Abstract
Context Sumac [Rhus coriaria L. (RC) (Anacardiaceae)] is used as a folk medicine in the treatment of diabetes in Turkey.
Objective This study investigates the in vivo healing and protective effects of lyophilized extract sumac against streptozotocin (STZ)-induced diabetic complications.
Materials and methods Toxicity test was conducted in three different dosages (250, 500 and 1000 mg/kg of plant extracts, respectively). Six groups of seven rats each were used in experiments. Groups were designed as Normal control, Diabetic (DM), DM + AC-20 mg/kg, DM + Extract-100 mg/kg, DM + Extract 250 mg/kg and DM + Extract 500 mg/kg group. Experimental diabetes [50 mg/kg, intraperitoneal (i.p.)] was induced by STZ. The effects of oral administration of the extract for 21 d on the level of serum glucose, insulin, C-peptide, lipid profile (LP), hepatic and renal damage biomarkers (HRDB), diabetic serum biomarkers (DSB), glycosylated haemoglobin (HbA1c), antioxidant defence system constituents (ADSCs), malondialdehyde (MDA) and α-glucosidase activity in small intestine tissue were evaluated.
Results The extract decreased the levels of blood glucose in diabetic groups (an average of 31%). Triglyceride, total cholesterol, high-density lipoprotein and low-density lipoprotein levels were balanced by plant extract (500 mg/kg) supplementation in the diabetic group. Decreased levels of aspartate aminotransferase (89%), alanine aminotransferase (91%), lactate dehydrogenase (35%), alkaline phosphatase (47%), creatinine (25%) and urea (29%) were detected in plant extract (500 mg/kg) supplemented diabetic group. Additionally, a considerable increase in the HRDB, DSB, LP, MDA and fluctuated ADSC levels were restored in RC-extract supplemented groups.
Conclusion RC lyophilized extract has a healing effect on diabetes and diabetes-related complications.
Introduction
Metabolic syndrome consists of hypertension, diabetes and dyslipidaemia caused by abdominal obesity is a complication of a modern lifestyle that includes overeating and underactivity. This syndrome is clinically important because of the associated cardiovascular risk accumulation, which exceeds that of the component parts (Ando & Fujita Citation2009). Diabetes is a group of metabolic diseases characterized by hyperglycaemia resulting from defects in insulin secretion, insulin action or both. The prevalence of diabetes for all age-groups worldwide was estimated to be 2.8% in 2000 and 4.4% in 2030. The total number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030 (Wild et al. Citation2004). Most patients with type 2 diabetes, which accounts for 90–95% of those with diabetes, are obese (American Diabetes Association Citation2012).
Metabolic syndrome is strongly linked to a “Western” lifestyle, characterized by low levels of physical activity and a diet rich in saturated fat and refined carbohydrate which is the main contributing cause of obesity. Obesity increases the risk of a number of diseases, including hypertension, diabetes, cancer and cardiovascular diseases (Jebb Citation2004). WHO estimated that there are currently 1.6 billion adults could be categorized as overweight while at least 400 million adults were considered obese. It is predicted that 2.3 billion would be overweight and >700 million obese by 2015 (WHO Citation2006).
Metabolic syndrome is also associated with overproduction of reactive oxygen species (ROS). Oxidative stress can be defined as a state of an imbalance towards the factors that generate reactive oxygen radicals and away from the factors that protect cellular macromolecules from these reactants including antioxidants like superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidases (GSH-Px). These factors that generate ROS exist as products of normal cellular physiology as well as from various exogenous sources. Excess ROS production can severely impair the cell and lead to macromolecular damage, dysfunction and death. Under conditions of oxidative stress, free radicals that are not reduced or removed from the cellular environment can cause damage to all cellular macromolecules including nucleic acids, lipids and proteins. Certain pathological conditions, such as obesity, hyperglycaemia and hyperlipidaemia, have shown to promote oxidative stress through elevated ROS production and/or reduced antioxidant defence (Styskal et al. Citation2012). ROS are clearly one of the key factors in metabolic syndrome. The levels of ROS are increased in obesity, which is an essential component of metabolic syndrome. ROS overproduction can activate the cortisol-mineralocorticoid receptor which can cause a number of disorders including insulin resistance and organ damage (Ando & Fujita Citation2009).
The oral administration in the management of metabolic syndrome-related disorders has stimulated great interest in recent years. Currently, commercially available drugs for the treatment of type II diabetes are acarbose, miglitol and voglibose (Bedekar et al. Citation2010), orlistat, sibutramine and rimonabant for the treatment of obesity (Padwal & Majumdar Citation2007). At present, because of high costs and potentially hazardous side effects (liver problems, diarrhoea and so forth) of these drugs, the potential of natural products for the treatment of metabolic syndrome-related disorders is under exploration. Plants contain high levels of bioactive compounds might be a significant factor in the regulation of metabolic syndrome-related disorders. Herbal medicines from natural sources have been in existence for thousands of years. Even in modern times, herbal therapies continue to play an essential role in health care/promotion. Globally plant sources used as drugs demonstrate that 80% of these have had an ethnomedical use (Fabricant & Farnsworth Citation2001).
Traditional medicinal plants have played an important role in Turkey. During many centuries, indigenous people of Turkey have been used herbal medicines for their health care. Among these herbal medicines Rhus coriaria L. (Anacardiaceae), known as sumac, is a plant species that is used in the treatment of diabetes. Sumac is a wild edible-medicinal plant growing in tropical and temperate regions worldwide, often growing in non-agriculturally viable regions (Rayne & Mazza Citation2007). Sumac is traditionally used as a herbal medicine in the treatment of various disorders including diabetes, cancer, stroke, diarrhoea, hypertension, dysentery, haematemesis, ophthalmia, stomachache, headaches diuresis, atherosclerosis, measles, smallpox, liver disease, aconuresis, teeth and gum ailments, dermatitis and liver disease in Mediterranean countries (Abu-Reidah et al. Citation2015). In Turkey, a decoction prepared from sumac is used in the treatment of diabetes and toothache as a traditional herbal medicine by local people of Southeastern Anatolia. Sumac is also used as a spice by local people.
Several research papers have been published describing health properties of sumac including antioxidant (Lee et al. Citation2002), antifibrogenic (Lee et al. Citation2003), antitumourigenic activities (Park et al. Citation2004) and hypoglycaemic (Giancarlo et al. Citation2006). Furthermore, R. coriaria is known to possess non-mutagenic, fever-reducing, DNA protective, antiseptic, antifungal, antibacterial, antioxidant, anti-ischemic, hypouricaemic, hypoglycaemic and hepatoprotective properties, which support its traditional uses (Abu-Reidah et al. Citation2015). However, very limited information is available on in vivo antioxidant, antihyperlipidaemic and antidiabetic activities of sumac.
Within this study, lyophilized hydrophilic extract obtained from the fruit of R. coriaria was evaluated for its ameroliating properties including antioxidant, antihyperlipidaemic and antidiabetic activities in vivo.
Materials and methods
Plant material and preparation of lyophilized extract
Plant samples were collected from Turkey (Haci Hamza village, Dargeçit/Mardin) in July 2013 (GPS coordinates 37° 33′ 39″ N 41° 45′ 53″ E). The identity of the plant materials was confirmed by Fevzi Ozgokce, PhD, at the Biological Sciences Department, Science Faculty, Yuzuncu Yil University, Turkey, and a voucher specimen stored at the university’s herbarium (Herbarium code: VANF-163744). The plant materials were promptly washed with distilled water and were left at room temperature in the dark until dry. Subsequently, the samples were ground and stored at −20 °C until analyzed.
The lyophilized extract was prepared as described previously (Dalar & Konczak Citation2013) with some modifications. Briefly, the ground plant materials were mixed with a five-fold volume of purified water (g/ml), shaken for 2 h at room temperature (22 °C) and centrifuged for 20 min with the supernatant collected. The solvent of supernatants were evaporated under reduced pressure at 40 °C. The derived fraction was dissolved in purified water and freeze-dried under a vacuum at −51 °C to obtain a fine lyophilized powder.
Animals
Fifty-four Wistar albino male rats 4–6 months of age, weighing 200–300 g, were provided by the Experimental Animal Research Center, Yuzuncu Yil University. The animals were housed at 20 ± 2 °C in a daily light/dark (∼16/8) cycle. The ethic regulations were followed in accordance with national and institutional guidelines for the protection of animal welfare during experiments. This study was approved by The Ethic Committee of the Yuzuncu Yil University (Protocol number: 27552122-264).
Induction of diabetes
The animals were fasted for 12 h prior to the induction of diabetes. Streptozotocin (STZ) freshly prepared in citrate buffer (0.1 M, pH 4.5) was administered intraperitoneally (i.p.) at a single dose of 50 mg/kg. Seventy-two hours after, blood glucose levels of STZ-treated fasted rats greater than 200 mg/dl were considered as diabetic and used in this study.
Chemicals
The chemicals of the technical grade used in this study were supplied by Sigma Chemical Co. (St. Louis, MO). Kits for antioxidant enzyme analysis were supplied by Randox Laboratories Ltd. The kit of α-glucosidase was supplied from BioVision (Catalog # K690–100, Milpitas, CA).
Acute toxicity test
The method of Ibeha and Ezeaja (Citation2011) were employed in this study. Twelve rats were randomly grouped into three groups; rats were dosed with 250, 500 or 1000 mg/kg of the extract orally via gastric gavage. The animals were given food and water ad libitum. No signs of toxicity and mortality were observed over a period of 72 h.
Experimental design
The rats were randomly divided into six groups each containing seven (n = 7) rats.
Normal control group (NC): NC rats received citrate buffer (pH 4.5) (1 ml/kg, i.p.).
Diabetes mellitus group (DM): DM rats received STZ in a single dose (50 mg/kg, i.p.). Seventy-two hours later, the glucose levels in blood samples taken from the tail vein were determined by Accu-Chek Go (Roche) brand glucometer equipment and through its strips. The rats with blood glucose 200 mg/dl and above were regarded as diabetic.
Diabetes mellitus + Acarbose (DM + AC-20) (20 mg/kg b.w.) group: Acarbose (20 mg/kg, per day) was treated to diabetic rats via orally during 21 d.
Diabetes mellitus + Extract (DM + Extract-100) (100 mg/kg b.w.) group: R. coriaria lyophilized extract (100 mg/kg, per day) was treated to diabetic rats via orally during 21 d.
Diabetes mellitus + Extract (DM + Extract-250) (250 mg/kg b.w.) group: R. coriaria lyophilized extract (250 mg/kg, per day) was treated to diabetic rats via orally during 21 d.
Diabetes mellitus + Extract (DM + Extract-500) (500 mg/kg b.w.) group: R. coriaria lyophilized extract (500 mg/kg, per day) was treated to diabetic rats via orally during 21 d.
Preparation of tissues supernatant and erythrocyte pellets
At the end of experiments, the rats were anesthetized by injection of ketamine (5 mg/100 g body weight) i.p. The blood samples were obtained from a cardiac puncture. Blood samples were put immediately into two silicon disposable glass tubes with ethylenediaminetetraacetic acid (EDTA) and without EDTA. The first tubes were used for the determination of glycosylated haemoglobin (HbA1c) levels and erythrocyte pellets. Second tubes were centrifuged at 4000 g for 15 min at 4 °C for serum. Then, the pellets were washed three times with physiological saline (0.9% NaCl). The tissues at the small intestine, brain, kidney and liver were dissected and put in petri dishes. After washing the tissues with physiological saline (0.9% NaCl), samples were taken and kept at −78 °C during the analysis. The tissues were homogenized for 5 min in 50 mm ice-cold potassium dihydrogen phosphate (KH2PO4) solution (1:5 w/v) using stainless steel probe homogenizer (20 kHz frequency ultrasonic, Jencons Scientific Co., Bedfordshire, UK) for 5 min, and then centrifuged at 7000×g for 15 min. All processes were carried out at 4 °C. Supernatants and erythrocyte pellets were used to determine antioxidant defence system (ADS) constituents and malondialdehyde (MDA) contents (Dogan & Celik Citation2012). Also, α-glucosidase activity was measured in small intestine tissue supernatant.
Biochemical analysis
The erythrocyte, liver, brain, kidney and small intestine in tissues supernatants MDA concentration, reduced glutathione (GSH) concentration, glutathione-S-transferase (GST) activity, glutathione reductase (GR) activity, GSH-Px activity and SOD activity were carried out as described previously (Dogan & Celik Citation2012). CAT activity was determined using the method described by Aebi (Citation1974) based on that of the rate of H2O2 consumption and as the decrease in absorbance at 240 nm. α-glucosidase activity was measured in small intestine tissue samples colorimetrically at 410 nm by α-glucosidase hydrolyzes the substrate mix to release the p-nitrophenol (BioVision kits).
Measurement of biochemical parameters
Serum biochemical parameters level were measured by an Auto Analyzer (COBAS 8000/Roche/Germany/Serial No. 1296-08) using the Roche kits (Rotkreuz, CHE). Insulin (CEA448Ra, Buckingham, UK) and c-peptide (CEA447Ra, Buckingham, UK) levels were measured in the 450 absorbance by Enzyme-linked Immunosorbent Assay kit.
Analysis of data
All data were expressed as mean ± standard deviation. The statistical analyses were made using the Minitab 13 (Minitab, State College, PA) for windows packet program. One-way analysis of variance (ANOVA) statistical test was used to determine the differences between means of the experimental groups accepting the significance level at p≤0.05.
Results
Acute toxicity studies
Optimum tolerance was detected in animals against the application of lyophilized extracts (250, 500 and 1000 mg/kg, respectively). The non-lethal dose was obtained until 1 g/kg lyophilized extract. Not any noticeable signs of toxicity and mortality were observed after daily administration of the extract orally for 3 d.
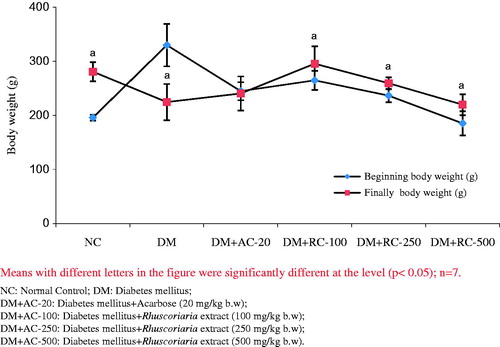
Effect of the extract on body weight
Changes in initial and final body weight in control and experimental groups were presented in . Significant weight loss was observed in final diabetic rats compared with initial animals. Treatment with extract improved the body weight as compared with initial diabetic animals.
Effect of the extract on hyperglycaemia
As shown in , there was no significant difference between NC and DM groups blood glucose levels at 1, 3 and 6 h, respectively, compared with zero-hour (0 h). On the contrary, DM + AC group glucose level significantly (p < 0.05) decreased at 1 and 3 h as compared with 0 h after administration of acarbose. In addition, a significant glucose level decrease was observed in DM + Extract (100 mg), DM + Extract (250 mg) and DM + Extract (500 mg) groups (p < 0.05) after 3 h compared to zero-time. Additionally, blood glucose level significantly decreased in DM + Extract (250 mg) group after 14 and 21 d ( and ).
Table 1. Effects of plant extract on blood glucose levels.
Effect of the extract on lipid profile
presented the change levels of triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL-c) and high-density lipoprotein (HDL-c). In all diabetic groups, TG, TC and LDL-c levels were significantly increased (p < 0.05) compared to the NC group, whereas the HDL-c was significantly decreased (p < 0.05) in the DM + AC group compared to the NC group. On the other hand, the administration of acarbose and/or plant extract, significant decrease were detected in TG, TC and LDL-c levels (p < 0.05) on DM + AC (20 mg), DM + Extract (100 mg), DM + Extract (250 mg) and DM + Extract (500 mg) groups compared to the DM group ().
Table 2. Effects of plant extract on lipid profile.
Effect of the extract on liver and renal serum biomarkers
As presented in , the activities of serum plasma liver damage enzymes such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) and renal serum biomarkers such as creatinine (CRE) and urea significantly increased in the DM group compared to the NC group. Interestingly, oral administration of the plant extract in diabetic groups for 21 d significantly restored the enzyme levels close to NC group ().
Table 3. Effects of plant extract on liver and renal serum biomarkers.
Effect of the extract on serum glucose, glycosylated Hb, serum insulin, serum c-peptide levels and α-glucosidase activity in small intestine tissue
As shown in , STZ caused a significant elevation in the levels of the HbA1c and α-glucosidase activity but serum insulin levels observed a significant decrease in the DM group when compared to the NC group. On the other hand, diabetic rats were when administrated by RC-extract, the extract caused a significant decrease in the levels of HbA1c and α-glucosidase activity but RC-extract caused a significant increase in serum insulin levels in the extract supplement diabetic rats comparison to DM group. Also, C-peptide level did not change significantly in all groups when compared with each other ().
Figure 2. The effect of lyophilized fruit extract of sumac on the glucose levels of rats >21 d of treatment.
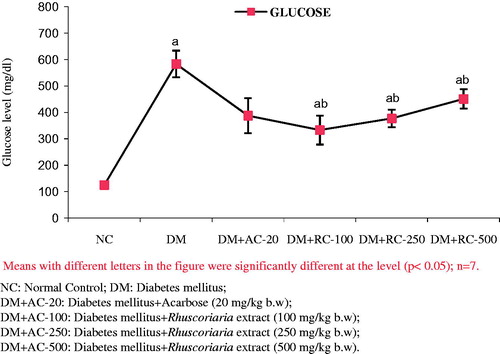
Table 4. Effects of plant extract on serum glucose, glycosylated Hb, serum insulin, serum C-peptide levels and α-glucosidase activity in small intestine tissue.
Effect of the extract on lipid peroxidation and antioxidant defence systems
With regard to MDA content and ADS constituent, the results of experiment showed that the treatment of rats with STZ and STZ + RC-extract/acarbose caused changes in MDA concentration and antioxidative defence systems such as GSH, GST, GR, CAT, GPx and SOD in small intestine, brain, kidney, erythrocyte and liver in comparison to control groups. According to the results, the increased MDA content due to oxidative stress induced by STZ were found to be decreased in RC-extract treated groups. Namely, MDA content increased significantly in the brain, kidney, erythrocyte and liver tissues exposed to DM group, whereas decreased in the DM + RC-extract groups rats. Further, the MDA content significantly decreased in the brain, kidney and erythrocyte tissues of DM + RC-extract groups as compared with those of DM rats. With regard to antioxidant defence systems, GR, CAT, GPx and SOD activities decreased significantly in the brain, kidney, erythrocyte and liver of DM group while a significant increase was observed for GST. While GSH decreased significantly in the brain, kidney and liver of the DM group, it did not change significantly in the small intestine and erythrocyte tissues as compared with the normal control group. On the other hand, the RC-extract-supplemented DM groups generally exhibited a significant increase in the ADS compared to diabetic control ().
Table 5. Effects of plant extract on lipid peroxidation and antioxidant defence systems.
Discussion
Overexposures to oxidative stress caused by environmental pollutants and chemical agents are thought to increase the risk from cancer, diabetes, etc. Hence, efforts are needed to provide effective protection from the damaging agents. Experimental studies have shown the influence of a number of plant chemicals in this regard. Based on the concept that agents that can activate the biochemical pathways for detoxification of hazardous compounds are potential chemopreventive agents for cancer and chronic diseases, we have investigated a number of plants in search of new sources for candidate compounds that will be able to protect from irreversible DNA damage, carcinogenesis and diabetes. The first aim of this study was to investigate whether RC-extract could prevent hyperglycaemic, hyperlipidaemia and hepatotoxic of STZ, decrease content of the MDA and increase the antioxidant defence system in rats.
As shown in , a significant weight loss was observed in the final diabetic group compared to initial animals. Treatment with RC-extract increased the body weight compared to initial diabetic animals. The reasons behind such effect of diabetes mellitus and RC-extract have not been fully understood until now. However, there might be a positive role of RC-extract on weight gain and energy metabolism. Similar effects of RC-extract for their possible hypoglycaemic potential on rats have been reported previously (Daisy et al. Citation2009; El Shafey et al. Citation2013). Lipid profile results showed that the RC-extract might have positive effects on lipid metabolism. Additionally, the increase in TG, TC and LDL-C and the decrease in HDL-C in diabetic group compared to the normal control group were significant. Reasons behind such effect of RC-extract are still unclear. However, d-limonene (L-methyl-4-(1-methylethenyl)-cyclohexane) (a component of sumac essential oil) is a monocyclic monoterpene component of sumac which has hypocholesterolemic effects (Santiago et al. Citation2010). The hypocholesterolemic effect of sumac extracts and its bioactive components have been reported previously in rabbits, mice and broiler chickens (Golzadeh et al. Citation2012). Although the plasma cholesterol lowering effects of sumac have been shown in rabbits, mice and broiler chickens, there is no or limited study on the effects of sumac extract on serum TC, TG, serum blood sugar levels and other serum lipoproteins. Santiago et al. (Citation2010) reported that lower blood cholesterol concentrations could be mediated by the stimulation of 3-hydroxy-3-methylglutaryl CoA reductase activity. Moreover, d-limonene has resulted in lower blood sugar and insulin concentrations, and pancreatic β-cell mass in the rat (Santiago et al. Citation2011). Hence, RC-extract reduces the serum TG, TC and LDL-C concentrations, which can be related to the stimulated activity of the HMG-CoA reductase enzyme.
A remarkable elevation in AST, ALT, ALP, LDH, CRE and urea were observed in diabetic (DM) group rats. Treatment with RC-extract or acarbose significantly decreased AST, ALT, ALP, LDH, CRE and urea activities (). It is well known that hepatic and renal damage biomarkers such as these enzymes have been considered as indicators of the hepatic or renal dysfunction and damage. In addition, the increase in the levels of AST and ALT in the serum of diabetic rats is mainly due to the leakage of these enzymes from the liver cytosol into the bloodstream (Navarro et al. Citation1993). Furthermore, ALT and AST levels are also of a value indicating the existence of liver diseases, as this enzyme is present in large quantities in the liver. ALT increases in serum when cellular degeneration or destruction occurs in this organ (Hassoun & Stohs Citation1995). LDH activity increases after liver, muscle and heart damage (Aldrich Citation2003). The increase in plasma LDH activity may be due to the hepatocellular necrosis leading to leakage of the enzyme to the bloodstream (Wang & Zhai Citation1988). Raised ALP activity indicates bone and liver diseases or bile tract blockage (Mayne Citation1994). CRE and urea levels are used in the evaluation of renal disorders. An increase in these serum parameters reflects active renal damage. In the diabetic state blood urea nitrogen and CRE are noticeable indexes for assessment of glomerular filtration rate (He et al. Citation2006). Similar to our results, some earlier studies revealed the increases in AST, ALT, ALP (Daisy et al. Citation2009), LDH, CRE and urea activity in the serum plasma of STZ-induced diabetic rats (Ozkol et al. Citation2013). STZ treatment has a significant role in the alteration of liver and renal functions since the activity of AST, ALT, LDH, ALP, CRE and urea significantly higher than those of normal value and this may lead to decay of the tissue. In addition, the results indicate that RC-extract might have a healing effect on renal and hepatic injury in STZ-induced diabetic rats.
Diabetes mellitus is a chronic metabolic disorder characterized by high blood glucose and glycosylated haemoglobin (HbA1c) levels. In the current study, a significant increase of serum blood glucose and HbA1c levels were determined in the DM group compared to the NC group (). Also, significant increases of α-glucosidase activity in small intestine tissue and remarkable reductions of insulin levels were determined in the DM group compared to the NC group. The high level of glucose led to an elevation in HbA1c. HbA1c is useful in the demonstration of glycaemic control over a period of 2–3 months, which is the lifespan of red blood cells (Heibashy Citation2005). C-peptide is formed from the specific enzymatic cleavage of proinsulin and is released from the β-cells of the pancreas in equimolar amounts with insulin (Rubenstein et al. Citation1969). Insulin is an important parameter in diabetic patients. The reduced glucose levels suggested that RC-extract might exert through the insulin release by the stimulation of a regeneration process and revitalization of the remaining β-cells. This was clearly evidenced by the increased levels of plasma insulin in diabetic rats treated with plant extract which may stimulate insulin secretion from regenerated β-cells or caused the release of insulin from the residual β-cells. Some antidiabetic plants may exert their action by stimulating the function or number of β-cells, and thus increasing insulin release (Daisy et al. Citation2009; Patel et al. Citation2014). Another parameter is the α-glucosidase inhibitors, such as acarbose, voglibose and miglitol, which is widely used either alone or in combination with insulin secretagogues in patients with type 2 diabetes (Bedekar et al. Citation2010). α-Glucosidase is a key enzyme involved in the sugar metabolism, which is considered as a good model for studying the effect of nutraceuticals on type 2 diabetes (Kim et al. Citation2005). Phytochemicals from plants have been reported to be a rich source of α-glucosidase inhibitors (Bedekar et al. Citation2010). In this context, intestinal α-glucosidases play a critical role in carbohydrate digestion and absorption, and therefore, the inhibition of α-glucosidases provides an effective antidiabetic option by targeting postprandial hyperglycaemia. Recent evidence has shown that some plant constituents such as pine bark, green tea and ginseng are potent inhibitors of α-amylase and α-glucosidase (Bedekar et al. Citation2010). In the small intestine, absorption is higher in the distal portion, with recent technological advances it is taken forward with the surgery of the distal part of the small intestine.
Oxidative stress is implicated in the aetiology and progression of DM and its complications which affect many organs of the body such as liver, kidney, brain, eyes and so forth. The present study demonstrated that the RC-extract could have an antioxidative role in diabetic rats. As presented in , the concentration of MDA in the tissues of DM + RC-extract supplement groups differed from that of the DM group. Application of RC-extract significantly decreased the MDA contents in diabetic groups (). The increased MDA content might have resulted from an increase of ROS as a result of a stress condition in the diabetic rats. There is increasing evidence that complications associated with diabetes may be related to oxidative stress induced by the production of free radicals. Several studies have shown elevated MDA levels in the diabetic state (Daisy et al. Citation2009; El Shafey et al. Citation2013). This escalation indicates a significant rise in ROS. Hyperglycaemic conditions of diabetes not only produce excessive ROS but also impair the antioxidant defence system (Liu et al. Citation2008). On the other hand, compared to control groups a similar trend of significantly decreased ADS constituents were observed in the DM groups, whereas supplementation of the plant extract restored ADS constituents towards to control group ().
It was reported that STZ-induced DM could cause a reduction in ADS levels, such as GSH, GR, GST and CAT (Jin et al. Citation2008). The decreased ADS levels of diabetic rats are compatible with the findings of this study. Such antioxidant effect of the RC-extract can be explained by the presence of bioactive chemical components present in the RC-extract. Phytochemical characterization investigations carried out on the sumac extract revealed that this plant contains various phytochemical compounds, including organic acids (Mavlyanov et al. Citation1997), gallic acid, anthocyanins, hydrolyzable tannins (Kosar et al. Citation2007), flavones (Rayne & Mazza Citation2007; Bursal & Köksal Citation2011). A recent study showed that the sumac extract contains a rich mixture of phytochemicals, such as tannins, (iso)flavonoids and terpenoids (Abu-Reidah et al. Citation2015).
Conclusion
The present study demonstrated the antidiabetic properties of lyophilized hydrophilic extract obtained from R. coriaria fruit in STZ-diabetic without toxic effects. We have observed that the RC-extract might have antidiabetic activities by reducing effects on the levels of serum enzymes, renal function markers, α-glucosidase and lipid peroxidation, whereas the RC-extract uptake could have an antioxidative role in rats. In addition, the results suggest that regular intake of the RC-extract may be useful for diabetes-related complications.
Funding information
The authors are grateful to the Yuzuncu Yil University Grant Commission for providing financial assistance during the tenure of research numbered with YYÜ-BAP-2013-FBE-D064.
Disclosure statement
This study represents the main part of PhD dissertation of Abdulahad Dogan. None of the authors has a commercial interest, financial interest and/or other relationship with manufacturers of pharmaceuticals, laboratory supplies, and/or medical devices or with commercial providers of medically related services. The authors report that they have no conflicts of interest.
References
- Abu-Reidah IM, Ali-Shtayeh MS, Jamous RM, Arráez-Román D, Segura-Carretero A. 2015. HPLC-DAD-ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 166:179–191.
- Aebi H. 1974. Catalase. In: Bergemeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press. p. 673–684.
- Aldrich JE. 2003. Clinical enzymology. In: Anderson SC, Cockayne S, editors. Clinical chemistry: concept and applications. New York: McGraw Hill. p. 261–284.
- American Diabetes Association (ADA). 2012. Diagnosis and classification of diabetes mellitus. Diab Care. 35:64–71.
- Ando K, Fujita T. 2009. Metabolic syndrome and oxidative stress. Free Radic Biol Med . 47:213–218.
- Bedekar A, Shah K, Koffas M. 2010. Natural products for type II diabetes treatment. Adv Appl Microbiol. 71:21–73.
- Bursal E, Köksal E. 2011. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.). Food Res Int. 44:2217–2221.
- Daisy P, Eliza J, Mohamed Farook KA. 2009. A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J Ethnopharmacol. 126:339–344.
- Dalar A, Konczak I. 2013. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind Crop Prod. 44:383–390.
- Dogan A, Celik I. 2012. Hepatoprotective and antioxidant activities of grapeseeds against ethanol-induced oxidative stress in rats. Br J Nutr. 107:45–51.
- El Shafey AAM, El-Ezabi MM, Selim MME, Ouda HHM, Ibrahim DS. 2013. Effect of Gymnema sylvestre R. Br. leaves extract on certain physiological parameters of diabetic rats. J King Saud Univ Sci. 25:135–141.
- Fabricant DS, Farnsworth NR. 2001. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 109:69–75.
- Giancarlo S, Rosa LM, Nadjafi F, Francesco M. 2006. Hypoglycaemic activity of two spices extracts: Rhus coriaria L. and Bunium persicum Boiss. Nat Prod Res. 20:882–886.
- Golzadeh M, Farhoomand P, Daneshyar M. 2012. Dietary Rhus coriaria L. powder reduces the blood cholesterol, VLDL-c and glucose, but increases abdominal fat in broilers. S Afr J Anim Sci. 42:398–405.
- Hassoun EA, Stohs SJ. 1995. Comparative studies on oxidative stress as a mechanism for the fetotoxic of TCDD, endrin and lindane in C57BL/6J and DBA/2J mice. Teratology. 51:186–192.
- He CY, Li WD, Guo SX, Lin SQ, Lin ZB. 2006. Effect of polysaccharides from Ganoderma lucidum on streptozotocin induced diabetic nephropathy in mice. J Asian Nat Prod Res. 8:705–711.
- Heibashy MIA. 2005. Effect of vanadium and taurine on lipid profile and some parameters indicative of myocardial status in male rats with streptozotocin-induced diabetes. J Egypt Ger Soc Zool. 47:117–138.
- Ibeha BO, EzeaJa MI. 2011. Preliminary study of antidiabetic activity of the methanolic leaf extract of Axonopus compressus (P. Beauv) in alloxan-induced diabetic rats. J Ethnopharmacol. 138:713–716.
- Jebb S. 2004. Obesity: causes and consequences. Women’s Health Med. 1:38–41.
- Jin L, Xue HY, Jin LJ, Li SY, Xu YP. 2008. Antioxidant and pancreas-protective effect of aucubin on rats with streptozotocin induced diabetes. Eur J Pharmacol. 582:162–167.
- Kim YM, Jeong YK, Wang MH, Lee WY, Rhee HI. 2005. Inhibitory effect of pine extract on alpha-glucosidase activity and postprandial hyperglycemia. Nutrition. 21:756–761.
- Kosar M, Bozan B, Temelli F, Baser KHC. 2007. Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem. 103:952–959.
- Lee JC, Lim KT, Jang YS. 2002. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and antiapoptotic properties. Biochem Biophys Acta. 1570:181–191.
- Lee SH, Nan JX, Zhao YZ, Woo SW, Park EJ, Kang TH, Seo GS, Kim YC, Sohn DH. 2003. The chalcone butein from Rhus verniciflua shows antifibrogenic activity. Planta Med. 69:990–994.
- Liu WH, Hei ZQ, Nie H, Tang FT, Huang HQ, Li XJ, Deng YH, Chen SR, Guo FF, Huang WG, et al. 2008. Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin Med J (Engl). 121:706–712.
- Mavlyanov SM, Islambekov Sh Yu, Karimdzhanov AK, Ismailov AI. 1997. Anthocyanins and organic acids of the fruits of some species of sumac. Chem Nat Compd. 33:209.
- Mayne PD. 1994. Clinical chemistry in diagnosis and treatment. London, UK: CRC Press.
- Navarro CM, Montilla PM, Martín A, Jimenez J, Utrilla PM. 1993. Free radicals scavenger and antihepatotoxic activity of Rosmarinus. Planta Med. 59:312–314.
- Ozkol H, Tuluce Y, Dilsiz N, Koyuncu I. 2013. Therapeutic potential of some plant extracts used in Turkish traditional medicine on streptozocin-induced type 1 diabetes mellitus in rats. J Membr Biol. 246:47–55.
- Padwal RS, Majumdar SR. 2007. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet. 369:71–77.
- Park KY, Jung GO, Lee KT, Choi J, Choi MY, Kim GT, Jung HJ, Park HJ. 2004. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J Ethnopharmacol. 90:73–79.
- Patel AN, Bandawane DD, Mhetre NK. 2014. Pomegranate (Punica granatum Linn.) leaves attenuate disturbed glucosehomeostasis and hyperglycemia mediated hyperlipidemia and oxidativestress in streptozotocin induced diabetic rats. Eur J Integr Med. 6:307–321.
- Rayne S, Mazza G. 2007. Biological activities of extracts from sumac (Rhus spp.): a review. Plant Foods Hum Nutr. 62:165–175.
- Rubenstein AH, Clark JL, Melani F, Steiner DF. 1969. Secretion of proinsulin, C-peptide by pancreatic beta cells and its circulation in blood. Nature. 224:697–699.
- Santiago JVA, Jayachitra J, Shenbagam M, Nalini N. 2010. D-Limonene attenuates blood pressure and improves the lipid and antioxidant status in high fat diet and L-NAME treated rats. J Pharm Sci. 11:752–758.
- Santiago JVA, Jayachitra J, Shenbagam M, Nalini N. 2011. Dietary D-limonene alleviates insulin resistance and oxidative stress-induced liver injury in high-fat diet and L-NAME-treated rats. Eur J Nutr. 51:57–68.
- Styskal J, Remmen HV, Richardson A, Salmon A. 2012. Oxidative stress and diabetes: what can we learn insulin resistance from antioxidant mutant mouse models? Free Radic Biol Med. 52:46–58.
- Wang X, Zhai W. 1988. Cellular and biochemical factors in bronchoalveolar lavage fluids of rats exposed to fenvalerate. Zhongguo Yaolixue Yu Dulixue Zoghi. 2:271–276.
- WHO. 2006. Obesity and overweight. Geneva, Italy: World Health Organization.
- Wild S, Roglic G, Green A, Sicree R, King H. 2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 27:1047–1053.