Abstract
Context The genus Anthemis L. (Asteraceae) comprises about 195 species which are widely used in the pharmaceutical, cosmetic and food industries.
Objective Anthemis mirheydari Iranshar, an endemic plant from Iran, was investigated for its cytotoxic properties and chemical constituents.
Materials and methods The whole parts of the plant (320 g) were extracted by dichloromethane and methanol for four days, successively. The cytotoxic activity of both dichloromethane and methanol extracts were assayed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide colorimetric methods against three human cancer cell lines including LS180, MCF-7 and MOLT-4. Different concentrations (10–100 μg/mL) of the plant extracts were tested to obtain IC50 values. The dichloromethane extract of A. mirheydari was subjected to silica gel-column and thin layer chromatography for purification of its chemical constituents and the isolated compounds were further tested against MOLT-4 cells. The structures of the pure compounds were elucidated using different spectral data including nuclear magnetic resonance and electron impact mass spectra.
Results The IC50 values of the dichloromethane extract were 30.8 ± 6.7, 25.2 ± 6.5 and 8.6 ± 1.1 μg/mL (means ± standard error) for the above-mentioned cell lines, respectively. Two triterpenoids, taraxasterol (1) and pseudotaraxasterol (2), one sterol, β-sitosterol (3) and one coumarin, 7-methoxycoumarin (4) were isolated from the extract. The IC50 of the mixture of compounds 1 and 2 as well as compounds 3 and 4 were higher (>100 μM) than that reported for the dichloromethane extract against MOLT-4 cells.
Conclusion The dichloromethane extract was the most active one among the tested material.
Keywords:
Introduction
The family Asteraceae is the most species-rich plant family (Bremer Citation1994). The genus Anthemis, the second largest genus in the Asteraceae family, comprises about 195 species which are widely used in the pharmaceutical, cosmetic, and food industries (Uzel et al. Citation2004; Amjad et al. Citation2013). Anthemis has a circum-Mediterranean distribution but its centre of the diversity is in Southwest of Asia, from which more than 150 species are reported (Presti et al. Citation2010). Thirty-nine annual and perennial species of the genus Anthemis are found in Iran, including 15 endemic (Mozaffarian Citation1996). The cytotoxic activity of different species of Anthemis is attributed to the presence of different types of natural products such as sesquiterpenes lactones (Collu et al. Citation2008; Saroglou et al. Citation2010; Bai et al. Citation2011). The essential oils of Anthemis xylopoda O. Schwarz (Asteraceae) from Turkey consist of mostly monoterpenoids that exhibit potent antibacterial properties (Uzel et al. Citation2004). Two sesquiterpenoids and two flavonoids were isolated from the flowers of Anthemis odontostephana Boiss. (Asteraceae) (Shokoohinia et al. Citation2015). Nine sesquiterpenoids were reported from Anthemis melanolepis Boiss. (Asteraceae), some of which showed antimicrobial activity with both Gram positive and Gram negative bacteria and fungi and possessed cytotoxic potential in human cancer cell lines (Saroglou et al. Citation2010).
We report here the chemical constituents and cytotoxic activities of methanol and dichloromethane (DCM) extracts of entire A. mirheydari plants, which is an annual and endemic specie growing wild in the southern parts of Iran. To the best of our knowledge, there are no phytochemical or biological reports on this plant except one abstract describing the antibacterial and chemical composition of the essential oil of the plant analyzed by GC-MS (Farjam et al. Citation2012).
Materials and methods
General experimental procedures
NMR spectra of the purified compounds were recorded on Bruker Avance 500 and 400 spectrometers (Bruker Biospin, Karlsruhe, Germany), operating at resonance frequencies of 500.13 and 400 MHz for 1H and 125.75 and 100 MHz for 13C, respectively. Samples were measured at 298 K in CDCl3. Tetramethylsilane (TMS) was used as an internal standard for referencing 1H and 13C NMR spectra. Standard tubes (5 mm i.d.) and capillary tubes (2 mm i.d.) were used for all NMR measurements. Data acquisition as well as processing was accomplished using Bruker Topspin 2.1.
Electron impact mass spectra (EI-MS) were recorded on an Agilent 5975C inert GC/MSD instrument. The chromatography separations were performed using gravity column chromatography using silica gel 60 (0.2–0.04 mm particle size), flash column chromatography using silica gel 60 (0.040–0.063 mm particle size) and thin layer chromatography (TLC) using silica gel 60 F254 pre-coated plates (0.25 mm film thickness). The adsorbents were purchased from Merck, Darmstadt, Germany.
Plant material and extraction procedure
All parts of A. mirheydari Iranshahr in the flowering stage were collected from Jahrom (N 28°28′38.45″, E 53°34′0.76″; at 1200 m altitude) in Fars Province, Iran, in March 2013 and was identified by Mehdi Zare (plant taxonomist) in Medicinal and Natural Products Chemistry Research Center (MNCRC), Shiraz University of Medical Sciences. A voucher specimen (PC-92-2-3-1) has been deposited at the herbarium of MNCRC. The air-dried powdered plant (320 g) was macerated for four days in DCM (3 L) and MeOH (3 L) successively at room temperature. Filtration and evaporation of the DCM and MeOH extracts in reduced pressure at 40 °C resulted in 5.6 and 3.4 g gummy material, respectively.
Isolation and purification of the plant extracts
The bioactive DCM extract was subjected to column (60 × 3.5 cm) chromatography over silica gel (100 g, 0.2–0.04 mm). The elution of the column was performed using n-hexane with 10% gradient of DCM up to 100% and then followed by increasing the polarity of the mobile phase with MeOH to afford 27 fractions. The fraction eluted with hexane-DCM (10:90, F10) was dissolved in n-hexane and the solution was kept in the refrigerator overnight. A white precipitate (4 mg) was collected and then identified as a 1:1 mixture of 1 and 2 (the ratio was deduced on the basis of the proton's signal integration in the 1H NMR spectrum). The fractions obtained from the CC eluted with DCM (70 mg) were purified on Preparative Layer Chromatography (PLC) (Merck F254) using DCM-acetone (98:2) as the mobile phase. Compounds 3 (3.8 mg) (Rf = 0.64) and 4 (10.1 mg) (Rf = 0.70) were obtained as white powder.
The spectral data
Taraxasterol (1): C30H50O, EIMS (rel. int. %): m/z 426 [M]+ (77), 357 (20), 315 (15), 257 (12), 207 (89), 204 (35), 189 (100), 175 (30). 1H NMR (400 MHz, CDCl3): δH 3.22 (1H, m, H-3), 0.96 (3H, d, J = 4.4 Hz, H-29), 4.66 (2H, s, H-30), 0.71 (3H, s, H-23), 0.77 (3H, s, H-24), 0.86 (3H, s, H-25), 0.97 (3H, s, H-26), 0.98 (3H, s, H-28) (Lee et al. Citation2010; Mouffok et al. Citation2012). Attached Proton Test (ATP) 13C NMR (100 MHz, CDCl3): δC 38.9(C-1), 27.4 (C-2), 78.9 (C-3), 38.8 (C-4), 55.3 (C-5), 18.3 (C-6), 34.1 (C-7), 40.9 (C-8), 50.5 (C-9), 37.1 (C-10), 21.4 (C-11), 25.6 (C-12), 38.6 (C-13), 42.0 (C-14), 26.7 (C-15), 39.2 (C-16), 34.4 (C-17), 48.7 (C-18), 38.3 (C-19), 154.7 (C-20), 25.5(C-21), 39.4 (C-22), 28.0 (C-23), 15.4 (C-24), 15.9 (C-25), 16.3 (C-26), 14.8 (C-27), 19.5 (C-28), 26.1 (C-29), 107.1 (C-30) (Mahato & Kundu Citation1994; Khalilov et al. Citation2003).
Pseudotaraxasterol (2): C30H50O, EIMS (rel. int. %): m/z 426 [M]+ (77), 357 (20), 315 (15), 257 (12), 207 (89), 204 (35), 189 (100), 175 (30). 1H NMR (400 MHz, CDCl3): δH 3.22 (1H, m, H-3), 0.99 (3H, d, J = 6.2 Hz, H-29), 5.2 (1H, d, J = 8 Hz, H-21), 0.95 (3H, s, H-23), 0.76 (3H, s, H-24), 0.84 (3H, s, H-25), 1.03 (3H, s, H-26), 0.94 (3H, s, H-27), 0.71 (3H, s, H-28), 1.64 (3H, s, H-30) (Ding et al. Citation2000). 13C NMR (100 MHz, CDCl3): δC 38.8 (C-1), 27.4 (C-2), 78.9 (C-3), 38.9 (C-4), 55.3 (C-5), 18.3 (C-6), 34.2 (C-7), 41.1 (C-8), 50.5 (C-9), 37.1 (C-10), 21.6 (C-11), 27.6 (C-12), 39.2 (C-13), 42.3 (C-14), 27.0 (C-15), 36.7 (C-16), 34.4 (C-17), 48.7 (C-18), 36.3 (C-19), 139.9 (C-20), 118.9 (C-21), 42.2 (C-22), 28.0 (C-23), 15.4 (C-24), 16.3 (C-25), 16.1 (C-26), 14.8 (C-27), 17.7 (C-28), 22.6 (C-29), 21.8 (C-30) (Ding et al. Citation2000).
β-Sitosterol (3): C29H50O EIMS (rel. int. %): m/z 414 (100), 396 (62), 381 (33), 351 (14), 341 (44), 314 (64). 1H NMR (400 MHz, CDCl3): δH 3.51 (1H, m, H-3), 5.3 (1H, d, J = 5.1 Hz, H-6), 0.86 (3H, s, H-18), 1.00 (3H, s, H-19), 0.92 (3H, s, H-21), 0.81 (3H, d, J = 6.8 Hz, H-26), 0.83 (3H, d, J = 6.8 Hz, H-27) (Mouffok et al. Citation2012). 13C NMR (100 MHz, CDCl3): δC 37.2 (C-1), 31.6 (C-2), 71.8 (C-3), 42.3 (C-4), 140.7 (C-5), 121.7 (C-6), 31.9 (C-7), 31.9 (C-8), 50.1 (C-9), 36.5 (C-10), 21.1 (C-11), 39.8 (C-12), 42.2 (C-13), 56.0 (C-14), 24.3 (C-15), 28.3 (C-16), 56.0(C-17), 11.9 (C-18), 19.4 (C-19), 36.2 (C-20), 18.8 (C-21), 33.9 (C-22), 26.0 (C-23), 45.8 (C-24), 29.1 (C-25), 19.8 (C-26), 19.0 (C-27), 23.0 (C-28), 12.0 (C-29) (Kovganko et al. Citation1999; De-Eknamkul & Potduang Citation2003; Mouffok et al. Citation2012).
7-Methoxycoumarin (4): C10H8O3, EIMS (rel. int. %):m/z 176 [M+] (100), 148 (85), 133 (94), 105 (20), 77 (24), 51 (16). 1H NMR (500 MHz, CDCl3), δH 6.25 (1H, d, J = 9.5 Hz, H-3), 7.64 (1H, d, J = 9.5 Hz, H-4), 7.38 (1H, d, J = 8.5 Hz, H-5), 6.84 (1H, dd, J = 6.0, 2.4 Hz, H-6), 6.81 (1H, d, J = 2.4 Hz, H-8), 3.87 (3H, s, H-1′). 13C NMR (125 MHz, CDCl3), δC 161.2 (C-2), 112.6 (C-3), 143.4 (C-4), 128.7 (C-5), 113.1 (C-6), 162.8 (C-7), 100.9 (C-8), 155.9 (C-9), 112.6 (C-10), 55.7 (C-1′) (Elgamal et al. Citation1979; Benkiki et al. Citation2007; Weber et al. Citation2008; Askari et al. Citation2009).
Cytotoxicity assay
Cell lines
LS180 (human colon adenocarcinoma), MCF-7 (human breast adenocarcinoma) and MOLT-4 (human acute lymphoblastic leukaemia) cells were obtained from the National Cell Bank of Iran, Pasteur Institute, Tehran, Iran. LS180 and MCF-7 cells were grown in monolayer cultures, while MOLT-4 cells were grown in suspension. RPMI 1640 supplemented with 10% FBS, and 100 units/mL penicillin-G and 100 μg/mL streptomycin was used as the growth medium for all cell lines and they were maintained at 37 °C in humidified air containing 5% CO2.
Cell viability following exposure to plant extracts or isolated compounds were measured by the MTT method as follows (Mosmann Citation1983; Firuzi et al. Citation2010): Suspensions of LS180, MCF-7 and MOLT-4 cells (100 μl) were plated in 96-well microplates at a density of 50 000 cells/mL. After overnight incubation, 3–4 different concentrations of the extracts or pure compounds were added to the wells in duplicate. Extracts and isolated compounds were first dissolved in DMSO and then diluted in the growth medium at the desired concentration. The level of DMSO in the wells was kept under 0.25%. Cells were further incubated for 72 h and their growth medium was replaced with medium containing 0.5 mg/mL MTT. Plates were then incubated for another 4 h at 37 °C, the media was removed and 200 μL DMSO was added to each well to dissolve the formazan crystals formed in the viable cells. The light absorbance was measured at 570 nm with background correction at 655 nm using a Bio-Rad microplate reader (Model 680). The percent inhibition of viability for each concentration of the extract was calculated with reference to the control (cells not exposed to any extract) and IC50 values were calculated with the software Curve Expert version 1.34 for Windows. Each experiment was repeated 3–4 times.
Result and discussion
The cytotoxic activity of methanol and DCM extracts of A. mirheydari was evaluated against three human cancer cell lines, LS180 (human colon adenocarcinoma), MCF-7 (human breast adenocarcinoma) and MOLT-4 (human acute lymphoblastic leukaemia). IC50 values of the DCM extract were determined as 30.8 ± 6.7, 25.2 ± 6.5 and 8.6 ± 1.1 μg/mL, respectively in the three lines (mean ± SE), while the MeOH extract showed no activity against any of the cell lines up to the concentration of 100 μg/mL. These findings demonstrated the higher cytotoxic effects of non-polar fractions and components of the DCM extract compared to the more polar components of the methanol extract. Therefore, we subjected the DCM extract to different chromatography techniques to separate the non-polar constituents of the active extract.
Two triterpenoids, taraxasterol (1) and pseudotaraxasterol (2), were purified using silica gel column chromatography (CC). In addition to these compounds, β-sitosterol (3) and 7-methoxycoumarin (4) were purified using preparative thin layer chromatography (PTLC) on silica gel plates (). The structures of all the compounds were elucidated using different spectral data including 1H NMR, 13C NMR and EI-MS and comparing their spectral data with those described in the literature (Elgamal et al. Citation1979; Mahato & Kundu Citation1994; Kovganko et al. Citation1999; Ding et al. Citation2000; De-Eknamkul & Potduang Citation2003; Khalilov et al. Citation2003; Benkiki et al. Citation2007; Weber et al. Citation2008; Askari et al. Citation2009; Mouffok et al. Citation2012). The isolated compounds (1–4) were tested against the most susceptible cells, MOLT-4, however, they did not show promising activities with IC50 values higher than 100 μM.
Figure 1. The chemical structures of pentacyclic triterpenoids (1–2), and the coumarin (4) isolated from A. mirheydari.
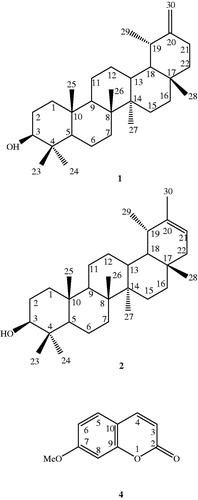
In a previous study, four pentacyclic triterpene alcohols with anti-inflammatory activity, including pseudotaraxasterol (ψ-taraxast-20-en-3β-ol) and taraxasterol [taraxast-20 (30)-en-3β-ol], were reported as the major components in the flower extracts of different plant species of the Compositeae (Asteraceae) family (Akihisa et al. Citation1996).
Compound 1 was isolated from the flowers of Cynara scolymus L. (Asteraceae) (artichoke), Chrysanthemum morifolium Ramat. (Asteraceae) (Yasukawa et al. Citation1996), and Anthemis austriaca Jacq. (Asteraceae) (Staneva et al. Citation2004). Taraxasterol showed interesting biological activities such as strong inhibitory activity against 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice comparable to indomethacin. Furthermore, its inhibitory activities against tumour promotion induced by phorbol ester on mice skin, has suggested 1 as a potent antitumour agent (Yasukawa et al. Citation1996). Compound 1 also exhibited cancer chemopreventive properties in cells and mouse models of carcinogenesis (Takasaki et al. Citation1999). Compounds 1 and 2 were isolated from the tubular flowers of Carthamus tinctorius L. (Asteraceae), C. morifolium and Helianthus annuus L. (Asteraceae) and demonstrated remarkable activity against TPA-induced inflammatory ear edema in mouse. The 50% inhibitory dose (ID50) values were reported to be 0.3 and 0.4 mg/ear for 1 and 2, respectively (Akihisa et al. Citation1996). The same research group described the anti-tubercular activity of 1 and 2 against Mycobacterium tuberculosis with the MIC values of 64 and >64 μg/mL, respectively (Akihisa et al. Citation2005). As for the anticancer activity of compound 1, it has shown cytotoxicity against PC3 (human prostate cancer) (IC50 15.8 μg/mL) and HT-29 (human colon cancer) (IC50 38.3 μg/mL) (Boutaghane et al. Citation2013), and MCF-7 (breast cancer) (IC50 53.3 μM) cells (Lee et al. Citation2010) in previous studies; however, in another study on KB (human nasopharyngeal carcinoma) and P388 (murine leukaemia) cells, no anticancer effect was observed for this compound (Villarreal et al. Citation1994). Our findings in human leukaemia cells are in agreement with the later report, which has also used leukaemia cells for cytotoxicity assays (Villarreal et al. Citation1994).
β-Sitosterol (3), a common dietary phytosterol, was reported to have many biological properties including anticancer, antibacterial and antifungal activities. Although it is structurally similar to cholesterol, except for an additional ethyl side chain at the C-24 position, it has been shown to have important biological effects in humans (Choi et al. Citation2003). Previous studies have suggested that populations with the low breast cancer incidence often consume high phytosterols containing diets, including β-sitosterol (Muti et al. Citation2003). In vitro studies have shown that β-sitosterol inhibits 66% of the growth and stimulate the apoptosis of MDA-MB-231 human breast cancer cells in culture at 16 μM (Awad et al. Citation2000) and it also has a selective cytotoxic activity against Bowes (melanoma) cells with an IC50 value of 36.5 μM (Nguyen et al. Citation2004). Furthermore, compound 3 has exhibited inhibitory effect on tumour-promotion in JB6 mouse epidermal cells (IC50 39.6 μmol/L) (Gao et al. Citation2003).
7-Methoxycoumarin (herniarin, 4), a bioactive coumarin derivative, has shown several important biological activities (Askari et al. Citation2009), including marked anti-inflammatory activity, as an inhibitor of the eicosanoid-release from ionophore-stimulated mouse peritoneal macrophages (Silván et al. Citation1996).
A number of studies have examined the anticancer effect of compound 4 in cancer cells. This compound has shown anticancer effect against laryngeal cancer cells (LCC) in a dose-dependent manner, but this effect has been lower than the effects of umbelliferone and scoparone (Kiełbus et al. Citation2013). In another report, 55% growth inhibition was observed at 100 μg/mL in EC-109 (esophageal squamous cell carcinoma) cells, however, compound 4 was also almost as effective against NIH-3T3 non-cancer cells, and the percent cell cytotoxicity was 57.21 ± 1.6 at 50 μM (Hong & Ying Citation2015). Cytotoxic effect of compound 4 has been evaluated against CH1 (ovarian carcinoma), A549 (lung cancer) and SK-MEL-28 (melanoma) cell lines using MTT assay. IC50 values against these cell lines were considerably higher than the standard chemotherapeutic agent, cisplatin (62.5, >250 and 250 μM, respectively) (Valiahdi et al. Citation2013), data are consistent with our findings about this compound.
Conclusion
Considering the presence of the bioactive compounds 1–4 in the DCM extract of A. mirheydari, we propose that some of the high cytotoxic activity of the DCM extract is due to the presence of these compounds. Because of higher activity of the DCM extract compared to those reported for all of the isolated compounds, there may be some other active substances such as well-known sesquiterpenoids, the chemical markers of the genus Anthemis, in the above extract (Shokoohinia et al. Citation2015). Therefore, we propose that A. mirheydari could be a new source for anti-cancer natural products and a new potential anti-cancer medicinal plant worthy of future investigations. The high cytotoxic effect of the plant extract is comparable with those measured for the standard cytotoxic agent, cisplatin (4.2–9.4 μg/mL).
Funding information
We would like to express our acknowledgement to the Alexander von Humboldt foundation for a research group linkage program grant (3.4 - IRN/1101775) and also the Shiraz University of Medical Sciences, Vice-Provost for Research for the financial support of this project.
Disclosure statement
The authors report no declarations of interest.
References
- Akihisa T, Franzblau SG, Ukiya M, Okuda H, Zhang F, Yasukawa K, Suzuki T, Kimura Y. 2005. Antitubercular activity of triterpenoids from Asteraceae flowers. Biol Pharm Bull. 28:158–160.
- Akihisa T, Yasukawa K, Oinuma H, Kasahara Y, Yamanouchi S, Takido M, Kumaki K, Tamura T. 1996. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry. 43:1255–1260.
- Amjad L, Rezvani Z, Madani M. 2013. The effect of methanolic extract of Anthemis gayana on Candida Spp. Inter J Agri Cro Sci. 5:1140–1144.
- Askari M, Sahebkar A, Iranshahi M. 2009. Synthesis and purification of 7-prenyloxycoumarins and herniarin as bioactive natural coumarins. Iran J Basic Med Sci. 12:63–69.
- Awad A, Downie AC, Fink CS. 2000. Inhibition of growth and stimulation of apoptosis by beta-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int J Mol Med. 5:541–546.
- Bai N, He K, Roller M, Lai CS, Shao X, Pan MH, Bily A, Ho CT. 2011. Flavonoid glycosides from Microtea debilis and their cytotoxic and anti-inflammatory effects. Fitoterapia. 82:168–172.
- Benkiki N, Kabouche Z, Bruneau C. 2007. Two coumarins and a thienylbutylamide from Anacyclus cyrtolepioides from the Algerian Septentrional Sahara. Chem Nat Compd. 43:612–613.
- Boutaghane N, Voutquenne-Nazabadioko L, Simon A, Harakatd D, Benlabede K, Kabouche Z. 2013. A new triterpenic diester from the aerial parts of Chrysanthemum macrocarpum. Phytochem Lett. 6:519–525.
- Bremer K. 1994. Branch support and tree stability. Cladistics. 10:295–304.
- Choi YH, Kong KR, Kim Y, Jung KO, Kil JH, Rhee SH, Park KY. 2003. Induction of Bax and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int J Oncol. 23:1657–1662.
- Collu F, Bonsignore L, Casu M, Floris C, Gertsch J, Cottiglia F. 2008. New cytotoxic saturated and unsaturated cyclohexanones from Anthemis maritima. Bioorg Med Chem Lett. 18:1559–1562.
- De-Eknamkul W, Potduang B. 2003. Biosynthesis of beta-sitosterol and stigmasterol in Croton sublyratus proceeds via a mixed origin of isoprene units. Phytochemistry. 62:389–398.
- Ding HY, Lin HC, Teng CM, Wu YC. 2000. Phytochemical and pharmacological studies on Chinese Paeonia species. J Chin Chem Soc-Taip. 47:381–388.
- Elgamal M, Elewa N, Elkhrisy E, Duddecka H. 1979. 13C NMR Chemical shifts and carbon-proton coupling constants of some furocoumarins and furochromones. Phytochemistry. 18:139–143.
- Farjam M, Izadi SF, Bazregar M. 2012. Antimicrobial and antioxidant activity and composition of the essential oils from aerial parts of Anthemis mirheydari Iranshahr growing in Iran. Res Pharm Sci. 7:S7
- Firuzi O, Asadollahi M, Gholami M, Javidnia K. 2010. Composition and biological activities of essential oils from four Heracleum species. Food Chem. 122:117–122.
- Gao H, Wu L, Kuroyanaci M, Harada K, Kawahara N, Nakane T, Umehara K, Hirasawa A, Nakamura Y. 2003. Antitumor-promoting constituents from Chaenomeles sinensis koehne and their activities in JB6 mouse epidermal cells. Chem Pharm Bull. 51:1318–1321.
- Hong L, Ying S. 2015. Ethanol extract and isolated constituents from Artemisia dracunculus inhibit esophageal squamous cell carcinoma and induce apoptotic cell death. Drug Res. 65:101–106.
- Khalilov L, Khalilova A, Shakurova E, Nuriev IF, Kachala VV, Shashkov AS, Dzhemilev UM. 2003. PMR and 13C NMR spectra of biologically active compounds. XII. Taraxasterol and its acetate from the aerial part of Onopordum acanthium. Chem Nat Compd. 39:285–288.
- Kiełbus M, Skalicka-woźniak K, Grabarska A, Jeleniewicz W, Dmoszynska-Graniczka M, Marston A, Polberg K, Gawda P, Klatka J, Stepulak A. 2013. 7-Substituted coumarins inhibit proliferation and migration of laryngeal cancer cells in vitro. Anticancer Res. 33:4347–4356.
- Kovganko N, Kashkan ZN, Borisov E, Batura EV. 1999. 13C NMR spectra of β-sitosterol derivatives with oxidized rings A and B. Chem Nat Compd. 35:646–649.
- Lee D-Y, Jung L, Park JH, Yoo K-H, Chung I-S, Baek N-I. 2010. Cytotoxic triterpenoids from Cornus kousa fruits. Chem Nat Compd. 46:142–145.
- Mahato SB, Kundu AP. 1994. 13C NMR Spectra of pentacyclic triterpenoids a compilation and some salient features. Phytochemistry. 37:1517–1575.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63.
- Mouffok S, Haba H, Lavaud C, Benkhaled M. 2012. Chemical constituents of Centaurea omphalotricha Coss. & Durieu ex Batt. & Trab. Rec Nat Prod. 6:292–295.
- Mozaffarian V. (1996). A dictionary of iranian plant names. Farhang Mo'aser: Tehran.
- Muti P, Awad AB, Schünemann H, Fink CS, Hovey K, Freudenheim JL, Wu YW, Bellati C, Pala V, Berrino F. 2003. A plant food-based diet modifies the serum beta-sitosterol concentration in hyperandrogenic postmenopausal women. J Nutr. 133:4252–4255.
- Nguyen A-T, Malonne H, Duez P, Vanhaelen-Fastre R, Vanhaelen M, Fontaine J. 2004. Cytotoxic constituents from Plumbago zeylanica. Fitoterapia. 75:500–504.
- Presti RML, Oppolzer S, Oberprieler C. 2010. A molecular phylogeny and a revised classification of the Mediterranean genus Anthemis s.l. (Compositae, Anthemideae) based on three molecular markers and micromorphological characters. Taxon. 59:1441–1456.
- Saroglou V, Karioti A, Rancic A, Dimas K, Koukoulitsa C, Zervou M, Skaltsa H. 2010. Sesquiterpene lactones from Anthemis melanolepis and their antibacterial and cytotoxic activities. Prediction of their pharmacokinetic profile. J Nat Prod. 73:242–246.
- Silván AM, Abad MJ, Bermejo P, Sollhuber M, Villar A. 1996. Antiinflammatory activity of coumarins from Santolina oblongifolia. J Nat Prod. 59:1183–1185.
- Shokoohinia Y, Sajjadi SE, Jassbi AR, Moradi H, Ghassemi N, Schneider B. 2015. Sesquiterpenes and flavonoids of Anthemis odontostephana Boiss. var. odontostephana. Chem Nat Compd. 51:491–494.
- Staneva J, Trendafilova-Savkova A, Todorova MN, Evstatievab L, Vitkova A. 2004. Terpenoids from Anthemis austriaca Jacq. Z Naturforsch. 59:161–165.
- Takasaki M, Konoshima T, Tokuda H, Masuda K, Arai Y, Shiojima K, Ageta H. 1999. Anti-carcinogenic activity of Taraxacum plant. II. Biol Pharm Bull. 22:606–610.
- Uzel A, Guvensen A, Cetin E. 2004. Chemical composition and antimicrobial activity of the essential oils of Anthemis xylopoda O. Schwarz from Turkey. J Ethnopharmacol. 95:151–154.
- Valiahdi SM, Iranshahi M, Sahebkar A. 2013. Cytotoxic activities of phytochemicals from Ferula species. DARU J Pharm Sci. 21:39–45.
- Villarreal ML, Alvarez L, Alonso D, Navarro V, García P, Delgado G. 1994. Cytotoxic and antimicrobial screening of selected terpenoids from Asteraceae species. J Ethnopharmacol. 42:25–29.
- Weber B, Herrmann M, Hartmann B, Joppe H, Schmidt CO, Bertram H-J. 2008. HPLC/MS and HPLC/NMR as hyphenated techniques for accelerated characterization of the main constituents in Chamomile (Chamomilla recutita [L.] Rauschert). Eur Food Res Technol. 226:755–760.
- Yasukawa K, Akihisa T, Oinuma H, Kaminaga T, Kanno H, Kasahara Y, Tamura T, Kumaki K, Yamanouchi S, Takido M. 1996. Inhibitory effect of taraxastane-type triterpenes on tumor promotion by 12-O-tetradecanoylphorbol-13-acetate in two-stage carcinogenesis in mouse skin. Oncology. 53:341–344.
