Abstract
Context Plants and plant products have been used in traditional medicine as anthelmintic agents in human and veterinary medicine. Three species of Senna plant, S. alata (L), S. alexandrina (M) and S. occidentalis (L.) Link (Fabaceae) have been shown to have a vermicidal/vermifugal effect on a zoonotic tapeworm Hymenolepis diminuta (Rudolphi) (Cyclophyllidean).
Objective The present study validates the mode of action of these Senna plants on the parasite. The alcoholic leaf extract was determined to obtain information on the intracellular free calcium concentration level.
Materials and methods Hymenolepis diminuta was maintained in Sprague–Dawley rat model for 2 months. Live parasites collected from infected rat intestine were exposed to 40 mg/mL concentration of each plant extracts prepared in phosphate buffer saline at 37 °C, till parasite gets paralyzed. The rate of efflux of calcium from the parasite tissue to the medium and the level of intracellular Ca2+ concentration were determined by an atomic absorption spectroscopy.
Results This study revealed that exposure of the worms to the plant extract leads to disruption in intracellular calcium homeostasis. A significant increase (44.6% and 25%) of efflux in Ca2+ from the tissue to the incubated medium was observed. Senna alata showed high rate of efflux (5.32 mg/g) followed by S. alexandria and S. occidentalis (both 4.6 mg/g) compared with control (3.68 mg/g).
Discussion and conclusion These results suggest that leaf extracts caused membrane permeability to Ca2+ after vacuolization of the tegument under stress and the extracts may contain compound that can be used as a chemotherapeutic agent.
Introduction
A number of medicinal plants have been used in various traditional practices globally and some of them have indicated to have considerable anthelmintic activities against a wide range of helminth parasites. Senna plants are widely distributed in the tropical and subtropical countries, including India, and West Bengal in particular. It has attracted the attention of many scientists because of its usage in folklore medicine. Few of them, such as S. alata (L), S. alexandrina (M) and S. occidentalis (L) of the family Fabaceae have been shown to have vermifugal/vermicidal activity against helminth parasites. Their crude extracts caused rapid muscular contraction followed by flaccid paralysis in Heterakis gallinarum, Raillietina tetragona and Catatropis sp. (Kundu et al. Citation2014) and disruption in the tegumental architecture (vacuolization, blebbings and clumping of microthriches) in R. tetragona (Kundu & Lyndem Citation2012) and H. diminuta (Kundu et al. Citation2012, Citation2015).
Praziquantel (PZQ) drug induced paralysis, tegumental disruption and sustained muscular contraction in Schistosoma mansoni (Becker et al. Citation1980; Fetterer et al. Citation1980; Mehlhorn et al. Citation1981). These responses are attributed to disturb/disrupted calcium homeostasis and are Ca2+-dependent processes (Day et al. Citation1992; Redman et al. Citation1996). Some authors also attributed to the suppression on the rate of Na2+ influx in parasitic helminth (Pax et al. Citation1978). Ca2+ is an essential and versatile intercellular messenger of helminth parasite, which plays an important role in releasing neurotransmitter from nerve terminals and neuromuscular coordination (Katz & Miledi Citation1967). Cytoplasmic Ca2+ has been proposed as a key regulator of numerous cellular processes and probably plays one or more important role in every cell type (Redman et al. Citation1996). Measurement of the key variable, intracellular free Ca2+ concentration is always technically demanding.
Thus, it seemed desirable to ascertain whether the changes in Ca2+ homeostasis are associated with the onset of paralytic state in the zoonotic tapeworm H. diminuta during treatment with these plants in the quest to find the plausible mode of action of crude extracts.
Materials and methods
Plant material
Live parasites were collected from infected rat intestine after autopsied the animal which was inoculated with cysticercoids (larval stage of H. diminuta) maintained by routine passage through rat and the beetle intermediate host (Tribolium sp.) in the laboratory for 2 months till maturity. All experimental protocols with rat were approved by the Institutional Animal Ethics Committee (IAEC) of Visva-Bharati University. The alcoholic leaf extracts of S. alata, S. alexandrina and S. occidentalis were obtained as per the method described earlier (Kundu et al. Citation2012). In brief, fresh leaves were collected from the three plants (already identified with voucher nos. VBSL1, VBSL3 and VBSL2, kept at National Herbarium, Botanical Survey of India, Kolkata), washed and sundried. The dried leaves were crushed to powder and run in Soxhlet apparatus containing 90% ethanol for overnight. The ethanol retrieved from there was then passed in rotary evaporator until the crude extract was obtained. The reference drug Praziquantel (PZQ) was obtained from Chandrabhaghat Pharma Pvt. Ltd., Mumbai, India. About 2 g wet weight (ww) parasite (5–6 worms) were exposed to 40 mg/mL concentration of three Senna leaf crude extracts, and 5 μg/mL of PZQ prepared in 10 mL of 0.1 molar PBS (pH 7.4) dissolved in 1% dimethylsulphoxide (DMSO) at 37 ± 1 °C with simultaneously maintaining control in PBS with 1% DMSO. The 40 mg/mL concentration was determined through earlier studies as the onset of paralysis (1.68, 2.32 and 3.86 h) for S. alata, S. alexandrina and S. occidentalis showed closed range of paralytic time with PZQ (0.31 h) at 5 μg/mL of concentration. The paralyzed treated worms and control were removed for measurement of Ca2+, Na2+ and K+.
Measurement of Ca2+, Na2+ and K+ in tissues
The whole-treated and control parasite tissue were digested in 10 mL of concentrated HNO3 and H2SO4 mixture (1:1) in 250 mL conical flask for overnight at 50 °C and the digested solution was kept for 5–8 h on a hot plate at 70–80 °C to allow the acid mixture for complete evaporation, followed by a gradual addition of 10–20 mL deionized double-distilled water. The solution was filtered through Whatman filter paper (110 mm Φ) and the volume was finally made to 100 mL by adding deionized double-distilled water. The final solution was used for the analysis of Ca2+, Na2+ and K+ on the basis of using a relative standard solution (a gradual dilution of 1000 ppm solution) of CaCl2 for Ca2+, NaCl for Na2+ and KCl for K+ with an atomic absorption spectrophotometer.
Measurement of Ca2+, Na2+ and K+ in the incubation medium
The whole medium (10 mL) from each treated and control parasite was collected in tubes immediately after paralysis of worms set in. It was then centrifuged at 600g for 20 min to precipitate out the debris present, if any. The supernatant was then processed for digestion, as mentioned above (in tissue), and its final volume was made to 100 mL for further analysis of effluxes of Ca2+, Na2+ and K+ to the medium. The rate of these ions effluxed into the incubated medium by treating parasite are expressed as per mg ww tissue per hour (h) of the paralytic time and the rate of effluxed was calculated, by dividing the amount of Ca2+, Na2+ and K+ per gram dry tissue weight effluxed into the medium at the paralytic time in each leaf extract. For control, the incubation medium was calculated as 4 h from starting the experiment (since within this period paralysis of all the parasites in the treated leaf extracts have occurred).
Results
Quantitative observations of vital trace elements (calcium, sodium and potassium) in the parasite tissue declared significantly as depicted in . The cestodes treated with leaf extracts and PZQ lowered the concentration of Ca2+, Na2+ and K+ in the parasite tissue. At the basal level, control worms contain 2.6, 24 and 18.6 mg/g ww of Ca2+, Na2+ and K+, respectively. However, after treatment, Ca2+ concentration dropped to 2.3, 2.3, 2.43 and 1.65 mg/g ww treated with S. alata, S. alexandrina, S. occidentalis and PZQ, respectively. Similarly, Na2 + concentration in plant treated as above and PZQ parasite tissue were recorded as 22, 22, 22.6 and 23.1 mg/g ww, respectively, while K+ concentration was observed at 10.1, 12, 13.6 and 10 mg/g ww.
Table 1. Effects of crude leaves extract of S. alata, S. alexandrina, S. occidentalis and PZQ on the concentration of Ca2+, Na2+ and K+ in the parasite tissue and their efflux by H. diminuta into the incubation medium.
In the incubated medium, the three ionic concentrations were found to increase compared with control. The control medium showed 3.68 mg/g ww of Ca2+ concentration, while in treated medium with S. alata, S. alexandrina, S. occidentalis and PZQ, the concentration of Ca2 + was observed as 5.32, 4.6, 4.6 and 6.1 mg/g ww, respectively. Furthermore, Na2+ and K+ concentrations in control medium were 21.66 and 7.2 mg/g ww, but in the incubated-treated medium, Na2+ concentration was 24, 24.2, 23.9 and 23.4 and K+ concentration observed as 10, 8.6, 8.3 and 10.5 mg/g ww in the respective incubated-treated medium ().
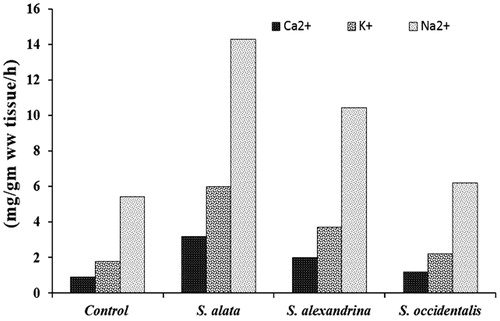
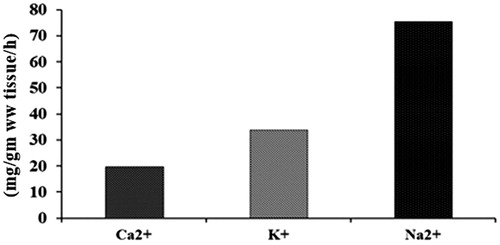
The rate of efflux of Ca2+, Na2+ and K+ from the parasite to the medium increased in S. alata (3.2, 14.3 and 6 mg/g wet wt. tissue/h) followed by S. alexandrina (2, 10.43 and 3.71 mg/g ww tissue/h) and S. occidentalis (1.2,6.2 and 2.2 mg/g ww tissue/h) compared with control (0.92, 5.42 and 1.8 mg/g ww tissue/h) () and the rate of efflux in PZQ treated parasite was 19.7, 75.48 and 33.87 mg/g ww tissue/h as depicted in .
Discussion
Our present study showed a significant decline of Ca2+, Na2+ and K+ concentrations in the parasite tissue treated with three crude leaf extract of Senna plants and PZQ compared with control. In corroboration with the decrease of Ca2+ concentration in the tissue, there was also a significant increase of Ca2+ concentration in the incubation medium, indicating thereby an increased efflux of Ca2+ into the medium during the treatments. Similar types of observations were also reported by Prichard et al. (Citation1982), Martinez et al. (Citation1992), Das et al. (Citation2006), and Dasgupta et al. (Citation2013) in helminth parasites. Simultaneously, our results also showed an increase of Na2+ and K+ concentration in the medium as also observed by Lalchhandama et al. (Citation2007) in R. echinobothrida. These findings could attribute to the action of leaf extracts on membrane permeability to Ca2+ after vacuolization of the tegument and the relation between the latter and the concentration of Ca2+ has been well demonstrated by Bricker et al. (Citation1983) on trematode parasites. These functional alterations in the membrane were also identified due to the effect of PZQ on voltage-gated and ligand-gated calcium channels, pumps and transporters as they are important entry for extracellular Ca2+ and regulating the levels of intracellular calcium (Andrews Citation1985; Kohn et al. Citation2001, Citation2003a,Citationb; Greenberg Citation2005). Another study was also reported by Apinhasmit and Sobhon (Citation1996) in depolymerization of the microtrabecular network through the induction of Ca2+ influx by PZQ that led to vacuolization, swelling, blebbing, tegumental disorganization and breakdown of myofilaments in the muscle cells.
The present study also showed significant decrease of Na2+ and K+ concentration in the treated parasitic tissues after treatment with three leaf extracts and PZQ, while there is a rise of these two ionic concentrations in the treated media. These observations were also reported by Das et al. (Citation2006). This may be due to the fact that all these trace elements which are responsible for muscular contraction may have followed the same pattern. Our findings strongly suggest that the alteration of the intracellular Ca2+, Na2+ and K+ homeostasis of these parasites is a promising strategy as a drug target.
Funding information
The authors’ gratefully acknowledge the University Grants Commission (UGC), New Delhi, for providing financial assistance through a major research project (No. UGC/SR/40-385/2011) sanctioned to Larisha M. Lyndem
Acknowledgements
The authors wish to thank the Department of Zoology, Center for Advanced Studies, Visva-Bharati for providing infrastructural support.
Disclosure statement
The authors report that they have no conflicts of interest.
References
- Andrews P. 1985. Praziquantel: mechanisms of anti-schistosomal activity. Pharmacol Ther. 29:129–156.
- Apinhasmit W, Sobhon P. 1996. Opisthorchis viverrini: effect of praziquantel on the adult tegument. Southeast Asian J Trop Med Public Health. 27:304–311.
- Becker B, Mehlhorn H, Andrews P, Thomas H, Eckert J. 1980. Light and electron microscopic studies on the effect of praziquantel on Schistosoma mansoni, Dicrocoelium dendriticum, and Fasciola hepatica (Trematoda) in vitro. Z Parasitenkd. 63:113–128.
- Bricker CS, Depenbusch JW, Bennett JL, Thompson DP. 1983. The relationship between tegumental disruption and muscle contraction in Schistosoma mansoni exposed to various compounds. Z Parasitenkd. 69:61–71.
- Das B, Tandon V, Saha N. 2006. Effect of isoflavone from Flemingia vestita (Fabaceae) on the Ca2+ homeostasis in Raillietina echinobothrida, the cestode of domestic fowl. Parasitol Int. 55:17–21.
- Dasgupta S, Roy B, Venkataswamy M, Giri BR. 2013. Effects of Acacia oxyphylla and Securinega virosa on functional characteristics of Raillietina echinobothrida (Phylum: Platyhelminthes; Class: Cestoidea), a poultry cestode parasite. J Parasit Dis. 37:125–130.
- Day TA, Bennett JL, Pax RA. 1992. Praziquantel: the enigmatic antiparasitic. Parasitol Today (Regul. Ed.). 8:342–344.
- Fetterer RH, Pax RA, Bennett JL. 1980. Praziquantel, potassium and 2,4-dinitrophenol: analysis of their action on the musculature of Schistosoma mansoni. Eur J Pharmacol. 64:31–38.
- Greenberg RM. 2005. Are Ca2+ channels targets of praziquantel action. Int J Parasitol. 35:1–9.
- Katz B, Miledi R. 1967. The timing of calcium action during neuromuscular transmission. J Physiol (Lond.). 189:535–544.
- Kohn AB, Anderson PA, Roberts-Misterly JM, Greenberg RM. 2001. Schistosome calcium channel beta subunits. Unusual modulatory effects and potential role in the action of the antischistosomal drug praziquantel. J Biol Chem. 276:36873–36876.
- Kohn AB, Roberts-Misterly JM, Anderson PA, Greenberg RM. 2003a. Creation by mutagenesis of a mammalian Ca(2+) channel beta subunit that confers praziquantel sensitivity to a mammalian Ca(2+) channel. Int J Parasitol. 33:1303–1308.
- Kohn AB, Roberts-Misterly JM, Anderson PA, Khan M, Greenberg RM. 2003b. Specific sites in the beta interaction domain of a Schistosome Ca2+ channel beta subunit are key to its role in sensitivity to the antischistosomal drug praziquantel. Parasitology 127:349–356.
- Kundu S, Roy S, Nandi S, Ukil B, Lyndem LM 2012. In vitro screening for cestocidal activity of three species of Cassia plants against the tapeworm Raillietina tetragona. J Helminthol. 87:154–159.
- Kundu S, Roy S, Lyndem LM. 2012. Cassia alata L.: potential role as anthelmintic agent against Hymenolepis diminuta. Parasitol Res. 111:1187–1192.
- Kundu S, Roy S, Lyndem LM. 2014. Broad spectrum anthelmintic potential of Cassia plants. Asian Pac J Trop Biomed. 4:S436–S441.
- Kundu S, Roy S, Nandi S, Ukil B, Lyndem LM. 2015. In vitro anthelmintic effects of Senna occidentalis (L.) link (Leguminosae) on rat tapeworm Hymenolepis diminuta. Int J Pharm PharmSci. 7:268–271.
- Lalchhandama K, Roy B, Kumar BD. 2007. In vitro anthelmintic activity of Acacia oxyphylla: changes in the levels of trace elements and activities of the tegumental enzymes of the cestode, Raillietina echinobothrida. Pharmacologyonline 2:307–317.
- Martinez ZG, Hoyo BC, Amezcua J, Gonzalez BD. 1992. Verapamil does not block the spastic response of Praziquantel on the larvae of Taenia pisiformis. Arch Med Res. 23:73–77.
- Mehlhorn H, Becker B, Andrews P, Thomas H, Frenkel JK. 1981. In vivo and in vitro experiments on the effects of praziquantel on Schistosoma mansoni. A light and electron microscopic study. Arzneimittelforschung 31:544–554.
- Pax RA, Bennett JL, Fetterer JL. 1978. A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn-Schmiedbergs Arch Pharmacol. 304:309–315.
- Prichard RK, Bachmann R, Hutchinson GW, Kohler P. 1982. The effect of praziquantel on calcium in Hymenolepis diminuta. Mol Biochem Parasitol. 5:297–308.
- Redman CA, Robertson A, Fallon PG, Modha J, Kusel R, Doenhoff MJ, Martin RJ. 1996. Praziquantel: an urgent and exciting challenge. Parasitol Today (Regul. Ed.). 12:14–20.