Abstract
Context Curcumin is widely used in China and India as a traditional herb but additional work is required to ascertain the folkloric claim of its antitumour and antioxidant activities.
Objective The present study determines the antitumour effect of curcumin against SKOV3 cell growth.
Materials and methods SKOV3 cells were incubated with curcumin (0, 20, 30 and 40 μM) for 72 h. The antiproliferative activity and the apoptosis rate were measured by MTT and flow cytometry. Expression of PI3K, T-Akt and p-Akt proteins was measured by western blotting.
Results The administration of curcumin (0, 20, 30 and 40 μM) inhibits SKOV3 cell growth (IC50 value= 24.8 μM) and increased apoptosis (32.5 and 85.7%). The activity of SKOV3 cell invasion (98.2 and 19.4%) was also decreased by curcumin administration (p < 0.05). Results of western blot analysis confirmed that the expression of p-Akt protein was decreased by curcumin (p < 0.05). It was also found that a high dose of curcumin (40 μM) can cause stronger antitumour activity (80.4%).
Conclusion Our results suggest that the curcumin induced SKOV3 apoptosis via modulation of the PI3K/Akt-signalling pathway.
Keywords:
Introduction
Unlike necrotic cell death, apoptosis is an active process of cell death that requires activation of associated genes and synthesis of their encoded proteins. The phosphoinositide 3-kinases (PI3K) are a conserved family of signal transduction enzymes which play a fundamental role in regulating cell survival. The PI3K and the downstream serine/threonine kinase Akt (also known as protein kinase B, PKB) modulate cellular activation, inflammatory response and apoptosis (Fruman & Cantley Citation2002; Zhang et al. Citation2008). More importantly, the PI3K/Akt-signalling pathway is frequently activated in many types of human cancers including colorectal carcinoma (Luo et al. Citation2003; Kritikou et al. Citation2014; Murugan et al. Citation2015) and has been linked to cancer development. Phosphoinositide 3-kinase (PI3K)-signalling pathway components are crucial to many aspects of cell growth and survival in colorectal carcinoma (CRC) via its regulation in diverse physiologic processes that include cell proliferation, cell-cycle progression, invasion and apoptosis (Kermorgant et al. Citation2001). This pathway controls several growth regulatory transcription factors.
Curcumin is the principal active component extracted from rhizomes of turmeric and is widely used in China and India as a traditional herb. Curcumin is mainly composed of curcuminoid (70%), bisdemethoxycurcumin (10–20%) and demethoxycurcumin (10%). It possesses diverse pharmacological activities, including antioxidant, anti-inflammatory, anticancer and antidepressant properties. Curcumin has also been reported to enhance wound healing (Sharma et al. Citation2005; Maheshwari et al. Citation2006). The use of curcumin as a promising new natural chemical for chemoprevention and cancer chemotherapy has been extensively studied over the past several years. The results of these studies have indicated that curcumin acts on multiple molecular targets to selectively kill tumour cells with low intrinsic toxicity (Ravindran et al. Citation2009).
The present study was designed to evaluate the antitumour activity of curcumin in ovarian cancer cells in vitro and to investigate the possible underlying cellular mechanisms by evaluating PI3K/Akt-signalling pathway activity after curcumin treatment. In addition, the anti-tumour activities of curcumin were also evaluated using a apoptotic assay, and detailed cell-cycle analysis.
Material and methods
Reagents and cell lines
Curcumin (98% purity) was purchased from Sigma, St. Louis, MO. Human ovarian cancer cell lines SKOV3 was cultured in RPMI 1640 (Invitrogen, Waltham, CA), supplemented with 10% heat-inactivated foetal bovine serum (FBS) (Invitrogen, Waltham, CA) and incubated at 37 °C.
Assay of the antiproliferative activity on SKOV3
Antiproliferative activities in vitro of curcumin on the human ovarian cancer cell line SKOV3 cells were determined by the MTT-based colorimetric method (Mosmann Citation1983). Briefly, SKOV3 cells were suspended in DMEM medium supplemented with 10% FBS, 100 IU/mL of penicillin and streptomycin at 37 °C in a humidified atmosphere containing 5% CO2.
The cell suspensions of SKOV3 cells were pipetted into a 96-well plate at a density of approximately 5000 cells/mL (100 μL/well) and cultured for 24 h. Then test samples at different concentrations (0, 20, 30 and 40 μM) were added into each well separately. After 72 h incubation, MTT reagent (0.5 mg/mL) was added to each well, and the plate was further incubated for 4 h. Then, the media were removed, and 150 μL of DMSO was added into each well for the dissolution of the formazan crystals. The absorbance of each well was read at 490 nm using the Varioskan Flash spectral scan multimode plate reader (Invitrogen, Waltham, CA). The antiproliferative activity was calculated according to the formula below:
where As is the absorbance of sample, Ac is the absorbance of the control (without sample) and Ab is the absorbance of the blank (the absorbance with DMSO).
Cell-cycle analysis
The protocol used has been described previously (Bradshaw et al. Citation2008) and is an adaptation of that used by Nicoletti (Citation1991). Cells were seeded in 6-well plates at densities of 1–2 × 105 cells/well (72 h treatments). Cells were incubated and allowed to attach overnight before treatment began. Following treatment, detached cells were collected and attached cells trypsinised, pooled samples were then washed (PBS; 4 °C), cells were centrifuged (1,500 rpm; 5 min; 4 °C) then resuspended in fluorochrome solution (50 μg/mL propidium iodide (PI), 0.1 mg/mL of ribonuclease A, 0.1% sodium citrate, 0.1% triton-X-100). Cell-cycle analyses were performed using a Beckman Coulter EPICS-XL flow cytometer (Beckman Coulter Inc., Brea, CA). Data were evaluated using EXPO32 software (Beckman Coulter Inc., Brea, CA).
Annexin V binding assay to detect apoptotic cells
After treatment of cancer cells with curcumin (0, 20, 30 and 40 μM) for 72 h, cells were detached from the culture tissue-flask with PBS containing 3 mM EDTA. These cells were then collected together with floating cells, washed twice with cold PBS and resuspended in binding buffer at a concentration of 1 × 106 cells per ml; 100 μL of solution was incubated for 30 min at 4 °C with 5 μL of Annexin V-PE antibody (BD Biosciences, San Jose, CA), and 5 μL of 7-amino-actynomycin D was then added. Cells were incubated for 15 min in darkness, and 400 μL of staining buffer was added before flow cytometry analysis. Apoptosis was analyzed by quadrant statistics as follows: Annexin V- and 7-AAD-negative cells are alive; Annexin V-positive and 7-AAD-negative cells are in early stages of apoptosis; Annexin V-negative and 7-AAD-positive cells are dead but not by apoptosis; and Annexin V-positive and 7-AAD-positive cells are in mid- or end-stage apoptosis.
In vitro invasion assay
In vitro invasion assay was performed using Transwell invasion chambers (BioCoat; BD Biosciences, San Jose, CA). SKOV3 cells were cultured in 24-well plates. An insert was used to divide each well of the plate into lower and upper chambers. The bottom of the insert comprised an 8.0 μm pore size PET membrane coated with Matrigel (BD Biosciences, San Jose, CA). The lower chamber was filled with 700 μL of DMEM supplemented with 0.1% bovine serum albumin (BSA) culture medium and human fibronectin (12.5 μg/mL). Subconfluent SKOV3 cells were harvested and resuspended in 500 μL of DMEM supplemented with 0.1% BSA containing curcumin (0, 20, 30 and 40 μM), and 5.0 × 104 cells/well were added to the upper chamber. After incubation for 23 h, the cells on the upper surface of the filters were removed with cotton swabs. Cells on the lower surface of the filters were fixed using 70% ethanol, stained with Giemsa stain, and five randomized fields were counted at 200 × magnification. The assay was conducted three times for each cell line.
Western blotting
In brief, 1 × 106 exponentially growing SKOV3 cells were treated with graded concentrations of curcumin (0, 20, 30 and 40 μM) for 72 h at 37 °C, and incubated in fresh medium for another 24 h. The cells collected after trypsinisation were pelleted down and suspended in lysis buffer consisting of 50 mM Hepes (Sigma, St. Louis, MO) (pH 7.4), 1% Triton-X 100 (SRL), 2 mM sodium orthovanadate (Sigma, St. Louis, MO), 100 mM sodium fluoride (Merk, Kenilworth, NJ), 1 mM edetic acid (SRL), 1 mM PMSF (phenylmethylsulphonyl fluoride) (SRL), 10 mg/L aprotinin (SRL), and 10 mg/L leupeptin (SRL) and lysed at 4 °C for 60 min. After centrifugation at 13 000 × g for 15 min, the protein content of the supernatant containing the cytosolic fraction was determined by Bradford reagent (Sigma, St. Louis, MO). The protein lysates were separated by electrophoresis in 12% SDS polyacrylamide gel (Sigma, St. Louis, MO) and transferred to a nitrocellulose membrane (Taurus Scientific, Cincinnati, OH). The membrane was blocked with 3% BSA (Genei, Redmond, Washington) and then incubated with the indicated primary antibodies against PI3K, Akt, p-Akt, Caspase-3, Bax, Bcl-2 and β-actin (as control) (Santa Cruz Biotechnologies, Santa Cruz, CA). After blocking with 3% BSA again, the membrane was incubated with secondary antibody (Sigma, St. Louis, MO) goat anti-mouse IgG conjugated with alkaline phosphatase (Santa Cruz Biotechnology, Santa Cruz, CA), and visualized using 5-bromo-4-chloro-3-indolyl phosphate nitro blue tetrazolium chloride (BCIP-NBT) (Chromous Biotech, Bangalore, India) as the alkaline phosphatase substrate. The gels were photographed by digital camera. The intensity of the bands was determined using the ‘Image J’ software (NIH, Bethesda, MD). Intensity of the band for control cells was taken as unity. The fold increase in the intensity for treated cells was determined by taking the ratio of the band intensity for treated cells/band intensity of control cells. Each experiment was repeated three-times. The statistical significance level of fold increase was tested by Student’s t-test.
Results and discussion
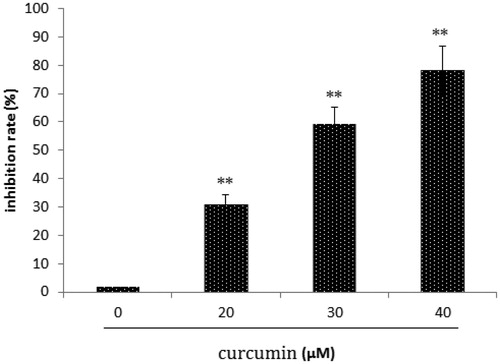
In SKOV3 cell lines treated with curcumin, we used a MTT assay to investigate the effect of curcumin treatment on SKOV3 cell proliferation and found that curcumin suppresses the growth of SKOV3 cells in a dose-dependent manner (; **p < 0.01).
Figure 1. Effect of curcumin on SKOV3 cell growth. Data are presented as mean ± SD (n = 3). **p < 0.01.
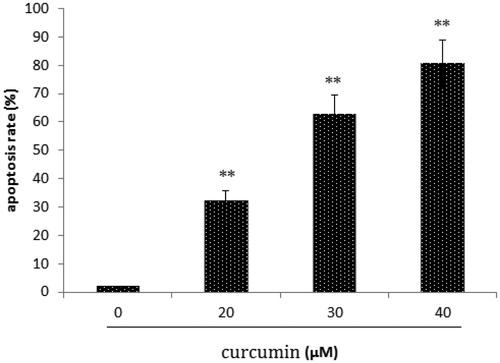
Cancer cell lines were treated with curcumin for 72 h to examine the capacity of curcumin to induce apoptosis. Untreated or curcumin-treated cancer cells were incubated with Annexin V-PE in a buffer containing 7-amino-actinomycin (7-AAD) and analyzed by flow cytometry. depicts representative results for SKOV3 cells. Curcumin increased apoptosis from 1.95% (untreated cells) to 80.77% in SKOV3 cells.
Figure 2. Effect of curcumin on SKOV3 cell apoptosis. Data are presented as mean ± SD (n = 3). **p < 0.01.
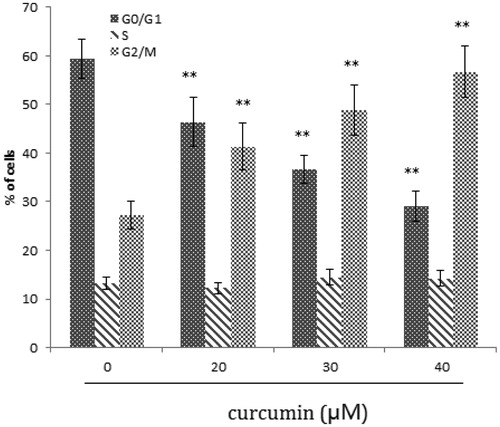
Our results showed that treatment with curcumin led to G2-M arrest in the cell (). G2-M blockage is a common feature of DNA minor groove binder (Yamori et al. Citation1999). Anticancer drugs like TAS-103, plumbagin, also arrest cell at G2-M, while drugs like camptothecin or resveratol arrest cells in G1-S phase (Kluza et al. Citation2000; Ahmed et al. Citation2001; Kuo et al. Citation2006). Unless damaged cells are effectively repaired, during arrest they proceed towards cell death. Twenty-four hours after treatment, there was an increase in hypodiploid population of SKOV3 cells that is the characteristic of apoptosis. Induction of apoptosis in cancer cells is an important goal for cancer therapy.
Figure 3. Effect of curcumin on SKOV3 cell cycle. Data are presented as mean ± SD (n = 3). **p < 0.01.
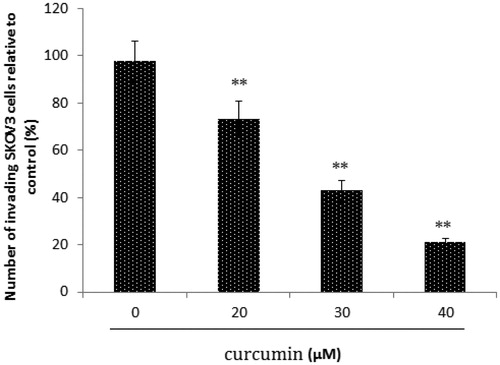
We evaluated the inhibitory effect of curcumin on tumour cell invasion by using SKOV3 cells (), and observed that the numbers of invading cells decreased as curcumin concentration increased. Therefore, SKOV3 cell invasion capacity is significantly suppressed by curcumin (; **p < 0.01).
Figure 4. Matrigel invasion assay. SKOV3 cells were treated with curcumin, and cell invasion was assessed using a Matrigel invasion assay. The number of invaded cells decreased as the concentration of curcumin increased. Data are presented as mean ± SD (n = 3). **p < 0.01.
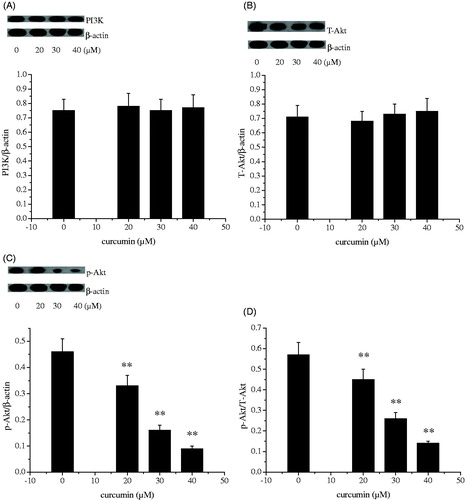
The PI3K/AKT pathway governs a plethora of cellular processes, including cell growth, proliferation and metabolism, in response to growth factors and cytokines. In the present study, the levels of PI3K and phospho-Akt were dose dependently and markedly decreased in the curcumin-treated SKOV3 cells as compared with the vehicle controls (p < 0.01). PI3K/Akt-signalling pathway had involved into ovarian cancer development. Deactivation of PI3K/Akt-signalling pathway is effective for therapy of ovarian cancer (Arafa et al. Citation2009; Wallin et al. Citation2010). In the current study, no significant changes of T-Akt expression were seen between the curcumin-treated cells and the control cells. Ratio of p-Akt to T-Akt dose dependently and markedly decreased with increasing concentration of curcumin treatment (). Our results showed that curcumin inhibited SKOV3 cell growth possibly acting as an inhibitor of PI3K/Akt-signalling pathway.
Figure 5. Effect of curcumin on PI3K (A), T-Akt (B), p-Akt (C) and p-Akt/T-Akt (D) protein expression. Data are presented as mean ± SD (n = 3). **p < 0.01.
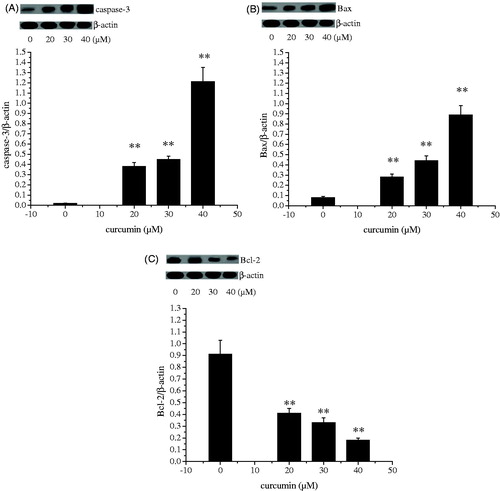
Bcl-2, a gene located at chromosome 18q21, encodes a 26-kD protein that blocks programmed cell death without affecting cellular proliferation. The Bax protein, a member of the Bcl-2 family, promotes apoptosis (Rosse et al. Citation1998; Salakou et al. Citation2007). The decision to initiate apoptosis is governed by the equilibrium between Bcl-2 and Bax, and drugs that stabilize the equilibrium can sustain cell survival, whereas interventions that shift the equilibrium toward free Bax promote the induction of apoptosis (Sharp et al. Citation2000; Zhao et al. Citation2003; Polster & Fiskum Citation2004). In many systems, members of the Bcl-2 family modulate apoptosis, with the Bax/Bcl-2 ratio serving to as a rheostat to determine cell susceptibility to apoptosis (Salakou et al. Citation2007). The caspases, especially caspase-3, are known to play a key role in the execution of apoptosis. Caspase-3 activation by cell death signals may happen via three routes: the mitochondrial (related to Bax and Bcl-2), the endoplasmic reticulum or a death receptor (FAS, FAS-L) (Chen et al. Citation1998; Benchoua et al. Citation2001; Ferrer & Planas Citation2003; Manabat et al. Citation2003). The expression levels of proteins present in cell death mechanism stages are observed in the Results section. When is examined, the bax protein expression level differed at a statistically significant level between groups (p < 0.01). The highest value was observed in the curcumin-treated group (40 μM). It is observed in that the bcl-2 protein expression level shows statistically significant differences among groups (p < 0.01). The bcl-2 protein expression levels of curcumin-treated groups (20–40 μM) were determined to be lower in comparison with those of the control group. When the protein expression level of caspase-3 protein which is an apoptotic protein is examined in , statistically significant difference was determined between the control and curcumin-treated groups. The highest value was observed in the curcumin-treated group (40 μM). Treatment of curcumin showed obvious effect on the phosphorylation of Akt, therefore, implying that the apoptotic effects of curcumin on SKOV3 cells mainly resulted from the PI3K/Akt-dependent apoptosis via caspase-3 and bax pathway.
Conclusion
We have demonstrated that curcumin inhibits the proliferation, invasion of SKOV3 cells and induce G2/M cell-cycle arrest in SKOV3 cells. The results of the present study indicated that curcumin inhibited cell-cycle progression at the G2/M phase by downregulating PI3K/Akt pathway which in turn increased caspase-3 and Bax levels. On the other hand, curcumin significantly downregulated the levels of Bcl-2 and coordinately inhibited anti-apoptotic effects.
Disclosure statement
The authors report that they have no conflicts of interest.
Reference
- Ahmed N, Adhami VM, Afaq F, Feyes DK, Mukhtar H. 2001. Resveratrol causes WAF-1/p21-mediatedG(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin Cancer Res. 7:1466–1473.
- Arafa el- SA, Zhu Q, Barakat BM, Wani G, Zhao Q, El-Mahdy MA, Wani AA. 2009. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 69:8910–8917.
- Benchoua A, Guegan C, Couriaud C, Hosseini H, Sampao N, Morin D, Onténiente B. 2001. Specific caspase pathways are activated in the two stages of cerebral infarction. J Neurosci. 21:7127–7134.
- Bradshaw TD, Stone EL, Trapani V, Leong C-O, Matthews CS, te Poele R, Stevens MFG. 2008. Mechanisms of acquired resistance to 2-(4-amino-3-methylphenyl)benzothiazole in breast cancer cell lines. Breast Cancer Res Treat. 110:57–68.
- Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. 1998. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 18:4914–4928.
- Ferrer I, Planas AM. 2003. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 62:329–339.
- Fruman DA, Cantley LC. 2002. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 14:7–18.
- Kermorgant S, Aparicio T, Dessirier V, Lewin MJM, Lehy T. 2001. Hepatocyte growth factor induces colonic cancer cell invasiveness via enhanced motility and protease overproduction. Evidence for PI3 kinase and PKC involvement. Carcinogenesis. 22:1035–1042.
- Kluza J, Lansiaux A, Wattez N, Mahieu C, Osheroff N, Bailly C. 2000. Apoptotic response of HL-60 human leukemia cells to the antitumor drug TAS-103. Cancer Res. 60:4077–4084.
- Kritikou I, Giannopoulou E, Koutras AK, Labropoulou VT, Kalofonos HP. 2014. The combination of antitumor drugs, exemestane and erlotinib, induced resistance mechanism in H358 and A549 non-small cell lung cancer (NSCLC) cell lines. Pharm Biol. 52:444–452.
- Kuo PL, Hsu YL, Cho CY. 2006. Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol Cancer Ther. 5:3209–3221.
- Luo J, Manning BD, Cantley LC. 2003. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 4:257–262.
- Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. 2006. Multiple biological activities of curcumin: a short review. Life Sci. 78:2081–2087.
- Manabat C, Han BH, Wendland M, Derugin N, Fox CK, Choi J, Holtzman DM, Ferriero DM, Vexler ZS. 2003. Reperfusion differentially induces caspase-3 activation in ischemic core and penumbra after stroke in immature brain. Stroke. 34:207–213.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63.
- Murugan AC, Karim MR, Yusoff MBM, Tan SH, Asras MF, Rashid SS. 2015. New insights into seaweed polyphenols on glucose homeostasis. Pharm Biol. 53:1087–1097.
- Nicoletti I. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 132:272–279.
- Polster BM, Fiskum G. 2004. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 90:1281–1289.
- Ravindran J, Prasad S, Aggarwal BB. 2009. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 11:495–510.
- Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. 1998. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 391:496–499.
- Salakou S, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos DS, Papapetropoulos T, Petsas T, et al. 2007. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis in vivo. In Vivo. 21:123–132.
- Sharma RA, Gescher AJ, Steward WP. 2005. Curcumin: the story so far. Eur J Cancer. 41:1955–1968.
- Sharp FR, Lu A, Tang Y, Millhorn DE. 2000. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 20:1011–1032.
- Wallin JJ, Guan J, Prior WW, Edgar KA, Kassees R, Sampath D, Belvin M, Friedman LS. 2010. Nuclear phospho-Akt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci Transl Med. 2:48–66.
- Yamori T, Matsunaga A, Sato S, Yamazaki K, Komi A, Ishizu K, Mita I, Edatsugi H, Matsuba Y, Takezawa K, et al. 1999. Potentantitumor activity of MS-247, a novel DNA minor groove binder, evaluated by an in vitro and in vivo human cancer cell line panel. Cancer Res. 59:4042–4049.
- Zhang Y, Foster R, Sun X, Yin Q, Li Y, Hanley G, Stuart C, Gan Y, Li C, Zhang Z, Yin D. 2008. Restraint stress induces lymphocyte reduction through p53 and PI3K/NF-kappaB pathways. J Neuroimmunol. 200:71–76.
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. 2003. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 85:1026–1036.