Abstract
Context Recently, adenosine triphosphate (ATP) was occasionally found to decrease the triglyceride (TG) levels in several hyperlipidemic patients in our clinical practice.
Objective The study investigates the anti-hyperlipidemic effects of ATP in a high-fat fed rabbit model and hyperlipidemic patients.
Materials and methods Twenty-four rabbits were randomly divided into three groups of eight animals each as follows: normal diet, high-fat diet and high-fat diet + ATP group. ATP supplementation (40 mg/day) was started at the 20th day and lasted for 10 days. Serum concentrations of total cholesterol (TC), TG, LDL-C, HDL-C were measured on the 20th day and 30th day. Heart, liver and aorta were subjected histopathological examination. Twenty outpatients diagnosed primary hyperlipidemia took ATP at a dose of 60 mg twice a day for 1 week.
Results Feeding rabbits with a high-fat diet resulted in a significant elevation of lipid parameters including TC, TG, LDL-C, VLDL-C compared to the normal diet group (p < 0.01). ATP treatment significantly decreased serum TG level (p < 0.01), whilst other parameters remained statistically unaltered. Meanwhile, ATP significantly reduced the thickness of fat layer in cardiac epicardium (p < 0.05) and pathological gradation of ballooning degeneration in hepatocytes (p < 0.05). After taking ATP for 1 week, hyperlipidemia patients exhibited a significant decrease of TG (p < 0.01), but other lipid parameters had no significant change.
Discussion and conclusion The study indicates that ATP selectively decreases serum TG levels in high-fat diet rabbits and hyperlipidemic patients. Therefore, ATP supplementation may provide an effective approach to control TG level.
Introduction
Hypertriglyceridemia is a condition in which triglyceride (TG) levels are elevated, often caused or exacerbated by uncontrolled diabetes mellitus, obesity and sedentary habits. At present, hypertriglyceridemia has become more generally accepted as a risk factor for cardiovascular diseases (CVDs) (Ninomiya et al. Citation2004; Miller et al. Citation2011). A meta-analysis has shown that per 1 mmol/L TG increment increases the risks of CVDs and all-cause deaths by 13% and 12%, respectively (Liu et al. Citation2013). Therefore, controlling TG may help to prevent CVDs and other causes of death. Besides intensive therapeutic lifestyle change, some drugs are recommended to control high TG. However, existing antihyperlipidemic drugs have limited efficacies and important side effects (Berglund et al. Citation2012), so that alternative TG-lowering agents are needed. Recently, adenosine triphosphate (ATP) was occasionally found to decrease the elevated TG levels in several hyperlipidemic patients in our clinical practice. The current study was designed to test the hypothesis that supplemental ATP would improve lipid profile in a hyperlipidemic rabbit model and patients with hyperlipidemia.
Materials and methods
Experimental animals and induction of hyperlipidemia
Twenty-four adult male and female New Zealand rabbits weighing 2.0–2.5 kg were purchased from Beijing ChangYang XiShan Animal Company (Beijing, China). Animals were fed a standard basal diet for 2 weeks for adaptation and were then randomly divided into three groups each containing eight rabbits, which were respectively fed with normal diet, high-fat diet containing 10% pig fat, 0.5% cholesterol and 9.5% egg yolk in standard diet and high-fat diet combined with ATP. ATP supplementation in high-fat diet rabbits was started at the 20th day after the blood samples were taken for the first time. ATP 40 mg was dissolved in 500 mL drinking water and animals had free access to the ATP-containing water for 10 days. Rabbits were maintained in a constant temperature (21–24 °C) with a 12 h light/dark cycle starting at 6 am. The study protocol was approved by the Hebei Medical University Institutional Animal Care and Use Committee.
Biochemical measurements
On the 20th day and 30th day (the end of ATP treatment), the rabbits were fasted for 12 h and blood samples were drawn from ear marginal veins under anaesthesia. Serum total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C), high-density lipoprotein cholesterol (HDL-C), apolipoproteins A (Apo A) and B (Apo B), lipoprotein (a) (LPa) levels were determined by routine enzymatic methods using commercial kits on a Hitachi 902 auto-analyser (Tokyo, Japan).
Histopathological studies
Heart, liver and aorta were removed after the blood collection at the end of study. Tissue samples were fixed for 48 h in 10% formalin-saline and followed by paraffin embedding. Sections of the tissue were prepared by using a rotary microtome and stained with haematoxylin and eosin dye, which was mounted in a neutral deparaffinated xylene medium for microscopic observations. To quantify the fat deposition in heart, the thickness of fat layer in left ventricular epicardium was measured under microscope at low magnification (5×). Serial photographs (12–15) were taken for one slice. The thickness of the fat layer was measured at the thickest port of the fat layer. A scoring method was performed to quantify histopathological damage of liver. Ten fields of view under microscope at high magnification (400×) for one sample were observed. The severity of degeneration in hepatocytes was graded 0–3, representing no, mild, moderate and severe ballooning degeneration, respectively.
Patients and clinical study protocol
Outpatients aged 32–68 years diagnosed primary hyperlipidemia for more than 2 years with combined hypercholesterolemia and hypertriglyceridemia were enrolled in the study. Exclusion criteria were patients taking other antihyperlipidemic agents in 2 weeks before enrolment and patients with obvious liver and kidney disease, pregnant women and breast-feeding women.
A group of 20 patients took ATP tablets manufactured by Guangzhou Baiyunshan Guanghua Pharmaceutical Company Ltd. (Guangzhou, China) at a dose of 60 mg twice a day by the oral route before breakfast and dinner for 1 week. Another parallel group of 30 patients orally took placebo tablets. The usual diet remained constant during the test. At the beginning and the end of the study, the fasting (after fasting for 12 h) serum concentrations of lipid parameters including TC, TG, LDL-C, VLDL-C, HDL-C, Apo-A, Apo-B and LPa were determined. Patient study was approved by the medical ethics committee of the local Board of Health.
Statistical analysis
Data for normal distribution were expressed as mean ± SD. Statistical analyses were conducted using SPSS software version 13.0 (SPSS Inc., Chicago, IL). Group comparisons were performed with one-way analysis of variance (ANOVA) with Dunnett’s post hoc tests. Paired t-tests were used for comparing means before and after treatment for the same patient. Data for non-normal distribution were presented as medians and interquartile range and differences among groups were evaluated using the non-parametric Wilcoxon test. A p value of < 0.05 was considered as statistically significant.
Results
ATP selectively decrease TG level in rabbits fed a high-fat diet
The lipid parameters measured on the 20th and 30th days are shown in . Feeding rabbits with a high-fat diet for 20 days resulted in a significant elevation of lipid parameters including TC, TG, LDL-C, VLDL-C compared to the normal diet group (p < 0.01) (). HDL-C remained unchanged between high-fat diet and normal diet group (p > 0.05). Ten days after ATP supplementation in high-fat diet animals significantly decreased serum TG level compared to high-fat diet (p < 0.01). Meanwhile, VLDL-C had a tendency to decrease after ATP treatment and showed no statistical difference compared to normal diet group (p > 0.05), but it did not reach statistical significance compared to high-fat diet, whereas ATP supplementation did not significantly affect other lipid parameters including TC, HDL-C, LDL-C, Apo-A, Apo-B and LPa ().
Table 1. Serum concentrations of lipids parameters in rabbits measured on the 20th day and 30th day (n = 8).
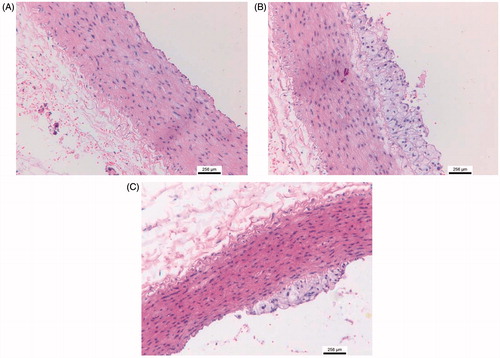
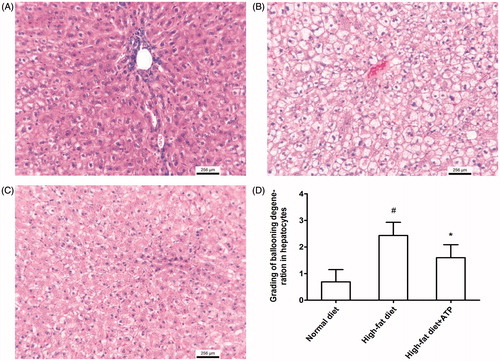
Pathological examination revealed that outer layer of hearts from high-fat diet animals had excessive fat deposition () and correspondingly histological section exhibited much more adipose tissue in epicardium compared to the normal diet animals (). ATP supplementation reduced the fat deposition evidenced by appearance of heart and histological sections (). The measurement for the thickness of fat layer in epicardium displayed a significant decrease in ATP supplementation rabbits compared to high-fat diet animals (p < 0.05, ). In addition, severe hydropic degeneration in hepatocyte was found in high-fat diet fed rabbit () and ATP supplementation obviously ameliorate histopathological damage of liver (p < 0.05, ). Furthermore, a large number of foam cells were observed in thoracic aorta from high-fat diet rabbits and ATP supplementation attenuated foam cell formation (). Taken together, the pathological results indicated that ATP diminished the lipid deposition in epicardium and pathological damage of liver caused by high-fat diet.
Figure 1. Macroscopic and histological photographs of rabbit hearts. Compared to a normal diet rabbit (A and D), high-fat diet animal (B and E) exhibited excessive fat deposition in epicardium; ATP supplementation rabbit (C and F) reduced the fat deposition. The measurement for the thickness of fat layer in epicardium displayed a significant decrease in ATP supplementation rabbit compared to high-fat diet animals (G). #p < 0.05 versus normal diet; *p < 0.05 versus high-fat diet. N = 8.
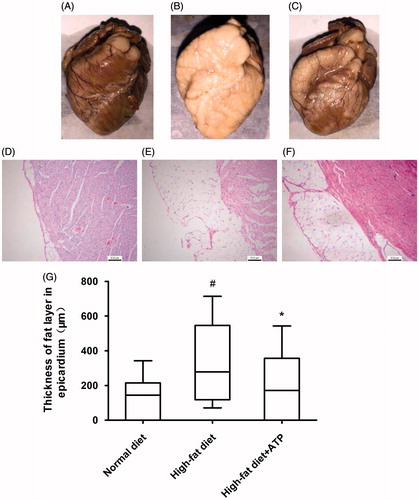
Figure 2. Histological findings in rabbit liver. (A) Representative histological photograph from a normal diet-fed rabbit. (B) Severe ballooning degeneration in hepatocyte in high-fat diet fed rabbit. (C) Mild ballooning degeneration in hepatocyte in a ATP supplementation rabbit. (D) ATP supplementation resulted in a significant reduction in grading of ballooning degeneration in hepatocytes. #p < 0.05 versus normal diet; *p < 0.05 versus high-fat diet. N = 8.
ATP decrease TG level in hyperlipidemic patients
Fasting serum lipid values in control and hyperlipidemia patients before and after ATP treatment are shown in . The baseline lipid parameters were not significantly different between the two groups (p > 0.05). After taking ATP for 1 week, hyperlipidemia patients exhibited a significant decrease of TG (p < 0.01) and other lipid parameters including TC, HDL-C, LDL-C, VLDL-C, Apo-A, Apo-B and LPa had no significant change (p > 0.05). The placebo administration did not have any effect on all of lipid parameters in control group. The results indicated a selective lowering-effect on circulating TG level by ATP.
Table 2. Effect of ATP supplementation on serum concentrations of lipids and lipoproteins in patients with hypertriglyceridemia.
Discussion
The present study indicated that high-fat and high-cholesterol diet feeding for 3 weeks raised the serum lipid profile (TC, TG, LDL-C and VLDL-C) in rabbits, which concomitantly characterized by excessive fat deposition in cardiac epicardium, a large number of foam cells in thoracic aorta and severe hydropic degeneration in hepatocyte. The main finding of the study is that ATP selectively decreases circulating TG level, without affecting other lipid parameters in high-fat diet rabbits. Meanwhile, ATP attenuated fat deposition in cardiac epicardium and reduced foam cells in thoracic aorta. It is well known that foam cell promotes the formation of fatty streak in blood vessels that is an early marker of atherosclerosis (Botham et al. Citation2007; Yu et al. Citation2013). It is generally accepted that total TG levels are an independent predictor for coronary heart diseases (Imke et al. Citation2005). Our results suggest that by selectively lowering TG level, ATP may decrease a risk for CVDs.
We further tested the effect of ATP on the lipid profile in hyperlipidemic patients. After taking ATP for 1 week, hyperlipidemia patients had a significant decrease in fast serum TG level. In contrast, other lipid parameters did not exhibit significant change after ATP treatment. The results are quite consistent with those in animal research. Therefore, ATP may be useful for dyslipidemic patients to control TG level.
Currently, the mechanism underlying the decrease of TG level by ATP is not known. ATP acts as the primary intracellular energy source and is involved in all aspects of biosynthesis and metabolism in cells. Extracellular ATP and its metabolites function as the main ligands involved in purinergic signalling, which involved in regulating a variety of biological processes including muscle contraction, vasodilation, neurotransmission and nociception (Agteresch et al. Citation1999). ATP and its metabolite adenosine can act through purinergic receptors in endothelial smooth muscle resulting in vasodilation and increased blood flow (Ellis et al. Citation2012). Oral ATP administration has been shown to increased blood flow and improve muscular function (Jäger et al. Citation2014), and are currently registered in France for the treatment of low back pain of muscular origin (Bannwarth et al. Citation2005). In addition, ATP can trigger signalling cascades for metabolic adaptation related to neuromuscular activity (phosphorylation of ERK1/2) (May et al. Citation2006). However, there are limited data related to the potential for extracellular ATP to improve lipid metabolism in hyperlipidemia. ATP and its metabolite adenosine may modulate the TG metabolism through purinergic receptors. Further studies are needed to investigate the regulation of purinergic signalling on TG homeostasis.
In conclusion, this study indicates that ATP selectively decreases serum TG levels in high-fat diet rabbits and hyperlipidemic patients. Therefore, ATP supplementation may provide an effective approach to control TG level.
Disclosure statement
The authors declare that there is no conflict of interests.
References
- Agteresch HJ, Dagnelie PC, van den Berg JW, Wilson JH. 1999. Adenosine triphosphate: established and potential clinical applications. Drugs. 58:211–212.
- Bannwarth B, Allaert FA, Avouac B, Rossignol M, Rozenberg S, Valat JP. 2005. A randomized, double-blind, placebo controlled triphosphate in study of oral adenosine subacute low back pain. J Rheumatol. 32:1114–1117.
- Berglund L, Brunzell J, Sacks FM. 2012. Patient information page from The Hormone Foundations. Patient guide to the assessment and treatment of hypertriglyceridemia (high triglycerides). J Clin Endocrinol Metab. 97:31A–32A.
- Botham KM, Moore EH, De Pascale C, Bejta F. 2007. The induction of macrophage foam cell formation by chylomicron remnants. Biochem Soc Trans. 35:454–458.
- Ellis CG, Milkovich S, Goldman D. 2012. What is the efficiency of ATP signaling from erythrocytes to regulate distribution of O(2) supply within the microvasculature? Microcirculation. 19:440–450.
- Imke C, Rodriguez BL, Grove JS, McNamara JR, Waslien C, Katz AR, Willcox B, Yano K, Curb JD. 2005. Are remnant-like particles independent predictors of coronary heart disease incidence? The Honolulu Heart study. Arterioscler Thromb Vasc Biol. 25:1718–1722.
- Jäger R, Roberts MD, Lowery RP, Joy JM, Cruthirds CL, Lockwood CM, Rathmacher JA, Purpura M, Wilson JM. 2014. Oral adenosine-5′-triphosphate (ATP) administration increases blood flow following exercise in animals and humans. J Int Soc Sports Nutr. 11:28. doi:10.1186/1550-2783-11-28.
- Liu J, Zeng FF, Liu ZM, Zhang CX, Ling WH, Chen YM. 2013. Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 12:159. doi:10.1186/1476-511X-12-159.
- May C, Weigl L, Karel A, Hohenegger M. 2006. Extracellular ATP activates ERK1/ERK2 via a metabotropic P2Y1 receptor in a Ca2+ independent manner in differentiated human skeletal muscle cells. Biochem Pharmacol. 71:1497–1509.
- Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, et al. 2011. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 123:2292–2333.
- Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. 2004. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 109:42–46.
- Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. 2013. Foam cells in atherosclerosis. Clin Chim Acta. 424:245–252.