated in mice as described earlier (Lorke Citation1983). The test was divided into two stages. In stage one, mice were randomly grouped (n = 3) to receive oral administrations of 10, 100 or 1000 mg/kg of LOE dissolved in distilled water, and the animals were monitored for 24 h for signs of toxicity and death. No death was recorded after 24 h. Since no death occurred in stage one, three higher doses, 1600, 2900 and 5000 mg/kg were administered to a fresh batch of animals at one dose per animal (n = 1) in stage two of the test, and the animals were observed for 24 h for signs of toxicity and death.
Pharmacological tests
Studies on antiplasmodial activity
Parasite
Chloroquine-sensitive rodent Plasmodium berghei NK65 (Plasmodiidae) obtained from National Institute for Medical Research, Lagos, Nigeria, was maintained alive by continuous intraperitoneal passage in healthy mice (Calvalho et al. Citation1991) every 5 d. The re-infected mice were kept at the Laboratory Animal Facility of the Department of Pharmacology and Toxicology, University of Nigeria, Nsukka.
Parasite inoculation
Prior to inoculation, parasitemia in the donor mouse was established by microscopic examination of Giemsa-stained thin blood smear. Subsequently, blood was collected from the tail vein of the donor mouse infected with parasites and diluted with normal saline to give a concentration of 108 parasitized erythrocytes per mL. Healthy animals were inoculated with 2 × 107 parasitized erythrocytes (i.e., 0.2 mL of 108 Plasmodium berghei parasitized erythrocytes/mL) through the intraperitoneal route (Calvalho et al. Citation1991; Basir et al. Citation2012).
Test on early infection (4-d suppressive test)
This was done as described earlier (Peters Citation1965; Knight & Peters Citation1980). Each healthy mouse was inoculated intraperitoneally as described above. They were randomly grouped (n = 6) to receive daily oral administrations of 200, 400 or 800 mg/kg of LOE, LOHF, LOEF and LOMF, respectively, for 4 d. The negative control group received the vehicle (3% Tween 80; 5 mL/kg), while the positive control group received chloroquine 5 mg/kg. On day 5 post-innoculation, thin blood film was prepared from the tail blood of each mouse. The thin films were fixed with methanol, stained with 10% Giemsa solution at pH 7.2 for 10 min (Cheesbrough Citation2004) and examined under a microscope. Parasitemia (%) was determined by counting the number of parasitized erythrocytes per 100 erythrocytes in a field under a light microscope at ×100 magnification, and the average count from four random fields was taken. Suppression of parasitemia (%) was determined using the relation:
Suppression of parasitemia (%) = 100[1 − (PT/PC)]; where PT is the parasitemia of the treated group, PC is the parasitemia of the control group.
Test on established infection (curative or Rane test)
This was done using the method described by Ryley and Peters (Citation1970). On day 1, each healthy mouse was inoculated as described above. On day 4 (72 h after inoculation), the mice were weighed and randomly grouped (n = 6) to receive daily administrations of 200, 400 or 800 mg/kg of LOE, LOHF, LOEF and LOMF, respectively, after collection of blood sample for the determination of parasitemia. The negative control group received the vehicle (5 mL/kg), while the positive control group received chloroquine 5 mg/kg. All the mice were treated orally for 4 d (days 4–7). On each day, parasitemia in each mouse was determined by thin blood smear, as earlier described. Parasitemia (%) was determined as described above. Inhibition of parasitemia (%) was calculated using the relation:
Inhibition of parasitemia (%) = 100[1 − (PT/PC)]; where PT is the parasitemia of the treated group, PC is the parasitemia of the control group.
Animals were monitored and time of death (days) recorded was used to calculate the survival time (ST) and the post-day 30 survival (%).
The ST of each group was calculated by finding the average survival time (days) post-inoculation using the relation:
The post-day 30 survival (%) was calculated using the relation: 100[a/b]; where a is the number of surviving animals after day 30, b is the number of animals in the group.
Test on residual infection (repository test)
The prophylactic activity of the extract was evaluated using the residual infection method described by Peters (Citation1965). Thirty healthy mice were randomly grouped (n = 6) to receive daily oral administrations of 200, 400 or 800 mg/kg of LOE, LOHF, LOEF and LOMF, respectively, for 4 d (days 1–4). The negative control group received the vehicle (5 mL/kg), while the positive control group received chloroquine (5 mg/kg). On day 5, all the mice were inoculated with parasite. After 72 h, parasitemia in each mouse was determined microscopically as earlier described. Inhibition of parasitemia (%) was calculated using the relation: inhibition of parasitemia (%) = 100[1 − (PT/PC)]; where PT is the parasitemia of the treated group, PC is the parasitemia of the control group.
Also each mouse was weighed on day 8 and the difference between the pre- (day 1) and post-treatment (day 8) body weights was calculated.
Statistical analysis
Data obtained were analyzed using one-way ANOVA in GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA) and subjected to Dunnett’s multiple comparison test. Results were presented as mean ± SEM, and differences between means of treated and control groups accepted significant at p < 0.001, p < 0.01 or p < 0.05.
Results
Phytochemical constituents
Preliminary phytochemical analysis showed that LOE tested positive to all the typical phytoconstituents assayed (). The LOHF gave positive reactions for resins, steroids and terpenoids, LOEF tested positive to flavonoids, resins, saponins, steroids, tannins and terpenoids, while LOMF gave positive reactions for alkaloids, flavonoids, saponins and tannins ().
Table 1. Phytochemical constituents of L. owariensis leaf.
HPLC fingerprint
Several peaks were observed in all the chromatograms which suggest the presence of many phytomolecules in the extracts.
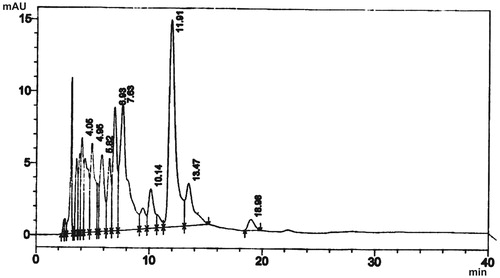
The HPLC fingerprint of LOE showed nine different components with retention time of 4.05, 4.95, 5.82, 6.93, 7.63, 10.14, 11.91, 13.47 and 18.98 min (). The major peak was at about 15 milliabsorbance unit (mAU) with a retention time of 11.91 min and the least peak at the highest retention time of 18.98 min, although less than the highest retention time of the two fractions ().
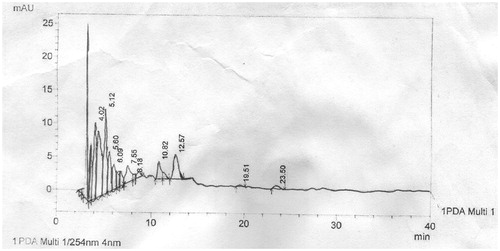
The HPLC fingerprint of LOEF showed a total of 10 components with retention time of 4.02, 5.12, 5.60, 6.09, 7.55, 8.18, 10.82, 12.57, 19.51 and 23.50 min (). This fraction gave components with the longest retention time with some components in trace amounts. The maximum peak was observed at 5.12 min ().
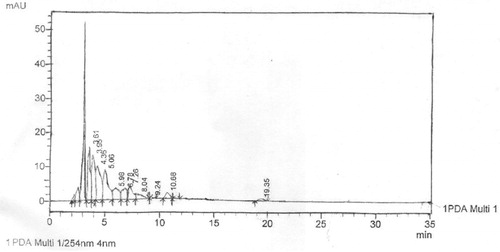
The HPLC fingerprint of LOMF revealed a total of 11 components which was the highest of the analytes with the maximum peak at 3.61 min (). This was also seen as the fastest component retained among the three samples analyzed. The other peaks were seen at 3.95, 4.36, 5.06, 5.98, 6.78, 7.26, 8.04, 9.24, 10.68 and 19.35 min ().
Acute toxicity and lethality (LD50)
Administration of LOE (10–5000 mg/kg) orally did not elicit signs of acute toxicity, and none of the animals died. The oral LD50 value of LOE in mice was thus established to be greater than 5000 mg/kg.
Effect of extract and fractions on early infection
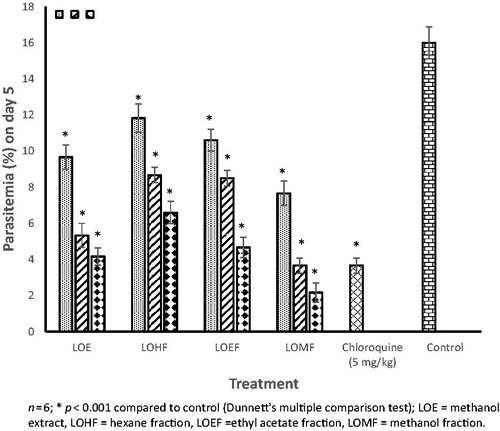
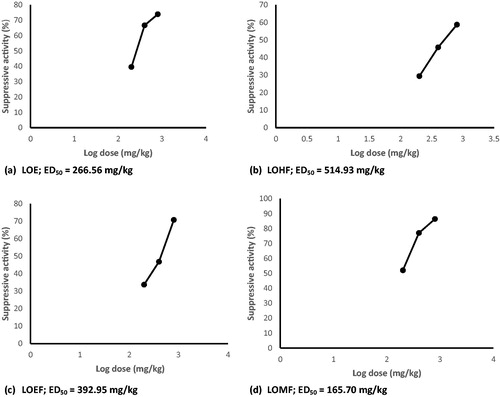
Administration of LOE and fractions elicited dose-related and significant (p < 0.001) suppressive activity in P. berghei-infected mice (). The degrees of suppression of parasitemia were 40–74, 29–59, 34–71 and 52–86% for LOE, LOHF, LOEF and LOMF, respectively. The ED50 values for suppressive activity were 266.56, 514.93, 392.95 and 165.70 mg/kg, for LOE, LOHF, LOEF and LOMF, respectively (). The magnitude of suppressive activity was of the order LOMF > LOE > LOEF > LOHF. The chemosuppression elicited by LOMF (800 mg/kg) (86.44%) was greater than that of chloroquine (77.06%).
Effect of extract and fractions on established infection
There was an increase in parasitemia level in all the mice in the negative control group; however, administration of LOE and fractions elicited dose-related and significant (p < 0.001) reduction in parasitemia (). The magnitude of inhibition of parasitemia, hence curative activity was of the order LOMF > LOE > LOEF > LOHF. The effect of LOMF was comparable with that of chloroquine.
Table 2. Curative activity of L. owariensis leaf extract and fractions in P. berghei malarial mice.
Furthermore, administration of LOE and fractions significantly (p < 0.001–0.05) increased the survival time of the mice compared with control (). Some of the extracts-treated mice survived beyond 30 d, as shown by the post-day 30 survival (%) index ().
Table 3. Effect of L. owariensis leaf extract and fractions on mortality of P. berghei malarial mice.
Effect of extract and fractions on residual infection
The LOE and fractions also elicited dose-related and significant (p < 0.001) prophylactic activity in P. berghei-infected mice (). Inhibition of parasitemia was 86–92, 75–85, 77–84 and 90–96% for LOE, LOHF, LOEF and LOMF, respectively. The magnitude of prophylactic activity as indicated by inhibition of parasitemia was of the order LOMF > LOE > LOEF > LOHF. The effect of LOMF was comparable with that of chloroquine.
Table 4. Prophylactic effect of L. owariensis leaf extract and fractions in P. berghei-infected mice.
In addition, there was significant (p < 0.001) and dose-related increase in the weight of Plasmodium-infected mice treated with the extract and fractions, compared with negative control mice that lost weight (). The increase in weight produced by the extract was of the order LOMF > LOE > LOEF > LOHF. The LOMF (800 mg/kg)-treated mice gained more weight than chloroquine-treated mice.
Discussion
Assessment of the antiplasmodial activity of L. owariensis leaf showed that the methanol extract and its fractions significantly reduced levels of parasitemia in P. berghei-infected mice, thereby demonstrating prophylactic, suppressive and curative effects.
Plasmodium berghei is one of the many species of malaria parasites that infect mammals other than humans, and one of the four species that have been described in West African rodents. Rodent malaria parasites are practical models for the experimental study of mammalian malaria, and have been demonstrated to produce malaria disease analogous to that of man and other primates in most essential aspects of structure, physiology and life cycle (Carter & Diggs Citation1977). Studies using rodent malaria parasites have contributed to man’s knowledge of the developmental biology of malaria parasites in general. Although rodent models do not produce exactly the same signs and symptoms observed in the human plasmodial infection, they have been demonstrated to produce disease features similar to those of human plasmodial infection, when infected with P. berghei (Thomas et al. Citation1998; Pierrot et al. Citation2003; Pedroni et al. Citation2006; Basir et al. Citation2012).
Antimalarials are categorized, according to the stage of the Plasmodium parasite they affect, into tissue schizontocides (act on the exoerythrocytic hepatic stage) used for casual prophylaxis or to prevent relapse, blood schizontocides (act on erythrocytic forms) used for suppressive and clinical cure, gametocytocides and sporontocides (Tracy & Webster Citation2001).
The LOE and fractions elicited dose-related and remarkably significant chemosuppression of parasitemia in early P. berghei infection, suggesting potential to elicit suppressive cure. Suppressive treatment with small doses of drugs, effective against erythrocytic stages, attempts to destroy parasites as they enter the blood stream and invade erythrocytes. It is intended to prevent parasitemia and clinical symptoms through early destruction of parasites in red blood cells. Suppressive cure is the complete elimination of malarial parasites from the body by continued treatment; the treatment course is usually longer than that used to achieve clinical cure. Suppressive treatment eliminates parasites when they leave the liver cells to invade the blood, hence any agent that provides suppressive cure will ultimately prevent the development of clinical malarial attack as the erythrocytic parasites are the ones that cause disease.
The ability of the extracts to afford suppressive cure will likely contribute to their efficacy in clinical attack, and is consistent with study results which demonstrated remarkably significant dose-related curative activity of extract and fractions. Clinical cure is achieved by interruption of erythrocytic schizogony and subsequent termination of acute clinical attacks, using larger doses of drugs. In P. berghei infection, the duration of the pre-erythrocytic stage is 48–52 h, while that of asexual erythrocytic cycle is 22–24 h (Carter & Diggs Citation1977; Killick-Kendrick Citation1978; Landau & Boulard Citation1978; Landau & Chabaud Citation1994), expectedly by 72 h post-inoculation, all infected mice had parasitemia which reduced on treatment. The ability of the extracts and fractions to evoke significant, consistent and progressive inhibition of parasitemia indicates interruption of erythrocytic schizogony and termination of clinical attacks, thereby achieving clinical cure.
In addition, the survival time after infection was dose dependently and significantly increased in treated mice compared with control animals suggesting potential to ameliorate symptoms of clinical malaria.
The extracts also significantly reduced parasitemia in the repository test, suggesting prophylactic efficacy. Prophylactic antimalarials are known to affect the tissue schizonts (exoerythrocytic stage), hence the prophylactic activity of the extract indicates inhibition of tissue schizonts and ability to prevent development of clinical malaria.
In addition, the treated mice gained weight, compared with the control mice that lost weight. Loss of body weight is one of the features of malarial mice (Langhorne et al. Citation2002), and earlier studies demonstrated a decrease in the body weight of malarial mice, compared with healthy mice (Basir et al. Citation2012); this may be secondary to loss of appetite, reduced food intake and disturbed metabolic functions associated with malaria disease. Furthermore, the persistence of malaria in the blood, especially in people who live in subtropical regions and endemic areas, leads to chronic malaria, with symptoms such as attacks of acute malaria interspersed with anaemia, weight loss or other infections (e.g. gastroenteritis) (Attwood Citation2011).
Acute toxicity and lethality (LD50) test on LOE established an oral LD50 value> 5000 mg/kg in mice, indicating a high degree of relative safety.
Preliminary phytochemical studies on the extract and fractions revealed the presence of typical phytoconstituents. For quality control and standardization, the LOE and two most active fractions, LOMF and LOEF, were separately subjected to HPLC fingerprinting and their chemical profiles were obtained as chromatograms. The results of the HPLC correlate with that of the phytochemical analysis showing the presence of many phytomolecules in the extracts. Analysis of the phytochemical constituents of LOE and fractions show that alkaloids, saponins, tannins and flavonoids abundant in the most active fraction, LOMF, may be largely responsible for the antiplasmodial effects of L. owariensis leaf. The antiplasmodial activity of various alkaloids (Saxena et al. Citation2003; Oliveira et al. Citation2009; Nasrullah et al. Citation2013), saponins (Oketch-Rabah et al. Citation1997; Traore et al. Citation2000), tannins (Asres et al. Citation2001) and flavonoids (Saxena et al. Citation2003; Batista et al. Citation2009; Zakaria et al. Citation2012) have been reported. Identification of the antiplasmodial constituent(s) of L. owariensis leaf is ongoing.
In conclusion, results of this study demonstrate the antiplasmodial activity of L. owariensis leaf and provide a pharmacological rationale for its ethnomedicinal use as antimalarial.
Disclosure statement
The authors report no conflict of interest.
References
- Anthony CB. 1995. Natural preservatives from Landolphia owariensis. Afr Dev J. 2:21–22.
- Asres K, Bucar F, Knauder E, Yardley V, Kendrick H, Croft SL. 2001. In vitro antiprotozoal activity of extract and compounds from the stem bark of Combretum molle. Phytother Res. 15:613–617.
- Attwood D. 2011. Malaria in South Sudan 2: clinical features and diagnosis. S Sudan Med J. 4:10–12.
- Basir R, Fazalul Rahiman SS, Hasballah K, Chong WC, Talib H, Yam MF, Jabbarzare M, Tie TH, Othman F, Moklas MAM, et al. 2012. Plasmodium berghei ANKA infection in ICR mice as a model of cerebral malaria. Iran J Parasitol. 7:62–74.
- Batista R, DeJesus Silva Júnior A, De Oliveira AB. 2009. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part II. Non-alkaloidal natural products. Molecules. 14:3037–3072.
- Burkill HM. 1985. The useful plants of west tropical Africa, Vol. 1. Kew, London: Royal Botanic Gardens. p. 162–185.
- Calvalho LH, Brandao MGL, Santos-Filho D, Lopes JLC, Krettli AU. 1991. Antimalarial activity of crude extracts from Brazilian plants studied in vivo in Plasmodium berghei-infected mice and in vitro against Plasmodium falciparum in culture. Braz J Med Biol Res. 24:1113–1123.
- Carter R, Diggs CL. 1977. Plasmodia of rodents. In: Kreier JP, editor Parasitic protozoa. Vol. III. New York: Academic Press. p. 359–465.
- Cheesbrough M. 2004. District laboratory practice in tropical countries. 2nd ed. Cambridge: Cambridge University Press. p. 239–258.
- Dalziel JM. 1937. The useful plants of West Tropical Africa. London: The Crown Agents for the Colonies.
- Ebi GC, Ofoefule SI. 1997. Investigations into the folkloric anti-microbial activities of Landolphia owariensis. Phytother Res. 11:149–151.
- Gill LS. 1992. Ethnomedicinal uses of plants in Nigeria. Benin City, Nigeria: UniBen Press.
- Harborne JBC. 1973. Phytochemical methods. London: Chapman and Hall.
- Held J, Kreidenweiss A, Mordmüller B. 2013. Novel approaches in antimalarial drug discovery. Expert Opin Drug Discov. 8:1325–1337.
- Iwu MM. 1978. Practical pharmacognosy manual of natural products. Vol. 2. Nsukka, Nigeria: Department of Pharmacognosy, University of Nigeria.
- Killick-Kendrick R. 1978. Taxonomy, zoography and evolution. In: Killick-Kendrick R, Peters W, editors. Rodent malaria. London: Academic Press. p. 1–52.
- Knight DJ, Peters W. 1980. The antimalarial activity of N-benzyloxydihydrotriazines. I. The activity of clociguanil (BRL 50216) against rodent malaria, and studies on its mode of action. Ann Trop Med Parasitol. 74:393–404.
- Landau I, Boulard Y. 1978. Lifecycles and morphology. In: Killick-Kendrick R, Peters W, editors. Rodent malaria. London: Academic Press. p. 53–84.
- Landau I, Chabaud A. 1994. Plasmodium species infecting Thamnomys rutilans: a zoological study. Adv Parasitol. 33:50–90.
- Langhorne J, Quin SJ, Sanni LA. 2002. Mouse models of blood-stage malaria infections: immune responses and cytokines involved in protection and pathology. In: Perlmann P, Troye-Blomberg M, editors. Malaria immunology. 2nd ed. Stockholm: Karger Publishers. p. 204–228.
- Lewis WH, Elvin-Lewis MPF. 1977. Medical botany: plants affecting man's health. New York: John Wiley & Sons.
- Lorke D. 1983. A new approach to practical acute toxicity testing. Arch Toxicol. 54:275–289.
- Nasrullah AA, Zahari A, Mohamad J, Awang K. 2013. Antiplasmodial alkaloids from the bark of Cryptocarya nigra (Lauraceae). Molecules. 18:8009–8017.
- Nwaogu LA, Alisi CS, Ibegbulem CO, Igwe CU. 2007. Phytochemical and antimicrobial activity of ethanolic extract of Landolphia owariensis leaf. Afr J Biotech. 6:890–893.
- Oke JM, Hamburger MO. 2002. Screening of some Nigerian medicinal plants for antioxidant activity using 2, 2-diphenylpicrylhydrazyl radical. Afr J Biomed Res. 5:77–79.
- Oketch-Rabah HA, Dossaji SF, Christensen SB, Frydenvang K, Lemmich E, Cornett C, Olsen CE, Chen M, Kharazmi A, Theander T. 1997. Antiprotozoal compounds from Asparagus africanus. J Nat Prod. 60:1017–1022.
- Okonkwo TJ, Osadebe PO. 2010. Hepato-protective effect of Landolphia owariensis P. Beauv. seed against CCl4-induced hepatopathy in rats. Port Harcourt Med J. 4:307–312.
- Okonkwo TJN, Osadebe PO. 2013. Isolation and characterization of potential bioactive compounds from Landolphia owariensis P. Beauv stringy seed pulp. Int J Appl Res Nat Prod. 6:28–38.
- Olaleye SB, Owoyele VB, Odukanmi AO. 2008. Antiulcer and gastric antisecretory effects of Landolphia owariensis extracts in rats. Niger J Physiol Sci. 23:23–26.
- Oliveira AB, Dolabela MF, Braga FC, Jacome RL, Varotti P, Povoa MM. 2009. Plant-derived antimalarial agents: new leads and efficient phythomedicines. Part I. Alkaloids. Acad Bras Cienc. 81:715–740.
- Owoyele BV, Olaleye SB, Oke JM, Elegbe RA. 2001. Anti-inflammatory and analgesic activities of leaf extract of Landolphia owariensis. Afr J Biomed Res. 4:131–133.
- Pedroni HC, Bettoni CC, Spalding SM, Costa TD. 2006. Plasmodium berghei: development of an irreversible experimental malaria model in Wistar rats. Exp Parasitol. 113:193–196.
- Peters W. 1965. Drug resistance in Plasmodium berghei Vincke and Lips, 1948. I. Chloroquine resistance. Exp Parasitol. 17:80–89.
- Pierrot C, Adam E, Laffite S, Godin C, Dive D, Capron M, Khalife J. 2003. Age related susceptibility and resistance to Plasmodium berghei in mice and rats. Exp Parasitol. 104:81–85.
- Ryley JF, Peters W. 1970. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol. 84:209–222.
- Saxena S, Pant N, Jain DC, Bhakuni RS. 2003. Antimalarial agents from plant sources. Curr Sci. 85:1314–1329.
- Thomas AM, Van Der Wel AM, Thomas AW, Janse CJ, Waters AP. 1998. Transfection system for animal models of malaria. Parasitol Today. 14:248–249.
- Tracy JW, Webster LT. 2001. Drugs used in the chemotherapy of protozoal infections: malaria. In: Hardman JG, Limbird LE, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill. p. 1069–1095.
- Traore F, Faure R, Ollivier E, Gasquet M, Azas N, Debrauwer L, Keita A, Timon-David P, Balansard G. 2000. Structure and antiprotozoal activity of triterpenoid saponins from Glinus oppositifolius. Planta Med. 66:368–371.
- Trease GE, Evans WC. 1983. Drugs of biological origin. In: Pharmacognosy. 12th ed. London: Balliere Tindall.
- World Health Organization. 2012. World malaria report 2012. Geneva, Switzerland: World Health Organization.
- World Health Organization. 2014. World malaria report 2014. Geneva, Switzerland: World Health Organization.
- Zakaria I, Ahmat N, Jaafar FM, Widyawaruyanti A. 2012. Flavonoids with antiplasmodial and cytotoxic activities of Macaranga triloba. Fitoterapia. 83:968–972.